Time to Emergence of the Lyme Disease Pathogen in Habitats of the Northeastern U.S.A.
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Model Overview
2.2. Sensitivity Analysis
2.3. MaxEnt for Host Distributions
2.4. Specific Locations
3. Results
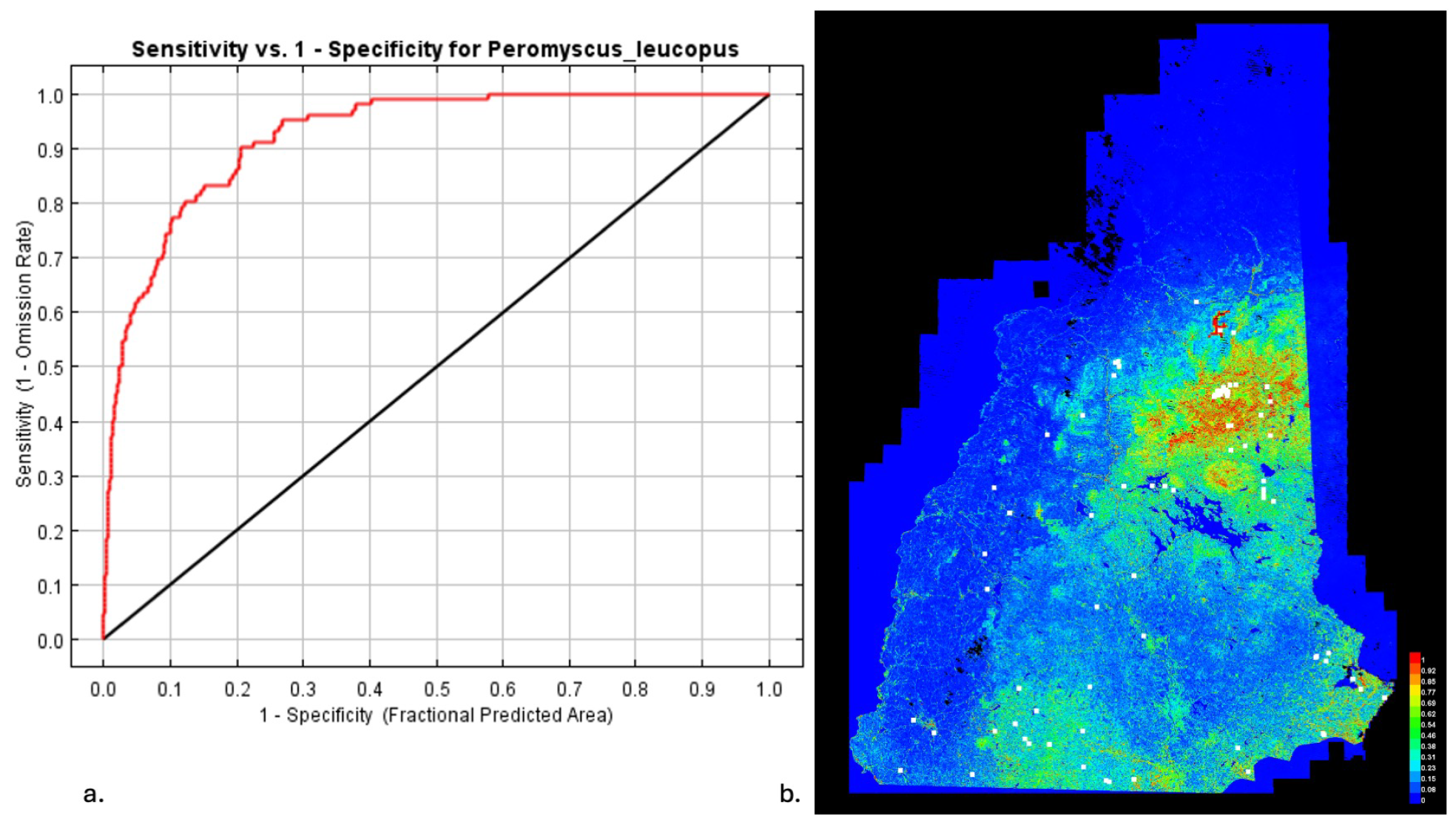
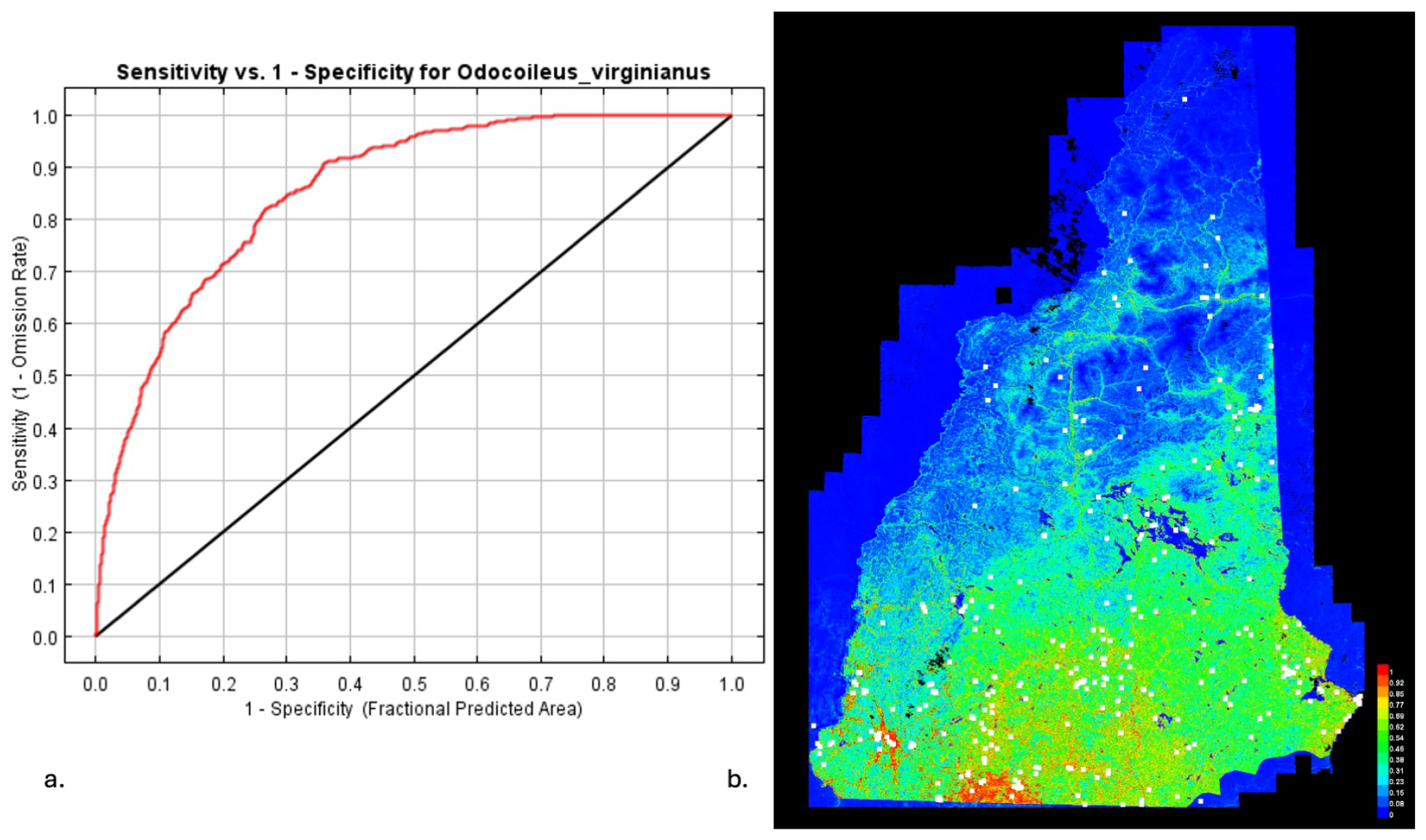
3.1. Model Performance
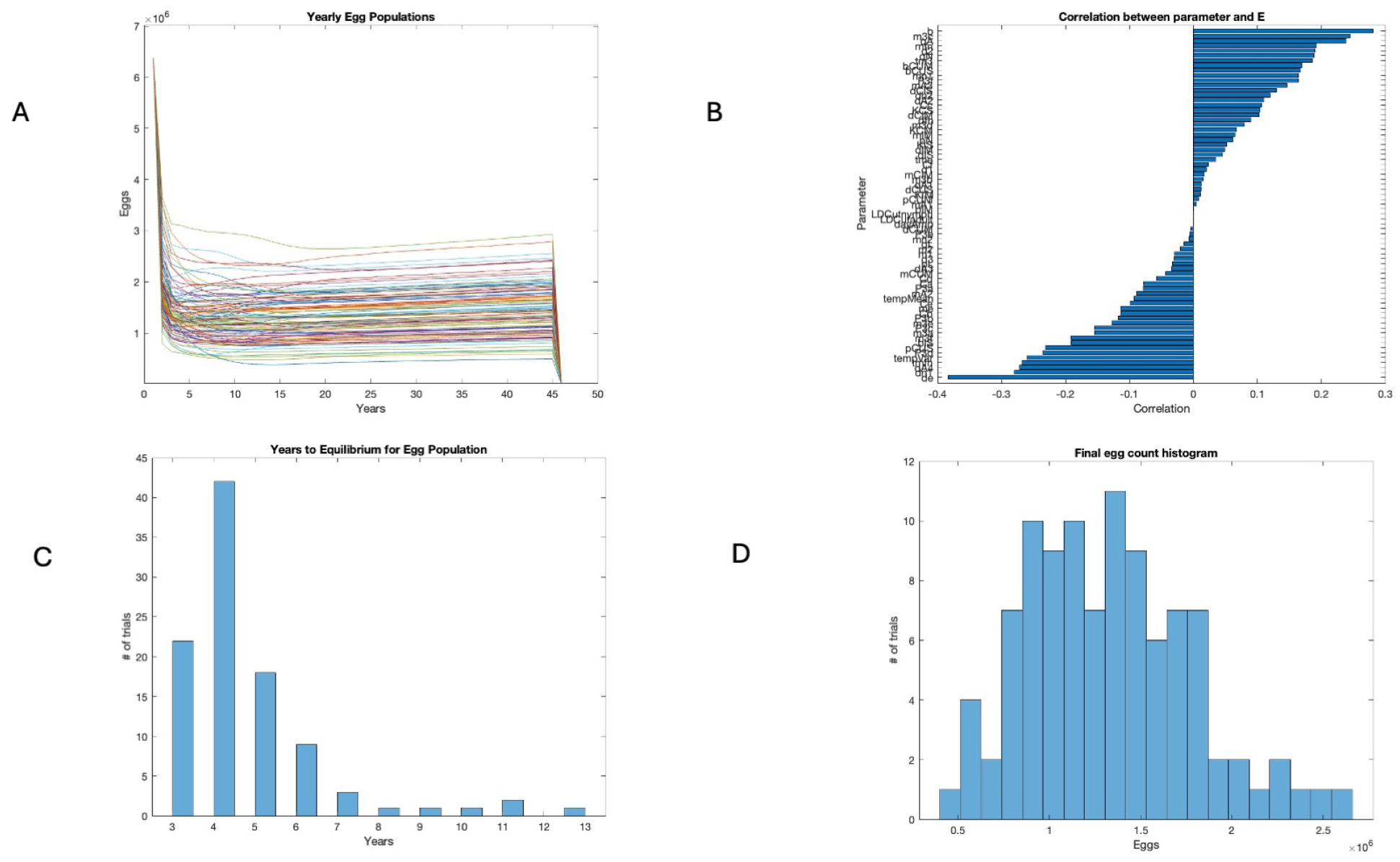
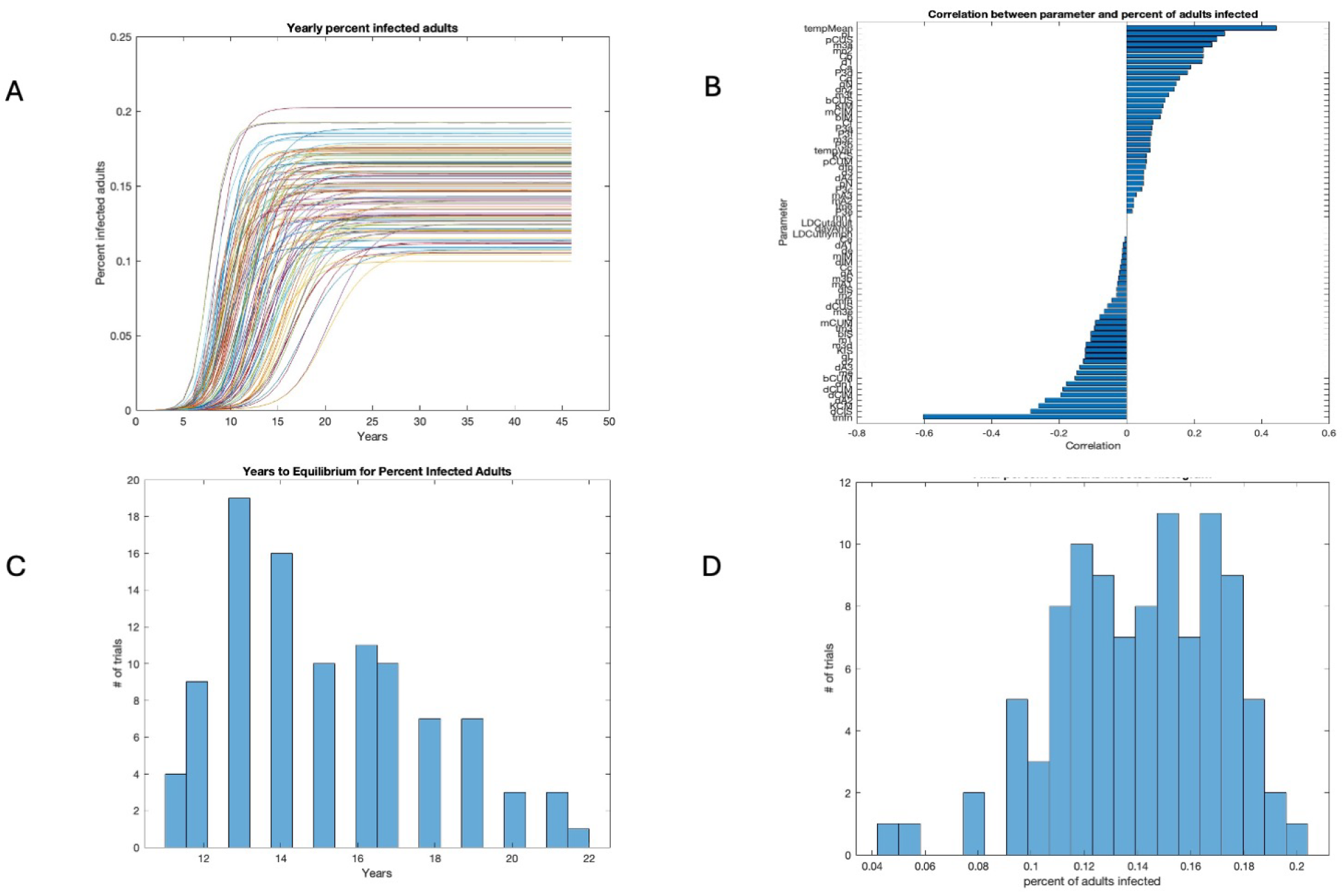
3.2. Sensitivity Analysis
3.3. Years to Equilibrium and Endemicity of Disease
3.4. MaxEnt for Host Distributions
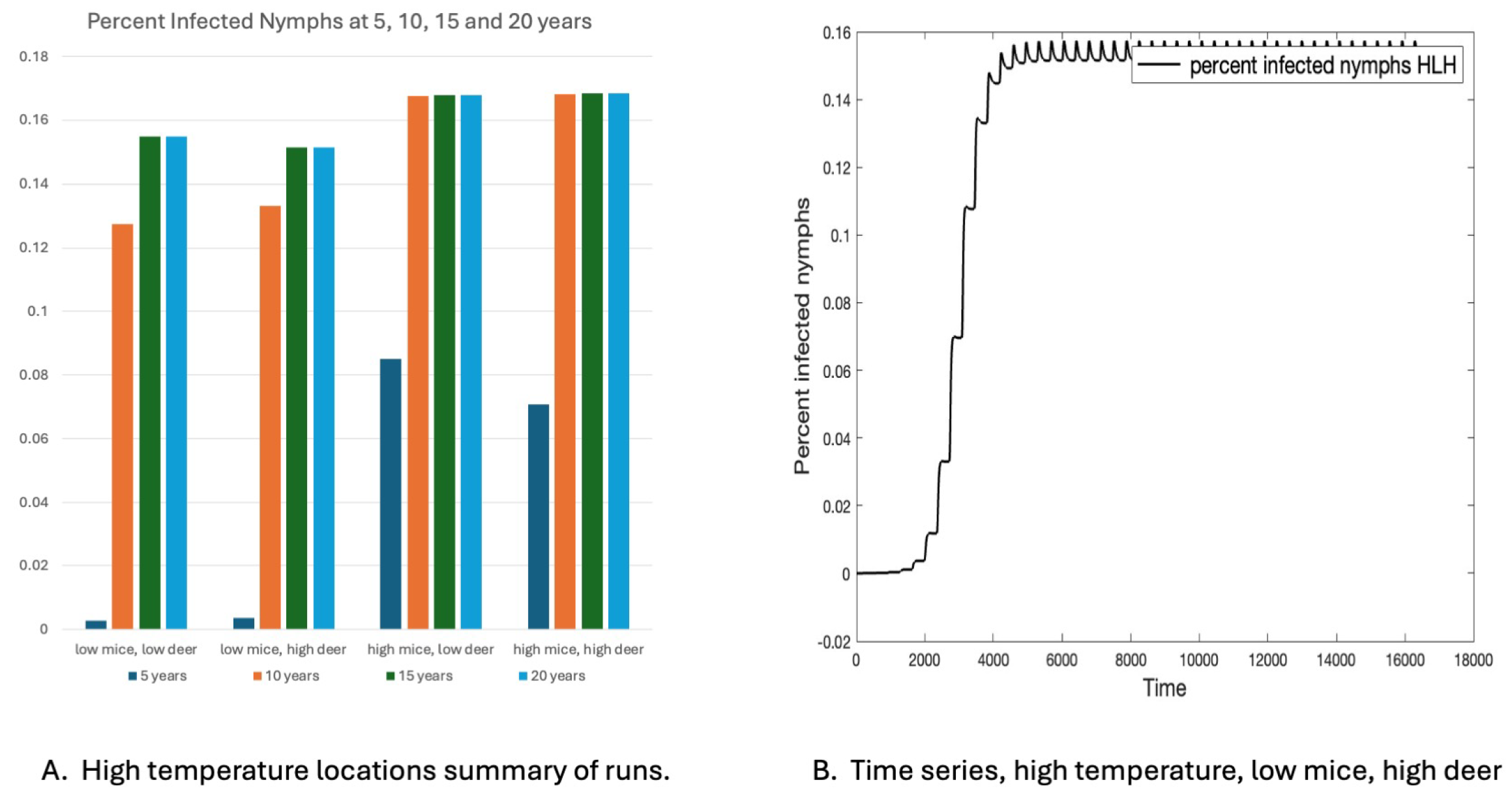
3.5. Comparison of Eight Locations
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix A.1. Equations and Parameters
Appendix A.2. Parameters
| Parameter | Value | Description |
|---|---|---|
| Tick parameters | ||
| b | 300 | egg production |
| 0.015 | egg death rate | |
| 0.01 | hardening larva death rate | |
| 0.033 | hardening larvae maturing to questing | |
| 0.094 | death rate of questing larvae | |
| 0.5 | success rate of questing larvae | |
| (0.51, 0.89, 0.73, 0.72, 0.73, 0.72) | death rate per day of feeding ticks on host X | |
| 0.5 | drop-off rate of feeding ticks | |
| daily probability of disease transmission from host to tick | ||
| 0.001 | death rate of engorged larvae | |
| 0.094 | death rate of questing nymphs | |
| 0.5 | success rate of questing nymphs | |
| 0.001 | death rate of engorged nymphs | |
| 0.094 | death rate of questing adults | |
| 0.5 | success rate of questing adults | |
| 0.006 | death rate of engorged adults | |
| (1, 1, 1) | questing tick success rate | |
| (1, 10, 15) | temperature cutoff for maturation (larvae, nymphs, adults) | |
| 0.001 | numerical stability | |
| Host parameters | ||
| (0.00261, 0.102, 0.00753, 0.176, n/a, n/a) | birth rate per day of uninfected host X, | |
| (0.000609, 0.00129, 0.00151, 0.00345, 0.00151, 0.00345) | daily death rate of host X | |
