Simple Summary
This study investigates the sublethal effects of abamectin and acetamiprid on the growth, development and reproduction of the bird cherry-oat aphid Rhopalosiphum padi (L.) (Hemiptera: Aphididae). R. padi is an important agricultural pest that poses a significant threat to cereal crops worldwide. We analyzed the life table and enzyme activity to understand the impact of sublethal concentrations (LC10 and LC30) of abamectin and acetamiprid on R. padi. Our findings demonstrate that sublethal concentrations of abamectin extended the longevity of R. padi F0 generation, whereas acetamiprid significantly reduced both longevity and fecundity. In the F1 generation, acetamiprid exposure markedly decreased adult longevity, fecundity and critical population parameters, while abamectin showed no significant effects on these metrics. Population projections further revealed substantially smaller total population sizes in the acetamiprid-exposed groups compared to the abamectin-treated and control groups. Furthermore, detoxification enzyme activities changed differently after treatments. These results indicate that sublethal concentrations of acetamiprid effectively suppress R. padi population growth, whereas abamectin exhibits limited inhibitory effects. This highlights the importance of considering sublethal impacts in optimizing integrated pest management strategies against R. padi.
Abstract
The bird cherry-oat aphid Rhopalosiphum padi (L.) poses a significant threat to wheat production, resulting in substantial yield reductions. Abamectin and acetamiprid are frequently utilized for management. This study assessed the sublethal effects of abamectin and acetamiprid on R. padi through life table analysis and enzyme activity assays. At 24 h, the LC10 and LC30 values for abamectin to R. padi were 0.063 mg/L and 0.252 mg/L, respectively, while, for acetamiprid, the corresponding values were 0.065 and 0.293 mg/L. The results indicated that exposure to sublethal concentrations of abamectin (AB-LC10) extended the longevity of R. padi F0 generation, while acetamiprid (AC-LC10 and AC-LC30) decreased it. Furthermore, the fecundity of the F0 generation was significantly reduced following exposure to AB-LC30, AC-LC10 and AC-LC30. In the F1 generation, exposure to sublethal concentrations of acetamiprid negatively impacted on R. padi, as evidenced by a significant reduction in longevity; fecundity and population parameters (R0, r, λ, sxj, lx, lxmx, vxj and exj). Conversely, sublethal concentrations of abamectin did not significantly affect these parameters. Additionally, population projections revealed a significantly smaller total population size of R. padi in the acetamiprid-exposed group compared to both the abamectin-exposed and control groups. Except these population-level effects, the activities of detoxification enzymes, including cytochrome P450 monooxygenases (P450), glutathione S-transferases (GST) and carboxylesterases (CarE), changed differently after treatments. These results suggest that sublethal concentrations of acetamiprid, but not abamectin, significantly inhibit the population growth of R. padi. These insights are crucial for R. padi control and facilitate the development of effective control strategies that take into account these sublethal effects in integrated pest management strategies targeting R. padi.
1. Introduction
The bird cherry-oat aphid, Rhopalosiphum padi (L.) (Hemiptera: Aphididae), is a destructive pest of cereal crop worldwide [1,2] and listed as a Category I crop pest by the Ministry of Agriculture and Rural Affairs of China (MARAC) in Announcement No. 333 [3]. In recent years, R. padi populations showed a trend of spreading from the southern to northern regions of China, gradually becoming the dominant wheat aphid species [4]. R. padi not only causes direct damage by sucking sap but also transmits barley yellow dwarf virus (BYDV), which can result in severe yield loss during outbreaks [5]. Although various management strategies exist, chemical insecticides remain the primary method for controlling wheat aphids in the field [5,6].
Abamectin, a biological pesticide, is widely used to control red spiders and aphids in wheat [7], while acetamiprid, a neonicotinoid insecticide, is extensively applied to control various aphids on crops [8,9]. In field applications, insecticides not only exert lethal effects on target pests but may also decline to sublethal concentrations over time due to environmental degradation and spatial heterogeneity [10]. However, the continuous selection under a long-term low or sublethal dose increases the risk of pests developing resistance to insecticides [10,11]. Indeed, field populations of R. padi have developed different levels of resistance to abamectin and acetamiprid, resulting in reduced efficacy [5,8]. Consequently, investigating the sublethal effect of insecticides on insects is crucial for both integrated pest management (IPM) strategies and insecticide resistance management.
Besides insecticide resistance, exposing insects to low or sublethal concentrations of insecticides may impact the behavior and physiological traits of surviving individuals that affect population dynamics [12,13,14]. The sublethal concentration of certain insecticides had negative impacts on the survival rate, developmental period, longevity, fecundity, behavior and demographic parameters [10,14]. For example, thiamethoxam at sublethal doses reduces the feeding behavior of R. padi [6,15]. Similarly, exposed to a sublethal concentration of acetamiprid significantly reduced the longevity, fecundity, population life-table parameters and population growth of two wheat aphids, Sitobion miscanthi Fabricius and Schizaphis graminum (Rondani) [16]. Conversely, some insecticides at sublethal doses may induce hormetic effects by the stimulation of biological processes [17,18]. Such hormetic effects have been documented in various aphids, including Aphis gossypii Glover exposed to imidacloprid, acetamiprid and thiamethoxam [19,20,21]; Myzus persicae (Sulzer) exposed to imidacloprid and flupyradifurone [22,23]; Aphis craccivora (Koch) exposed to flupyradifurone [24] and S. graminum exposed to thiamethoxam [25]. Additionally, effects induced by sublethal concentrations and doses of insecticides can be observed in subsequent progeny, even in the absence of direct insecticide exposure in the offspring generation [13,14,26,27]. These findings indicate that the sublethal effects of insecticides are variable and dependent on both the specific insecticide and the target pest. Therefore, understanding these sublethal and transgenerational effects is crucial for optimizing the rational and sustainable use of insecticides, as well as for improving IPM strategies.
In addition, sublethal concentrations of insecticides alter the activity of detoxifying enzymes such as cytochrome P450 (P450), glutathione S-transferase (GST) and carboxylesterase (CarE). Alterations in these enzyme activities serve as a biomarker for assessing insecticide exposure [28,29,30]. While the sublethal effects of abamectin and acetamiprid have been extensively studied in various agricultural pests [9,16,26,31], their potential sublethal effects on R. padi remain largely undocumented. Therefore, this study aimed to characterize the effects of two sublethal concentrations (LC10 and LC30) of abamectin and acetamiprid on the development, fecundity and detoxification enzyme activity of R. padi. The results not only contribute to the development of scientific and effective pest management strategies but also significantly enhance our understanding of sublethal effects.