| (10, 45, 3000, 3100) | carrying capacity for | |
| (239, 176.75, 11.4, 46.84) | on host tick capacity | |
| (0.117, 0.6635) | rate of tick-to-host infection | |
| Physical parameters | ||
| 11 | mean annual temperature | |
| 1 | scaled temperature variation | |
| day length for latitude of Hanover, NH | ||
| diapause cutoffs for nymphs | ||
| diapause cutoffs for adults | ||
| Initial conditions | ||
| 6,453,100 | initial number of eggs | |
| 856,100 | initial uninfected nymphs | |
| initial infected nymphs | ||
| 100 | initial infected feeding nymphs | |
| 291,360 | initial uninfected adults | |
| Other ticks | 0 | for January 1 of run |
| (10, 45, 3000, 3100, 0, 0) | initial hosts of type X |
Appendix A.3. Data Layers and Spatial Resolution Used in MaxEnt Analysis
Maximum Entropy Modeling Tables
| Original Data Layers | Resolution |
|---|---|
| Landsat 5 TM tiles | 30 m |
| Landsat 7 ETM+ tiles | 30 m |
| NLCD 2006-National | 30 m (derived from Landsat 5) |
| NLCD 2011-National | 30 m (derived from Landsat 5) |
| TRMM 3B42 RT tiles | 0.25o (∼20 km in NH) |
| NAIP 2009 tiles | 1 m |
| NAIP 2011 tiles | 1 m |
| BioClim (WorldClim) | |
| Intermediate (derived layers) | |
| Landsat 2009 early composite | 30 m |
| Landsat 2010 early composite | 30 m |
| Landsat 2011 early composite | 30 m |
| Landsat 2009 mid-composite | 30 m |
| Landsat 2010 mid-composite | 30 m |
| Landsat 2011 mid-composite | 30 m |
| Landsat 2009 late composite | 30 m |
| Landsat 2010 late composite | 30 m |
| Landsat 2011 late composite | 30 m |
| NLCD 2006-NH | 30 m (derived from Landsat 5) |
| NLCD 2011-NH | 31 m (derived from Landsat 5) |
| TRMM 2009 early composite | 20 km |
| TRMM 2010 early composite | 20 km |
| TRMM 2011 early composite | 20 km |
| TRMM 2009 mid-composite | 20 km |
| TRMM 2010 mid-composite | 20 km |
| TRMM 2011 mid-composite | 20 km |
| TRMM 2009 late composite | 20 km |
| TRMM 2010 late composite | 20 km |
| TRMM 2011 late composite | 20 km |
| NAIP 2009 and texture derivatives | 5 m |
| NAIP 2011 and texture derivatives | 5 m |
Appendix A.4. Environmental Variables
| Variable | Percent Contribution | Permutation Importance |
|---|---|---|
| Aerial Imagery 2011 Green Band (1 m) | 22.6 | 38 |
| Mean Temperature Wettest Quarter (1 km) | 20.3 | 7 |
| Precipitation of Coldest Quarter (1 km) | 13.1 | 3.9 |
| Min Temperature Coldest Month (1 km) | 10.9 | 17 |
| Landsat July 2009 EVI (30 m) | 8.3 | 4.6 |
| National Landcover Database 2011 (30 m) | 7.1 | 7.5 |
| Landsat May 2011 EVI (30 m) | 3.1 | 3.1 |
| Total Edge Low-Intensity Residential (30 m) | 2.9 | 4.3 |
| Precipitation Seasonality (1 km) | 2.7 | 4.7 |
| Core Area Evergreen Forest (30 m) | 2.4 | 1.3 |
| Total Edge Deciduous Forest (30 m) | 1.8 | 3.6 |
| GLCM Dvar on NAIP Green Band (1 m) | 1.6 | 2.1 |
| Core Area Deciduous Forest (30 m) | 1.4 | 0.5 |
| Entropy on NAIP Green Band (1 m) | 0.7 | 1 |
| GLCM Svar on NAIP Green Band (1 m) | 0.6 | 0.1 |
| GLCM Ent on NAIP Green Band (1 m) | 0.5 | 1.1 |
| Total Edge Evergreen Forest (30 m) | 0 | 0.1 |
| Variable | Percent Contribution | Permutation Importance |
|---|---|---|
| National Landcover Database 2011 (30 m) | 25.9 | 27.7 |
| Min Temperature Coldest Month (1 km) | 23.6 | 14.4 |
| Precipitation Seasonality (1 km) | 16.6 | 8.7 |
| Total Edge Low-Intensity Residential (30 m) | 10.3 | 4 |
| Aerial Imagery 2011 Green Band (1 m) | 6.3 | 3.6 |
| GLCM Dvar on NAIP Green Band (1 m) | 6.1 | 2.8 |
| Mean Temperature Wettest Quarter (1 km) | 2.9 | 5.7 |
| Landsat May 2011 EVI (30 m) | 1.3 | 3.3 |
| Precipitation of Coldest Quarter (1 km) | 1.2 | 9.5 |
| Core Area Evergreen Forest (30 m) | 2.4 | 1.7 |
| Core Area Deciduous Forest (30 m) | 2.4 | 5.6 |
| Total Edge Evergreen Forest (30 m) | 0.8 | 3.7 |
| GLCM Ent on NAIP Green Band (1 m) | 0.7 | 1 |
| Entropy on NAIP Green Band (1 m) | 0.7 | 1.4 |