2. Materials and Methods
2.1. Insects
R. padi used in the experiment was obtained from a laboratory colony maintained by Prof. Maohua Chen at the College of Plant Protection, Northwest A&F University (Yangling, China). Since 2019, the R. padi colony has been continuously reared in the laboratory on wheat seedlings (Changfeng 2112) without exposure to insecticides. The aphids were maintained in a climate chamber (Rxz-380B, Ningbo Jiangnan Instrument Factory, Ningbo, China) under controlled conditions: a temperature of 24 ± 1 °C, relative humidity of 60 ± 5% and a photoperiod of L:D = 16 h:8 h.
2.2. Insecticides and Reagents
Abamectin (97% pure) and acetamiprid (98% pure) were purchased from Hebei Weiyuan Biochemical Pesticide Co., Ltd., Shijiazhuang, China. The analytical-grade acetone, ethanol absolute and trisodium phosphate were obtained from Sinopharm Chemical Reagent Co., Ltd., Shanghai, China, and CDNB, Triton X-100, Tris-HCl, reduced glutathione and 7-ethoxycoumarin were sourced from Beijing Solarbio Science & Technology Co., Ltd., Beijing, China. The carboxylesterase (CarE) assay kit was purchased from the Nanjing Jiancheng Bioengineering Institute, Nanjing, China.
2.3. Bioassay
To assess the toxicity of abamectin and acetamiprid to R. padi, a leaf-dipping method was employed [32]. Stock solutions of abamectin and acetamiprid (10 g/L) were initially prepared by dissolving the insecticides in acetone. Subsequently, these stock solutions were diluted with aqueous solutions containing 0.01% Triton X-100 to create a range of concentrations. Wheat leaves with wingless adult aphids were then completely immersed in the prepared insecticide solutions for 10 s. Following immersion, excess solution was carefully removed using clean filter paper. The treated leaves, along with the aphids, were placed in a square plastic petri dish (10 × 10 cm) containing moistened filter papers to maintain humidity. These petri dishes were then returned to an incubator maintained under the conditions described previously. As a control, the aphids were treated with a solution of 0.01% Triton X-100 only. Each treatment was repeated three times, using at least 30 aphids on 10 leaves (10 cm) from 7–10-day-old wheat seedlings. Mortality data were recorded after 24 h of exposure, and concentration–mortality regressions were estimated by probit analysis using the PROBIT procedure in SAS statistical software version 9.4 (SAS Institute Inc., Cary, NC, USA) [33]. Aphids were considered dead if they failed to response when lightly touched with a fine brush. The LC10 and LC30 concentrations of abamectin and acetamiprid were chosen for evaluating the sublethal effects on the population parameters of R. padi.
2.4. Sublethal Effects on the Parent Generation of R. padi
The newly emergence apterous adults of R. padi were treated at sublethal concentrations (LC10 and LC30) of abamectin and acetamiprid, as determined in the toxicity assay, respectively. Aphids treated with 0.01% Triton X-100 only were set as the control. No less than 30 aphids were treated at each concentration, and each treatment was replicated 3 times. Treated aphids were individually transferred to petri dishes containing wheat leaves and incubated under the same environmental conditions. Fresh untreated wheat leaves were replaced daily (24 h) after treatment. The survival and nymphs produced were observed and recorded every 24 h until death.
2.5. Sublethal Effects on the Traits of the Offspring of R. padi
To evaluate the transgenerational effects of sublethal concentrations of abamectin and acetamiprid on the offspring, nymphs laid by adults under different treatments were collected. Namely, following the experimental protocol detailed above, adult parent aphids were exposed to sublethal concentrations of abamectin and acetamiprid. Twenty-four hours post-treatment, the surviving adults were individually transferred to new wheat leaves and maintained in separate plastic dishes. After a further 24 h, all adult aphids were removed, leaving only a single neonate nymph on each wheat leaf. Three groups of 30 nymph each were supplied with untreated wheat leaves until death. The aphids were examined every 12 h before molting and the onset of reproduction. Once the aphids reach adulthood, the nymphs produced every 24 h were removed using a fine brush and counted.
2.6. Age-Specific Life Table and Population Projection
The raw life table data of individual R. padi were analyzed based on the age stage, two-sex life table theory and utilized the method described by Chi (2023) [34]. To estimate the variance and standard error of the biological and population parameters, the bootstrap technique was employed, with 100,000 bootstraps performed [35]. The age stage-specific survival rate (sxj) represents the probability of R. padi surviving to age x and stage j; age-specific survival rate (lx) denotes the probability of the R. padi population surviving to age x; age-specific fecundity of females (fxj) signifies the mean number of offspring produced by a female R. padi at age x; age-specific total oviposition (mx) represents the mean number of offspring produced by an individual R. padi at age x. These parameters were calculated as referred from Bai et al. [36]. We used Consume-MSChart and Timing-MSChart to project the growth of different treatments over the next 30 days from an initial population [37].
2.7. Determination of Detoxification Enzyme Activities in R. padi
The enzyme activity was determined using the surviving parent adults at 24 h post-exposure. The activity of the P450 enzyme and GST enzyme was determined according to the method described by Hu et al. [38]. CarE enzyme activity was measured using the carboxylesterase activity assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China, Catalog No.: A133-1-1). The protein concentration was determined using the BCA Protein Assay Kit (Takara Biotechnology (Dalian) Co., Ltd., Dalian, China). Each crude enzyme, consisting of a homogenate of 20 apterous adult aphids, was replicated three times.
2.8. Data Analysis
The longevity and fecundity of the parent generation were analyzed for significance under different concentrations of abamectin and acetamiprid using one-way analysis of variance (ANOVA) with Tukey’s honest significant difference (HSD) tests. The software SAS version 8.1 was employed for this analysis. The developmental duration of each instar, lifespan, intrinsic rate of increase (r), net reproductive rate (R0), finite rate of increase (λ) and mean generation time (T) of the F1 generation were compared under different sublethal concentrations of AB and AC using the paired bootstrap test (p < 0.05). All data were presented as the mean of three replicates ± the standard error (SE). The survival curves and enzyme activities were plotted with GraphPad Prism software (version 10). The survival rates (lx), fecundities of female (fx), fecundities of population (mx), the net reproductive rate (lxmx), reproductive values (vxj) and predicted population dynamics of R. padi under different treatments were plotted with SigmaPlot version 12.5.