| Landsat July 2009 EVI (30 m) | 0.6 | 0.9 |
| Total Edge Deciduous Forest (30 m) | 0.5 | 4.2 |
| GLCM Svar on NAIP Green Band (1 m) | 0.5 | 1.9 |
References
- Burgdorfer, W.; Barbour, A.G.; Hayes, S.F.; Benach, J.L.; Grunwaldt, E.; Davis, J.P. Lyme disease-a tick-borne spirochetosis? Science 1982, 216, 1317–1319. [Google Scholar] [CrossRef] [PubMed]
- Say, T. An account of the arachnides of the United States. J. Acad. Nat. Sci. Phila. 1821, 2, 59–82. [Google Scholar]
- Dennis, D.T.; Hayes, E.B. Chapter: Epidemiology of Lyme borreliosis. In Lyme Borreliosis: Biology, Epidemiology, and Control; CABI: Wallingford, UK, 2002. [Google Scholar]
- Walter, K.S.; Pepin, K.M.; Webb, C.T.; Gaff, H.D.; Krause, P.J.; Pitzer, V.E.; Diuk-Wasser, M.A. Invasion of two tick-borne diseases across New England: Harnessing human surveillance data to capture underlying ecological invasion processes. Proc. R. Soc. B Biol. Sci. 2016, 283, 20160834. [Google Scholar] [CrossRef]
- Mead, P.; Hinckley, A.; Kugeler, K. Lyme Disease Surveillance and Epidemiology in the United States: A Historical Perspective. J. Infect. Dis. 2024, 230, S11–S17. [Google Scholar] [CrossRef]
- Steere, A.C.; Coburn, J.; Glickstein, L. The emergence of Lyme disease. J. Clin. Investig. 2004, 113, 1093–1101. [Google Scholar] [CrossRef]
- Fernández-Ruiz, N.; Estrada-Peña, A.; McElroy, S.; Morse, K. Passive collection of ticks in New Hampshire reveals species-specific patterns of distribution and activity. J. Med. Entomol. 2023, 60, 575–589. [Google Scholar] [CrossRef] [PubMed]
- Ostfeld, R. Lyme Disease: The Ecology of a Complex System; OUP USA: New York, NY, USA, 2011. [Google Scholar]
- Stafford, K.C., III. Reduced abundance of Ixodes scapularis (Acari: Ixodidae) with exclusion of deer by electric fencing. J. Med. Entomol. 1993, 30, 986–996. [Google Scholar] [CrossRef]