3. Results
3.1. Determination of the Toxicity of Abamectin and Acetamiprid to R. padi
The toxicity of abamectin and acetamiprid to R. padi adults is shown in Table 1. Analysis of the concentration–mortality data reveals that the LC10 and LC30 values for abamectin to R. padi were 0.063 mg/L (AB-LC10) and 0.252 mg/L (AB-LC30), respectively. In comparison, the LC10 and LC30 values for acetamiprid were 0.065 (AC-LC10) and 0.293 (AC-LC30) mg/L, respectively.

Table 1.
Toxicity of abamectin and acetamiprid to adults of Rhopalosiphum padi.
3.2. Sublethal Effects on the Survial, Longevity and Fecundity of the F0 Generation of R. padi
The survival curve of adult aphids exposed to sublethal concentrations of abamectin and acetamiprid is illustrated in Figure 1. All curves display a downward trend, in which the survival rate of the AC-LC10 and AC-LC30 treatment groups declined more rapidly over time than that of the control group. The survival rate of the AB-LC30 treatment group dropped sharply within 5 days after treatment and then tended to be gentle and higher than that of the control group. Similarly, the AB-LC10 treatment group was consistent with the control group within 12 days post-treatment and thereafter higher than the control group.
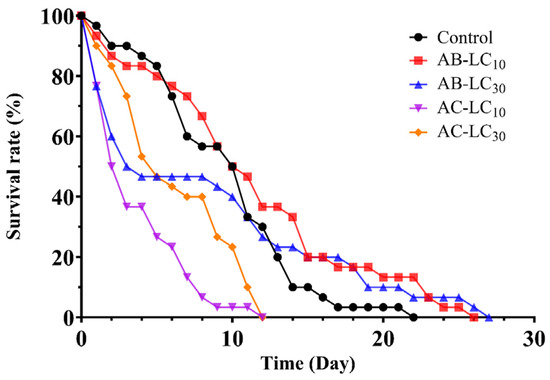
Figure 1.
Survival curves of Rhopalosiphum padi adults under sublethal concentrations of abamectin and acetamiprid.
For the F0 generation of R. padi, the longevity and fecundity of the adults were significantly affected by the sublethal concentrations of abamectin and acetamiprid that are evaluated in Table 2. The longevity was significantly extended after exposure to AB-LC10, and fecundity was significantly reduced after exposure to AB-LC30 compared to the control group. However, the longevity and fecundity were significantly decreased after treatment with AC-LC10 and AC-LC30 compared to the control group.

Table 2.
Sublethal effects of abamectin and acetamiprid on the longevity and fecundity of the Rhopalosiphum padi F0 generation.
3.3. Sublethal Effects on the Development and Fecundity of the F1 Generation of R. padi
There are different effects of abamectin and acetamiprid treatments on the development and reproduction of the F0 generation of R. padi in Table 3. Compared to the control, the AB-LC10 treatment significantly prolonged the average development period of the nymphal stage in R. padi F1 generation by 0.33 d, but the development times of the first to fourth instar nymphs were not significant between them. The AB-LC10 treatment significantly extended the average development period of the nymphal stage in the R. padi F1 generation by 0.33 d compared to the control, although there were no significant differences in the development times of the first to fourth instar nymphs. The AB-LC30 treatment significantly shortened the development times of the second and third instar nymphs by 0.21 and 0.13 d compared to the control, respectively. Except for the first instar nymphs, the AC-LC10 and AC-LC30 treatments significantly extended the development period of the second to fourth instar nymphs and nymphal stage in the R. padi F1 generation compared to the control. The adult longevity, total longevity and oviposition period of the R. padi F1 generation significantly increased in the AB-LC10 and AB-LC30 treatments but significantly decreased in the AC-LC10 and AC-LC30 treatments compared to the control. In addition, fecundity of the R. padi F1 generation decreased significantly after the AC-LC10 and AC-LC30 treatments, while there was no significant difference between the AB treatment and control.

Table 3.
Sublethal effects of abamectin and acetamiprid on the developmental period and fecundity of the Rhopalosiphum padi F1 generation.
3.4. Sublethal Effects on the Population Parameters of the R. padi F1 Generation
The sublethal effects of abamectin and acetamiprid on the life table of the R. padi F1 generation are shown in Table 4. Compared to the control, the mean generation time (T) was significantly prolonged under all treatments (F = 6.555; df = 4; p < 0.001). Abamectin treatments (AB-LC10 and AB-LC30) showed no significant effect on the life table parameters of the R. padi F1 generation, except the mean generation time (T). In contrast, acetamiprid treatments (AC-LC10 and AC-LC30) significantly negatively affected the life table parameters of the R. padi F1 generation.

Table 4.
Sublethal effects of abamectin and acetamiprid on the demographic parameters of the Rhopalosiphum padi F1 generation.
The maximum age stage-specific survival rate (sxj) of R. padi exposure to the control, AB-LC10, AB-LC30, AC-LC10 and AC-LC30 in the adult stages was 0.86, 0.96, 0.86, 0.67 and 0.67, respectively, indicating that only the acetamiprid-treated R. padi F1 generation reduced sxj compared to the control (Figure 2). The age-specific survival rate (lx) was higher in the abamectin-treated F1 generation compared to the control group but lower in the acetamiprid-treated R. padi F1 generation in Figure 3. The age-specific fecundity (mx) and the age-specific net maternity (lxmx) were similar in the control and abamectin-treated F1 generation. However, the mx and lxmx of AC-LC10 and AC-LC30 were significantly lower than those of the control in Figure 3. The age stage-specific life expectancy (exj) of the acetamiprid-treated R. padi F1 generation was lower than that of the control group, while the exj value of the abamectin-treated F1 generation was similar to that of the control group in different stages in Figure 4.
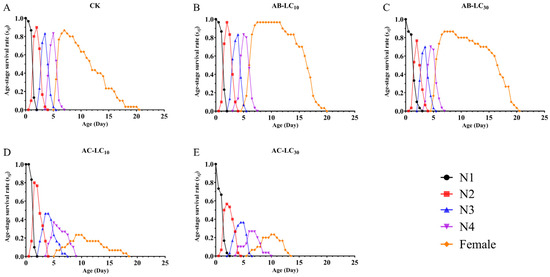
Figure 2.