- Lindsay, L.; Mathison, S.; Barker, I.; McEwen, S.; Surgeoner, G. Abundance of Ixodes scapularis (Acari: Ixodidae) larvae and nymphs in relation to host density and habitat on Long Point, Ontario. J. Med. Entomol. 1999, 36, 243–254. [Google Scholar] [CrossRef]
- LoGiudice, K.; Ostfeld, R.S.; Schmidt, K.A.; Keesing, F. The ecology of infectious disease: Effects of host diversity and community composition on Lyme disease risk. Proc. Natl. Acad. Sci. USA 2003, 100, 567–571. [Google Scholar] [CrossRef]
- Ogden, N.H.; Mechai, S.; Margos, G. Changing geographic ranges of ticks and tick-borne pathogens: Drivers, mechanisms and consequences for pathogen diversity. Front. Cell. Infect. Microbiol. 2013, 3, 46. [Google Scholar] [CrossRef]
- Ogden, N.; Lindsay, L.; Beauchamp, G.; Charron, D.; Maarouf, A.; O’Callaghan, C.; Waltner-Toews, D.; Barker, I. Investigation of relationships between temperature and developmental rates of tick Ixodes scapularis (Acari: Ixodidae) in the laboratory and field. J. Med. Entomol. 2004, 41, 622–633. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.; Ratti, V.; Kodali, A.; Winter, J.M.; Ayres, M.P.; Chipman, J.W.; Aoki, C.F.; Osterberg, E.C.; Silvanic, C.; Partridge, T.F.; et al. Effect of rising temperature on Lyme disease: Ixodes scapularis population dynamics and Borrelia burgdorferi transmission and prevalence. Can. J. Infect. Dis. Med. Microbiol. 2019, 2019, 9817930. [Google Scholar] [CrossRef] [PubMed]
- Ratti, V.; Winter, J.M.; Wallace, D.I. Dilution and amplification effects in Lyme disease: Modeling the effects of reservoir-incompetent hosts on Borrelia burgdorferi sensu stricto transmission. Ticks Tick-Borne Dis. 2021, 12, 101724. [Google Scholar] [CrossRef] [PubMed]
- Green, R.H. A multivariate statistical approach to the Hutchinsonian niche: Bivalve molluscs of central Canada. Ecology 1971, 52, 543–556. [Google Scholar] [CrossRef]
- Green, R.H. Multivariate niche analysis with temporally varying environmental factors. Ecology 1974, 55, 73–83. [Google Scholar] [CrossRef]