Age stage-specific survival rates (sxj) of the Rhopalosiphum padi F1 generation under sublethal concentrations of abamectin and acetamiprid. Abbreviations: CK, untreated control; N1–N4 refers to the 1st to 4th nymphs. (A) The sxj of untreated control group, (B) The sxj of AB-LC10 treatment group, (C) The sxj of AB-LC30 treatment group, (D) The sxj of AC-LC10 treatment group, (E): The sxj of AC-LC30 treatment group.
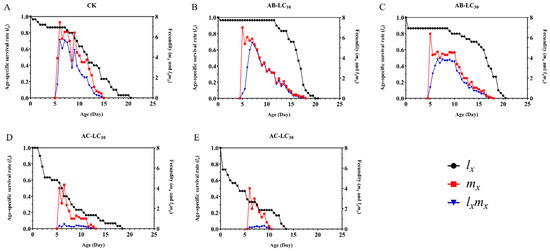
Figure 3.
Age-specific survival rate (lx), age-specific fecundity of the total population (mx) and age-specific maternity (lxmx) of the Rhopalosiphum padi F1 generation under sublethal concentrations of abamectin and acetamiprid. (A) The lx, mx and lxmx of untreated control group, (B) The lx, mx and lxmx of AB-LC10 treatment group, (C) The lx, mx and lxmx of AB-LC30 treatment group, (D) The lx, mx and lxmx of AC-LC10 treatment group, (E): The lx, mx and lxmx of AC-LC30 treatment group. Abbreviations: CK, untreated control.
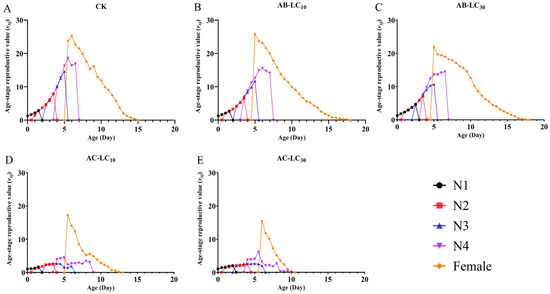
Figure 4.
Age stage-specific reproductive rate (vxj) of the Rhopalosiphum padi F1 generation under sublethal concentrations of abamectin and acetamiprid. (A) The vxj of untreated control group, (B) The vxj of AB-LC10 treatment group, (C) The vxj of AB-LC30 treatment group, (D) The vxj of AC-LC10 treatment group, (E): The vxj of AC-LC30 treatment group. Abbreviations: CK, untreated control; N1–N4 refers to the 1st to 4th nymphs.
The age stage-specific reproductive values (vxj) are shown in Figure 5. With the extension of age and stage, vxj gradually increased at first and then decreased in all the treatments. The peak vxj values occurred around the age of 5 d in all the treatments. However, the peak vxj values were 25.29, 25.80, 21.96, 17.23 and 15.48 offspring for the control, AB-LC10, AB-LC30, AC-LC10 and AC-LC30, respectively. Moreover, the sxj, lx, mx, lxmx, exj and vxj showed a decrease with the increasing acetamiprid concentration in R. padi.
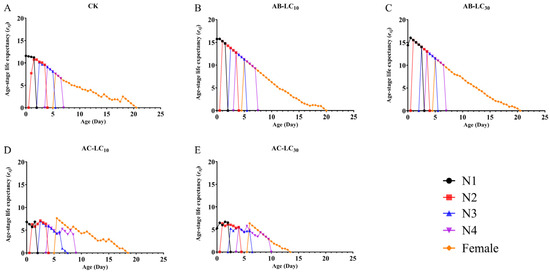
Figure 5.
Age stage-specific life expectancy (exj) of Rhopalosiphum padi F1 generation under sublethal concentrations of abamectin and acetamiprid. (A) The exj of untreated control group, (B) The exj of AB-LC10 treatment group, (C) The exj of AB-LC30 treatment group, (D) The exj of AC-LC10 treatment group, (E): The exj of AC-LC30 treatment group. Abbreviations: CK, untreated control; N1–N4 refers to the 1st to 4th nymphs.
3.5. Population Projection of R. padi Exposure to Abamectin and Acetamiprid
After beginning with an initial population, the projected population growth of R. padi for a 30-d period is shown in Figure 6. The results indicated that R. padi could complete four generations under the control treatment and AB-LC10 and AB-LC30 treatment within the next 30 days. However, only three generations can be completed under the AC-LC30 treatment during the same period. The population growth curves in R. padi exposure to AC-LC10 were nearly linear after 6 days. The population size of R. padi exposure to AB-LC10 and AB-LC30 was similar to the control. However, exposure to AC-LC10 (1.7) and AC-LC30 (0.9) resulted in a significantly lower population size compared to the control (6.5).
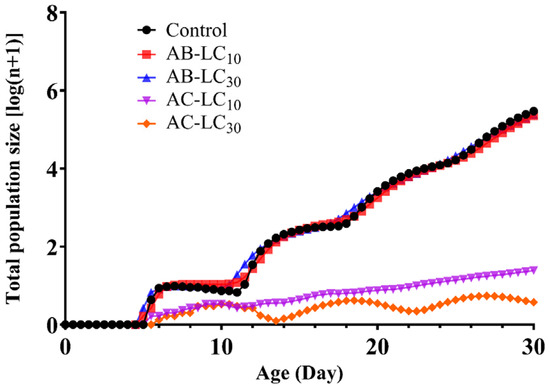
Figure 6.
Predicted population dynamics of Rhopalosiphum padi over the next 30 days under different treatments.
3.6. Detoxifying Enzymes Activity of the F0 Generation in R. padi
The enzyme activity exhibited varied responses following treatment with sublethal concentrations of abamectin and acetamiprid in Figure 7. For abamectin treatments, the activities of P450 showed no significant difference among the treatments and control, while the activities of GST significantly decreased compared to the control. In contrast, CarE activity was significantly higher in the AB-LC30 treatment than in the AB-LC10 treatment, although it still did not differ significantly from the control. Regarding acetamiprid treatments, the enzyme activities of P450 in AC-LC10 showed significant differences compared to AC-LC30 and the control, while GST showed no significant differences among the treatments and control. Additionally, CarE activity following acetamiprid treatment showed a similar pattern observed with abamectin.
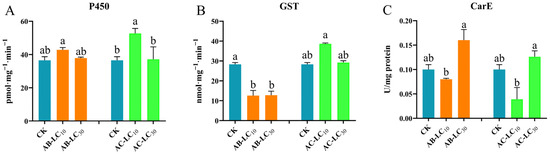
Figure 7.
Effects of sublethal concentrations of abamectin and acetamiprid on the detoxification enzyme activities of Rhopalosiphum padi from the parent generation. (A) P450 enzyme activity, (B) GST enzyme activity, (C) CarE enzyme activity. Abbreviations: CK, untreated control. The different letters above the bar represent significant differences among these treatments (p < 0.05).
4. Discussion
Arthropod populations are frequently exposed to low and/or sublethal concentrations of pesticides due to the uneven distribution and continuous degradation of active ingredients on plants following the initial pesticide applications [10,39]. These sublethal effects can manifest either as a negative inhibition or positive stimulation of population development and reproduction [10,40]. While many studies have traditionally focused on the effects within a single exposed generation, it is crucial to recognize that sublethal effects can also extend to subsequent generations [21,23,41]. Furthermore, the variability of sublethal effects is influenced by factors such as insect species, insecticide classes and concentrations [42]. In this study, we evaluated the sublethal and transgenerational effects of two insecticides, abamectin and acetamiprid, which are commonly used in wheat fields, on the aphid R. padi. Our findings indicate that sublethal concentrations of acetamiprid significantly negatively impacted the survival rate, longevity, development, reproductive and population traits of R. padi in both the F0 and F1 generations. Conversely, sublethal concentrations of abamectin did not exhibit a significant effect on R. padi. These results are critical for guiding the rational use of insecticides and for improving resistance management strategies.
Exposure to low or sublethal concentrations of pesticides can affect insect development and reproduction [10]. Our study demonstrated that the adult longevity and fecundity of the F0 generation of R. padi were significantly diminished when exposed to sublethal concentrations of acetamiprid (Table 2). These results indicate that sublethal concentrations of acetamiprid have adverse effects on R. padi, which is consistent with the results of previous research on other neonicotinoid insecticides. For instance, the adult longevity and reproduction of S. miscanthi and S. graminum were significantly reduced following exposure to a sublethal concentration of acetamiprid [16]. Similarly, the longevity and fecundity of the R. padi F0 generation were significantly decreased by exposure to sublethal doses (LD10, LD20 and LD30) of dinotefuran [1]. Moreover, studies also found that the development and fecundity of the F0 generation of Brevicoryne brassicae L. decreased after exposure to sublethal doses of imidacloprid and spirotetramat [13,43]. Many studies have demonstrated the adverse effect of abamectin on the fecundity of insects and mites, such as Cydia pomonella (L.) [26], Grapholita molesta (Busck) [31], Neoseiulus longispinosus (Evans) [44] and Phytoseiulus persimilis Athias-Henriot [45]. Similarly, the adult fecundity of the F0 generation of R. padi was significantly reduced when exposed to LC30 concentrations of abamectin. However, the longevity of the F0 generation of R. padi was significantly prolonged when exposed to the LC10 concentration of abamectin, which may be related to hormesis. The longevity extension has also been reported in Nilaparavata lugens (Stal) treated with LC25 abamectin [46]. Together with reduced fecundity, this suggests that abamectin stress at LC10 induces biological tradeoffs, diverting resources from reproduction to survival. In conclusion, the sublethal effects on the parent generation could vary among insecticide classes and concentrations, insect species and application methods [41,47].
Potential transgenerational effects of LC10 and LC30 of abamectin and acetamiprid on the fitness of R. padi were also investigated. Consistent with the results of the F0 generation, nymph development, adult longevity and fecundity of the F1 generation were significantly reduced following parental exposure to sublethal concentrations of acetamiprid compared to the control. Meanwhile, the population life table parameters, including the R0, r, λ, T, sxj, lx, lxmx, vxj and exj, showed significant decreases in the R. padi F1 generation-treated parent generation with sublethal concentrations of acetamiprid. These results are consistent with results in other wheat aphid species (S. miscanthi and S. graminum) after being exposed to sublethal concentrations of acetamiprid [16] and R. padi after being exposed to sublethal concentrations of pirimicarb [48]. These results suggest that sublethal concentrations of acetamiprid can inhibit the population of the R. padi F1 generation. However, low or sublethal doses of insecticides also cause an excitatory effect of toxicants in several pest species, which is beneficial for population development and leads to pest resurgence [11,49]. For instance, the fecundity and r of the Metopolophium dirhodum (Walker) F1 generation were significantly increased under sublethal doses of imidacloprid [50], as well as the M. persicae F1 generation at sublethal doses of flupyradifurone [23]. The present study observed that the total longevity and T of the F1 generation tended to be longer in the sublethal concentrations of abamectin treatment groups than in the control. Although no significant changes in fecundity were observed, the prolonged lifespan may lead to an extended period of detrimental effects, potentially resulting in more severe harm. Compared to the control and abamectin, the predicted population dynamics parameters of R. padi exposed to sublethal concentrations of acetamiprid exhibited a decline, indicating that low-concentration acetamiprid retains a certain efficacy in controlling R. padi populations. The prediction is based on laboratory experimental data for reference, but field conditions, including temperature and natural enemies, may also affect the population dynamics. Therefore, the transgenerational effects of insecticides on pests may influence the population dynamics and outbreaks of pest species and have great significance for IPM programs.
Insects often modulate the activities of detoxification enzymes in response to insecticide-induced stress [32,51]. Enhanced detoxification metabolism is one of the primary mechanisms of insecticides resistance [52]. The activity of detoxifying enzymes may be elevated or descending when insects are exposed to various insecticides. According to Zhang et al. [53], exposure to sublethal doses of metaflumizone and indoxacarb significantly increased the activities of P450 and GST in Spodoptera frugiperda (J.E. Smith). Jiang et al. [50] reported that sublethal concentrations of spinetoram treatments inhibited the activities of MFO, GST and CarE in Tuta absoluta (Meyrick) larvae. In the present study, exposure to a low concentration of acetamiprid (LC10 treatment) led to an increase in P450 enzyme activity in R. padi, while the activity of GST was significantly reduced compared to the control. Similarly, sublethal doses of imidacloprid inhibited the activity of the GST enzymes in S. avenae and R. padi [54]. In contrast, the GST activity was increased in R. padi treated by LC50 concentrations of chlorpyrifos, isoprocarb, imidacloprid and sulfoxaflor [32]. These seemingly contradictory results suggest that GST activity is influenced by factors such as the specific insecticide used, the exposure dose and the duration of exposure. The observed change in CarE activity may be related to the treated concentration [54]. Therefore, different insecticide types or doses may have varying effects on the activity of detoxification enzymes in insect species.