- Garbutt, K.; Zangerl, A. Application of genotype-environment interaction analysis to niche quantification. Ecology 1983, 64, 1292–1296. [Google Scholar] [CrossRef]
- Clark, J.D.; Dunn, J.E.; Smith, K.G. A multivariate model of female black bear habitat use for a geographic information system. J. Wildl. Manag. 1993, 57, 519–526. [Google Scholar] [CrossRef]
- James, D.A.; Lockerd, M.J. Chapter: Refinement of the Shugart-Patten-Dueser model for analyzing ecological niche patterns. In Wildlife 2000: Modelling Habitat Relationships of Terrestrial Vertebrates; University of Wisconsin Press: Madison, WI, USA, 1986; pp. 51–56. [Google Scholar]
- Noon, B. Summary: Biometric approaches to modeling—The researcher’s viewpoint. In Wildlife 2000: Modeling Habitat Relationships of Terrestrial Vertebrates; University of Wisconsin Press: Madison, WI, USA, 1986. [Google Scholar]
- Stauffer, D.F.; Best, L.B. Chapter: Effects of habitat type and sample size on habitat suitability index models. In Wildlife 2000: Modelling Habitat Relationships of Terrestrial Vertebrates; University of Wisconsin Press: Madison, WI, USA, 1986; pp. 71–77. [Google Scholar]
- Hermy, M.; Lewi, P.J. Multivariate Ratio Analysis: A Graphical Method for Ecological Ordination. Ecology 1991, 72, 735–739. [Google Scholar] [CrossRef]
- Shugart, H., Jr.; Urban, D. Chapter: Modeling habitat relationships of terrestrial vertebrates—The researcher’s viewpoint. In Wildlife 2000: Modelling Habitat Relationships of Terrestrial Vertebrates; University of Wisconsin Press: Madison, WI, USA, 1986; pp. 425–432. [Google Scholar]
- Lancia, R.; Adams, D.; Lunk, E. Chapter: Temporal and spatial aspects of species-habitat models. In Wildlife 2000: Modelling Habitat Relationships of Terrestrial Vertebrates; University of Wisconsin Press: Madison, WI, USA, 1986; pp. 65–69. [Google Scholar]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Palace, M.W.; McMichael, C.N.; Braswell, B.H.; Hagen, S.C.; Bush, M.B.; Neves, E.; Tamanaha, E.; Herrick, C.; Frolking, S. Ancient Amazonian populations left lasting impacts on forest structure. Ecosphere 2017, 8, e02035. [Google Scholar] [CrossRef]