These findings indicate that sublethal concentrations of acetamiprid effectively restrict the population growth of R. padi and remain as a highly effective insecticide for controlling R. padi consistent with applications in other wheat aphids: S. miscanthi and S. graminum [16]. Combined with a slow resistance evolution to acetamiprid in field populations [8], acetamiprid can be a potential management tool for wheat aphid control, particularly in regions where resistance to neonicotinoids and other insecticides has emerged. Although abamectin did not stimulate the fecundity of R. padi leading to the resurgence, it still needs to be used with caution in practical applications. Furthermore, to manage R. padi effectively and delay resistance, rotate insecticides with different modes of action, avoiding continuous use of the same type for extended periods.
5. Conclusions
In this study, we utilized the commonly used concentrations of LC10 and LC30 to investigate the sublethal impacts of abamectin and acetamiprid on R. padi. Abamectin inhibited the reproduction of R. padi in the F0 generation but had no significant influence on the development and reproduction of the F1 generation. Thus, it is important to ensure an appropriate interval and sufficient concentration or dosage when using abamectin. In contrast, acetamiprid is an effective insecticide for suppressing R. padi population growth at low concentrations. Sublethal concentrations of acetamiprid significantly reduced the fecundity of both the F0 and F1 generations, which was confirmed by the reduction in the population size and parameters R0, r and λ compared to the control group. Therefore, further field studies are needed to validate these findings and explore the underlying molecular mechanisms. The insights gained from this research may contribute to implement more effective and environmentally friendly pest management programs for R. padi.
Author Contributions
Conceptualization, B.W., H.H. and Y.L.; methodology, B.W., H.H., X.L. and Y.L.; software, B.W., H.H. and X.L.; validation, B.W., H.H. and X.L.; formal analysis, B.W. and H.H.; investigation, B.W., H.H. and X.L.; resources, X.Y. and Y.L.; data curation, B.W.; writing—original draft preparation, B.W.; writing—review and editing, X.Y. and Y.L.; visualization, B.W. and Y.L.; supervision, Y.L.; project administration, Y.L.; funding acquisition, Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (31801733) and the National College Students Innovation and Entrepreneurship Training Program of China (202310157030).
Data Availability Statement
The original data presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors are grateful to Maohua Chen (Northwest A&F University) for providing the R. padi population.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Deng, D.; Duan, W.; Wang, H.; Zhang, K.; Guo, J.; Yuan, L.; Wang, L.; Wu, S. Assessment of the effects of lethal and sublethal exposure to dinotefuran on the wheat aphid Rhopalosiphum padi (Linnaeus). Ecotoxicology 2019, 28, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lu, H.; Guo, K.; Yao, S.; Cui, F. Insecticide resistance status and detoxification enzymes of wheat aphids Sitobion avenae and Rhopalosiphum padi. Sci. China Life Sci. 2017, 60, 927–930. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Agriculture and Rural Affairs of the People’s Republic of China (MARAC). Announcement No. 333 of the Ministry of Agriculture and Rural Affairs of the People’s Republic of China; MARAC: Beijing, China, 2020. Available online: https://www.moa.gov.cn/nybgb/2020/202010/202011/t20201130_6357326.htm (accessed on 12 October 2024).
- Miao, J.; Guo, P.; Zhang, Y.; Tan, X.; Chen, J.; Li, Y.; Wu, Y. Effect of High Temperature and Natural Enemies on the Interspecies Competition Between Two Wheat Aphid Species, Rhopalosiphum padi and Sitobion miscanthi. J. Econ. Entomol. 2022, 115, 539–544. [Google Scholar] [CrossRef]
- Gong, P.; Li, X.; Gao, H.; Wang, C.; Li, M.; Zhang, Y.; Li, X.; Liu, E.; Zhu, X. Field evolved resistance to pyrethroids, neonicotinoids, organophosphates and macrolides in Rhopalosiphum padi (Linnaeus) and Sitobion avenae (Fabricius) from China. Chemosphere 2021, 269, 128747. [Google Scholar] [CrossRef] [PubMed]
- Gul, H.; Haq, I.U.; Güncan, A.; Abbas, A.; Khan, S.; Yaseen, A.; Ullah, F.; Desneux, N.; Liu, X. Thiamethoxam-Induced Intergenerational Sublethal Effects on the Life History and Feeding Behavior of Rhopalosiphum padi. Plants 2024, 13, 865. [Google Scholar] [CrossRef]
- Cui, L.; Wang, G.; Yang, D.; Nahiyoon, S.A.; Yan, X.; Yuan, H. Biocidal radiuses of abamectin, thiamethoxam and sulfoxaflor droplets controlling against wheat aphid (Sitobion avenae). PLoS ONE 2018, 13, e0205598. [Google Scholar] [CrossRef]
- Xu, T.; Zhang, S.; Liu, Y.; Ma, L.; Li, X.; Zhang, Y.; Fan, Y.; Song, D.; Gao, X. Slow resistance evolution to neonicotinoids in field populations of wheat aphids revealed by insecticide resistance monitoring in China. Pest Manag. Sci. 2022, 78, 1428–1437. [Google Scholar] [CrossRef]
- Zhang, A.; Xu, L.; Liu, Z.; Zhang, J.; Zhao, K.; Han, L. Effects of Acetamiprid at Low and Median Lethal Concentrations on the Development and Reproduction of the Soybean Aphid Aphis glycines. Insects 2022, 13, 87. [Google Scholar] [CrossRef] [PubMed]
- Desneux, N.; Decourtye, A.; Delpuech, J.M. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef]
- Guedes, R.N.C.; Walse, S.S.; Throne, J.E. Sublethal exposure, insecticide resistance, and community stress. Curr. Opin. Insect Sci. 2017, 21, 47–53. [Google Scholar] [CrossRef]
- Qu, Y.; Ullah, F.; Luo, C.; Monticelli, L.S.; Lavoir, A.-V.; Gao, X.; Song, D.; Desneux, N. Sublethal effects of beta-cypermethrin modulate interspecific interactions between specialist and generalist aphid species on soybean. Ecotox. Environ. Saf. 2020, 206, 111302. [Google Scholar] [CrossRef] [PubMed]
- Iftikhar, A.; Hafeez, F.; Aziz, M.A.; Hashim, M.; Naeem, A.; Yousaf, H.K.; Saleem, M.J.; Hussain, S.; Hafeez, M.; Ali, Q.; et al. Assessment of sublethal and transgenerational effects of spirotetramat, on population growth of cabbage aphid, Brevicoryne brassicae L. (Hemiptera: Aphididae). Front. Physiol. 2022, 13, 1014190. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Fu, Z.; Zhu, Y.; Gao, X.; Liu, T.-X.; Liang, P. Sublethal and transgenerational effects of afidopyropen on biological traits of the green peach aphid Myzus persicae (Sluzer). Pestic. Biochem. Physiol. 2022, 180, 104981. [Google Scholar] [CrossRef]
- Daniels, M.; Bale, J.S.; Newbury, H.J.; Lind, R.J.; Pritchard, J. A sublethal dose of thiamethoxam causes a reduction in xylem feeding by the bird cherry-oat aphid (Rhopalosiphum padi), which is associated with dehydration and reduced performance. J. Insect Physiol. 2009, 55, 758–765. [Google Scholar] [CrossRef]
- Li, X.; Zhang, W.; Zhang, H.; Zhang, X.; Song, C.; Zhang, P.; Li, G.; Zhu, X.; Zhang, B. The sublethal concentration of acetamiprid suppresses the population growth of 2 species of wheat aphids, Sitobion miscanthi and Schizaphis graminum (Hemiptera: Aphididae). J. Econ. Entomol. 2024, 117, 1315–1323. [Google Scholar] [CrossRef]
- Ayyanath, M.M.; Cutler, G.C.; Scott-Dupree, C.D.; Sibley, P.K. Transgenerational Shifts in Reproduction Hormesis in Green Peach Aphid Exposed to Low Concentrations of Imidacloprid. PLoS ONE 2013, 8, e74532. [Google Scholar] [CrossRef]
- Huangfu, N.; Guo, L.; Shang, J.; Wang, L.; Zhang, K.; Li, D.; Gao, X.; Zhu, X.; Ji, J.; Luo, J.; et al. Hormetic dose response induced by sublethal-dose sulfoxaflor leads to reproductive stimulation of Aphis gossypii. Pestic. Biochem. Physiol. 2024, 204, 106061. [Google Scholar] [CrossRef] [PubMed]
- Ullah, F.; Gul, H.; Desneux, N.; Gao, X.; Song, D. Imidacloprid-induced hormesis effects on demographic traits of the melon aphid, Aphis gossypii. Entomol. Gen. 2019, 39, 325–337. [Google Scholar] [CrossRef]
- Ullah, F.; Gul, H.; Desneux, N.; Qu, Y.; Xiao, X.; Khattak, A.M.; Gao, X.; Song, D. Acetamiprid-induced hormetic effects and vitellogenin gene (Vg) expression in the melon aphid, Aphis gossypii. Entomol. Gen. 2019, 39, 259–270. [Google Scholar] [CrossRef]
- Ullah, F.; Gul, H.; Tariq, K.; Desneux, N.; Gao, X.; Song, D. Thiamethoxam induces transgenerational hormesis effects and alteration of genes expression in Aphis gossypii. Pestic. Biochem. Physiol. 2020, 165, 104557. [Google Scholar] [CrossRef]
- Yu, Y.; Shen, G.; Zhu, H.; Lu, Y. Imidacloprid-induced hormesis on the fecundity and juvenile hormone levels of the green peach aphid Myzus persicae (Sulzer). Pestic. Biochem. Physiol. 2010, 98, 238–242. [Google Scholar] [CrossRef]
- Tang, Q.; Ma, K.; Chi, H.; Hou, Y.; Gao, X. Transgenerational hormetic effects of sublethal dose of flupyradifurone on the green peach aphid, Myzus persicae (Sulzer) (Hemiptera: Aphididae). PLoS ONE 2019, 14, e0208058. [Google Scholar] [CrossRef]
- Fouad, E.A.; El-Sherif, S.A.N.; Mokbel, E.-S.M.S. Flupyradifurone induces transgenerational hormesis effects in the cowpea aphid, Aphis craccivora. Ecotoxicology 2022, 31, 909–918. [Google Scholar] [CrossRef]
- Gul, H.; Haq, I.U.; Ullah, F.; Khan, S.; Yaseen, A.; Tariq, K.; Güncan, A.; Desneux, N.; Liu, X. Hormetic effects of thiamethoxam on Schizaphis graminum: Demographics and feeding behavior. Ecotoxicology 2024, 33, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Ju, D.; Liu, Y.-X.; Liu, X.; Dewer, Y.; Mota-Sanchez, D.; Yang, X.-Q. Exposure to lambda-cyhalothrin and abamectin drives sublethal and transgenerational effects on the development and reproduction of Cydia pomonella. Ecotoxicol. Environ. Saf. 2023, 252, 114581. [Google Scholar] [CrossRef]
- Gul, H.; Guncan, A.; Ullah, F.; Desneux, N.; Liu, X. Intergenerational Sublethal Effects of Flonicamid on Cotton Aphid, Aphis gossypii: An Age-Stage, Two-Sex Life Table Study. Insects 2024, 15, 529. [Google Scholar] [CrossRef]
- Haynes, K.F. Sublethal effects of neurotoxic insecticides on insect behavior. Annu. Rev. Entomol. 1988, 33, 149–168. [Google Scholar] [CrossRef] [PubMed]
- Booth, L.H.; Wratten, S.D.; Kehrli, P. Effects of reduced rates of two insecticides on enzyme activity and mortality of an aphid and its lacewing predator. J. Econ. Entomol. 2007, 100, 11–19. [Google Scholar] [CrossRef]
- Kumrungsee, N.; Pluempanupat, W.; Koul, O.; Bullangpoti, V. Toxicity of essential oil compounds against diamondback moth, Plutella xylostella, and their impact on detoxification enzyme activities. J. Pest Sci. 2014, 87, 721–729. [Google Scholar] [CrossRef]
- Su, S.; Jian, C.; Zhang, X.; Fang, S.; Peng, X.; Pinero, J.C.; Chen, M. Sublethal Effects of Abamectin on the Development, Reproduction, Detoxification Enzyme Activity, and Related Gene Expression of the Oriental Fruit Moth (Lepidoptera: Tortricidae). J. Econ. Entomol. 2021, 114, 2430–2438. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Su, S.; Zhang, C.; Chen, M. Identification and functional characterization of two sigma glutathione S-transferase genes from bird cherry-oat aphid (Hemiptera: Aphididae). J. Econ. Entomol. 2019, 112, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Finney, D.J. Statisical logic in the monitoring of reactions to therapeutic drugs. Method. Inform. Med. 1971, 10, 237–245. [Google Scholar]
- Chi, H. TWOSEX-MSChart: A Computer Program for the Age-Stage, Two-Sex Life Table Analysis. 2023. Available online: http://www.faas.cn/cms/sitemanage/index.shtml?siteId=810640925913080000 (accessed on 24 August 2024).