- McMichael, C.H.; Palace, M.W.; Bush, M.B.; Braswell, B.; Hagen, S.; Neves, E.G.; Silman, M.R.; Tamanaha, E.K.; Czarnecki, C. Predicting pre-Columbian anthropogenic soils in Amazonia. Proc. R. Soc. B Biol. Sci. 2014, 281, 20132475. [Google Scholar] [CrossRef] [PubMed]
- Howey, M.C.; Palace, M.W.; McMichael, C.H. Geospatial modeling approach to monument construction using Michigan from AD 1000–1600 as a case study. Proc. Natl. Acad. Sci. USA 2016, 113, 7443–7448. [Google Scholar] [CrossRef]
- Amaral, K.E.; Palace, M.; O’Brien, K.M.; Fenderson, L.E.; Kovach, A.I. Anthropogenic habitats facilitate dispersal of an early successional obligate: Implications for restoration of an endangered ecosystem. PLoS ONE 2016, 11, e0148842. [Google Scholar] [CrossRef]
- Winter, J.M.; Partridge, T.F.; Wallace, D.; Chipman, J.W.; Ayres, M.P.; Osterberg, E.C.; Dekker, E.R. Modeling the sensitivity of blacklegged ticks (Ixodes scapularis) to temperature and land cover in the northeastern United States. J. Med. Entomol. 2021, 58, 416–427. [Google Scholar] [CrossRef]
- Belozerov, V.N.; Fourie, L.J.; Kok, D.J. Photoperiodic control of developmental diapause in nymphs of prostriate ixodid ticks (Acari: Ixodidae). Exp. Appl. Acarol. 2002, 28, 163–168. [Google Scholar] [CrossRef]
- Thomas, O. An analysis of the mammalian generic names given in Dr. C. W. L. Gloger’s Naturgeschichte (1841). Ann. Mag. Nat. Hist. 1895, 6, 189–193. [Google Scholar] [CrossRef]
- Zimmerman, E.A.W. Geographische Geschichte des Menschen und der Vierfiissigen Thiere; In der Weygandschen Buchhandlung: Leipzig, Germany, 1780; Volume 2. [Google Scholar]
- MatLab; Mathworks: Natick, MA, USA, 2016.