- Huang, Y.B.; Chi, H. Life tables of Bactrocera cucurbitae (Diptera: Tephritidae): With an invalidation of the jackknife technique. J. Appl. Entomol. 2013, 137, 327–339. [Google Scholar] [CrossRef]
- Bai, B.; Zhang, S.P.; Li, Y.T.; Gao, P.; Yang, X.Q. Quercetin stimulates an accelerated burst of oviposition-based reproductive strategy in codling moth controlled by juvenile hormone signaling pathway. Sci. Total Environ. 2024, 913, 169643. [Google Scholar] [CrossRef]
- Chi, H.; Kavousi, A.; Gharekhani, G.; Atlihan, R.; Ozgokce, M.S.; Guncan, A.; Gokce, A.; Smith, C.L.; Benelli, G.; Guedes, R.N.C.; et al. Advances in theory, data analysis, and application of the age-stage, two-sex life table for demographic research, biological control, and pest management. Entomol. Gen. 2023, 43, 705–732. [Google Scholar] [CrossRef]
- Hu, C.; Liu, Y.X.; Zhang, S.-P.; Wang, Y.Q.; Gao, P.; Li, Y.T.; Yang, X.Q. Transcription Factor AhR Regulates Glutathione S-Transferases Conferring Resistance to lambda-Cyhalothrin in Cydia pomonella. J. Agric. Food Chem. 2023, 71, 5230–5239. [Google Scholar] [CrossRef]
- Gandara, L.; Jacoby, R.; Laurent, F.; Spatuzzi, M.; Vlachopoulos, N.; Borst, N.O.; Ekmen, G.; Potel, C.M.; Garrido-Rodriguez, M.; Boehmert, A.L.; et al. Pervasive sublethal effects of agrochemicals on insects at environmentally relevant concentrations. Science 2024, 386, 446–453. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Baldwin, L.A. Hormesis: The dose-response revolution. Annu. Rev. Pharmacol. Toxicol. 2003, 43, 175–197. [Google Scholar] [CrossRef]
- Gong, Y.; Cheng, S.; Desneux, N.; Gao, X.; Xiu, X.; Wang, F.; Hou, M. Transgenerational hormesis effects of nitenpyram on fitness and insecticide tolerance/resistance of Nilaparvata lugens. J. Pest Sci. 2023, 96, 161–180. [Google Scholar] [CrossRef]
- Müller, T.; Gesing, M.A.; Segeler, M.; Müller, C. Sublethal insecticide exposure of an herbivore alters the response of its predator. Environ. Pollut. 2019, 247, 39–45. [Google Scholar] [CrossRef]
- Lashkari, M.R.; Sahragard, A.; Ghadamyari, M. Sublethal effects of imidacloprid and pymetrozine on population growth parameters of cabbage aphid, Brevicoryne brassicae on rapeseed, Brassica napus L. Insect Sci. 2007, 14, 207–212. [Google Scholar] [CrossRef]
- Ibrahim, Y.B.; Yee, T.S. Influence of sublethal exposure to abamectin on the biological performance of Neoseiulus longispinosus (Acari: Phytoseiidae). J. Econ. Entomol. 2000, 93, 1085–1089. [Google Scholar] [CrossRef] [PubMed]
- Bostanian, N.J.; Akalach, M. The effect of indoxacarb and five other insecticides on Phytoseiulus persimilis (Acari: Phytoseiidae), Amblyseius fallacis (Acari: Phytoseiidae) and nymphs of Orius insidiosus (Hemiptera: Anthocoridae). Pest Manag. Sci. 2006, 62, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhou, C.; Yang, X.B.; Long, G.Y.; Jin, D.C. Effects of Insecticide Stress on Expression of NlABCG Transporter Gene in the Brown Planthopper, Nilaparvata lugens. Insects 2019, 10, 334. [Google Scholar] [CrossRef]
- Yin, X.H.; Wu, Q.J.; Li, X.F.; Zhang, Y.J.; Xu, B.Y. Sublethal effects of spinosad on Plutella xylostella (Lepidoptera: Yponomeutidae). Crop Prot. 2008, 27, 1385–1391. [Google Scholar] [CrossRef]
- Xiao, D.; Yang, T.; Desneux, N.; Han, P.; Gao, X. Assessment of Sublethal and Transgenerational Effects of Pirimicarb on the Wheat Aphids Rhopalosiphum padi and Sitobion avenae. PLoS ONE 2015, 10, e0128936. [Google Scholar] [CrossRef]
- Guedes, R.N.C.; Smagghe, G.; Stark, J.D.; Desneux, N. Pesticide-Induced Stress in Arthropod Pests for Optimized Integrated Pest Management Programs. Annu. Rev. Entomol. 2016, 61, 43–62. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Zhu, X.; Li, X.; Cheng, D.; Zhang, Y. Effects of acetamiprid-induced hormesis on the development and reproduction of the rose-grain aphid Metopolophium dirhodum (Hemiptera: Aphididae). Front. Physiol. 2023, 14, 1113464. [Google Scholar] [CrossRef]
- Jiang, M.; Qian, X.; Zhou, Z.; Liu, Y.; Zhang, M.; Yang, Y. Impacts of Sublethal Doses of Spinetoram on the Biological Traits and Detoxifying Enzymes of the Tomato Leaf Miner, Tuta absoluta (Lepidoptera: Gelechiidae). Insects 2024, 15, 990. [Google Scholar] [CrossRef]
- Amezian, D.; Nauen, R.; Le Goff, G. Transcriptional regulation of xenobiotic detoxification genes in insects-An overview. Pestic. Biochem. Physiol. 2021, 174, 104822. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, H.Z.; He, P.Y.; Cao, H.Q.; Zhang, W.N.; Peng, Y.C.; Sheng, C.W. Sublethal effect and detoxifying metabolism of metaflumizone and indoxacarb on the fall armyworm, Spodoptera frugiperda. Pestic. Biochem. Physiol. 2024, 201, 105879. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.H.; Zheng, X.S.; Gao, X.W. Sublethal effects of imidacloprid on the fecundity, longevity, and enzyme activity of Sitobion avenae (Fabricius) and Rhopalosiphum padi (Linnaeus). Bull. Entomol. Res. 2016, 106, 551–559. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).