- Daymet v. 4.1. Available online: https://daymet.ornl.gov (accessed on 11 January 2024).
- Fuda, B. New Hampshire White-Tailed Deer Assessment 2024; Technical Report; New Hampshire Fish and Game Department: Concord, NH, USA, 2024. [Google Scholar]
- Donnelly, T.M.; Quimby, F.W. Chapter: Biology and diseases of other rodents. In Laboratory Animal Medicine, 2nd ed.; Chapter 7; Academic Press: Cambridge, MA, USA, 2002; pp. 248–307. [Google Scholar]
- Wolff, J.O. The effects of density, food, and interspecific interference on home range size in Peromyscus leucopus and Peromyscus maniculatus. Can. J. Zool. 1985, 63, 2657–2662. [Google Scholar] [CrossRef]
- Wang, H.H.; Grant, W.; Teel, P. Simulation of climate–host–parasite–landscape interactions: A spatially explicit model for ticks (Acari: Ixodidae). Ecol. Model. 2012, 243, 42–62. [Google Scholar] [CrossRef]
- Wu, X.; Duvvuri, V.R.; Lou, Y.; Ogden, N.H.; Pelcat, Y.; Wu, J. Developing a temperature-driven map of the basic reproductive number of the emerging tick vector of Lyme disease Ixodes scapularis in Canada. J. Theor. Biol. 2013, 319, 50–61. [Google Scholar] [CrossRef]
- Simon, J.A.; Marrotte, R.R.; Desrosiers, N.; Fiset, J.; Gaitan, J.; Gonzalez, A.; Koffi, J.K.; Lapointe, F.J.; Leighton, P.A.; Lindsay, L.R.; et al. Climate change and habitat fragmentation drive the occurrence of Borrelia burgdorferi, the agent of Lyme disease, at the northeastern limit of its distribution. Evol. Appl. 2014, 7, 750–764. [Google Scholar] [CrossRef] [PubMed]
- Levi, T.; Keesing, F.; Oggenfuss, K.; Ostfeld, R.S. Accelerated phenology of blacklegged ticks under climate warming. Philos. Trans. R. Soc. Biol. Sci. 2015, 370, 20130556. [Google Scholar] [CrossRef] [PubMed]
- Bisanzio, D.; Fernández, M.P.; Martello, E.; Reithinger, R.; Diuk-Wasser, M.A. Current and future spatiotemporal patterns of Lyme disease reporting in the Northeastern United States. JAMA Netw. Open 2020, 3, e200319. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.; Johnson, R.; Magnarelli, L. Seasonal prevalence of Borrelia burgdorferi in natural populations of white-footed mice, Peromyscus leucopus. J. Clin. Microbiol. 1987, 25, 1564–1566. [Google Scholar] [CrossRef]
- Bunikis, J.; Tsao, J.; Luke, C.J.; Luna, M.G.; Fish, D.; Barbour, A.G. Borrelia burgdorferi infection in a natural population of Peromyscus leucopus mice: A longitudinal study in an area where Lyme borreliosis is highly endemic. J. Infect. Dis. 2004, 189, 1515–1523. [Google Scholar] [CrossRef]
- Son, H. New Hampshire Department Health & Human Services (DHHS) Data Portal. 2023. Available online: https://wisdom.dhhs.nh.gov/wisdom/ (accessed on 26 September 2023).
- Feldman, K.A.; Connally, N.P.; Hojgaard, A.; Jones, E.H.; White, J.L.; Hinckley, A.F. Abundance and infection rates of Ixodes scapularis nymphs collected from residential properties in Lyme disease-endemic areas of Connecticut, Maryland, and New York. J. Vector Ecol. 2015, 40, 198. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wallace, D.; Palace, M.; Price, L.E.; Shi, X. Time to Emergence of the Lyme Disease Pathogen in Habitats of the Northeastern U.S.A. Insects 2025, 16, 631. https://doi.org/10.3390/insects16060631
Wallace D, Palace M, Price LE, Shi X. Time to Emergence of the Lyme Disease Pathogen in Habitats of the Northeastern U.S.A. Insects. 2025; 16(6):631. https://doi.org/10.3390/insects16060631
Chicago/Turabian StyleWallace, Dorothy, Michael Palace, Lucas Eli Price, and Xun Shi. 2025. "Time to Emergence of the Lyme Disease Pathogen in Habitats of the Northeastern U.S.A." Insects 16, no. 6: 631. https://doi.org/10.3390/insects16060631
APA StyleWallace, D., Palace, M., Price, L. E., & Shi, X. (2025). Time to Emergence of the Lyme Disease Pathogen in Habitats of the Northeastern U.S.A. Insects, 16(6), 631. https://doi.org/10.3390/insects16060631






