Potentially Suitable Habitat for the Pest Histia rhodope Based on Its Host Plant Bischofia polycarpa and Climatic Factors in China
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Processing of Distribution Data
2.2. Optimization and Construction of the MaxEnt Model
2.3. Analysis of the Potential Distribution Under Current and Predicted Climate Change
2.4. Analysis of Centroid Migration Under Predicted Climate Change
3. Results
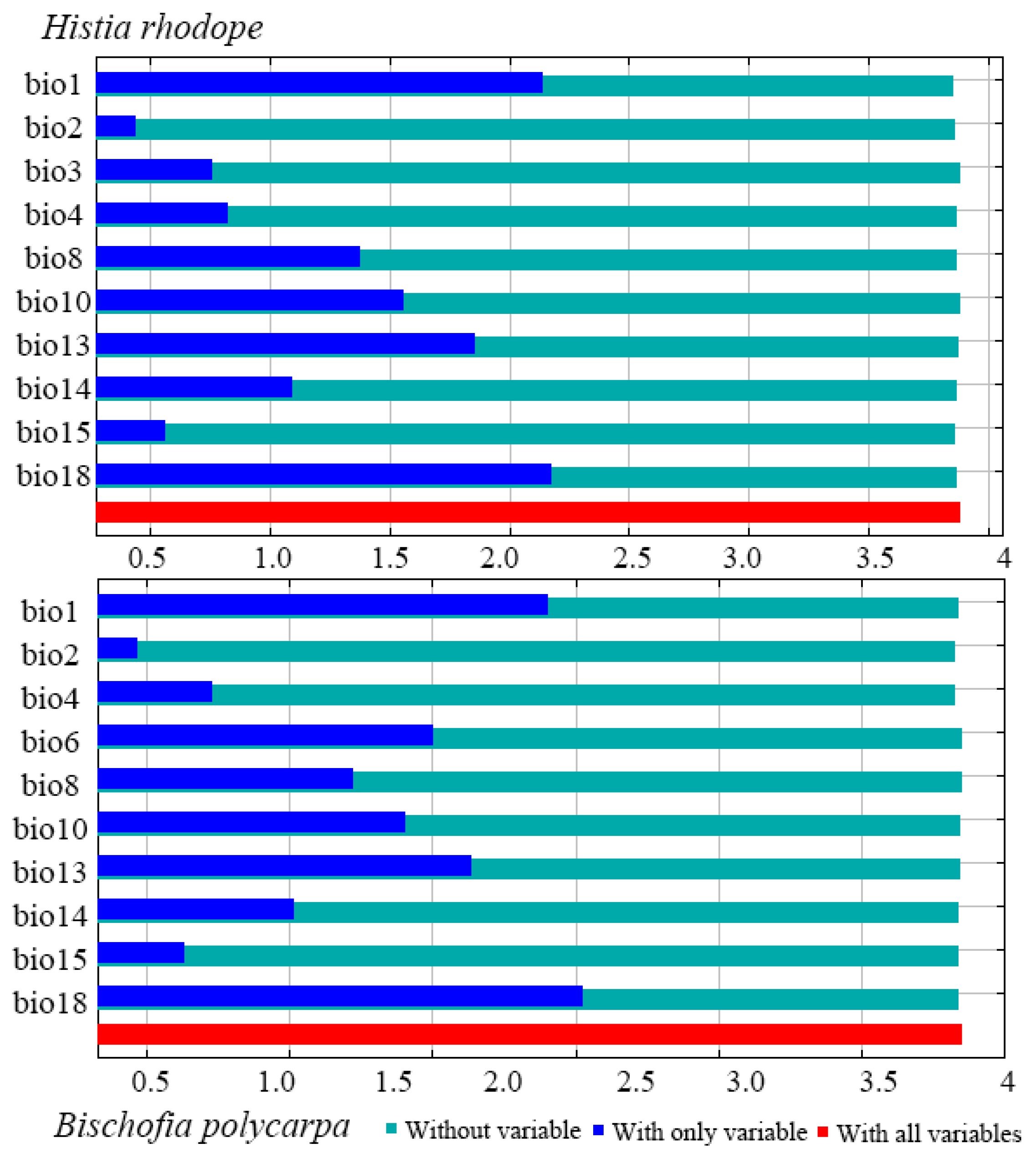
3.1. Screening and Importance of Key Environmental Factors
3.2. Prediction of the Distribution of Suitable Habitats for H. rhodope and B. polycarpa Under Current Conditions
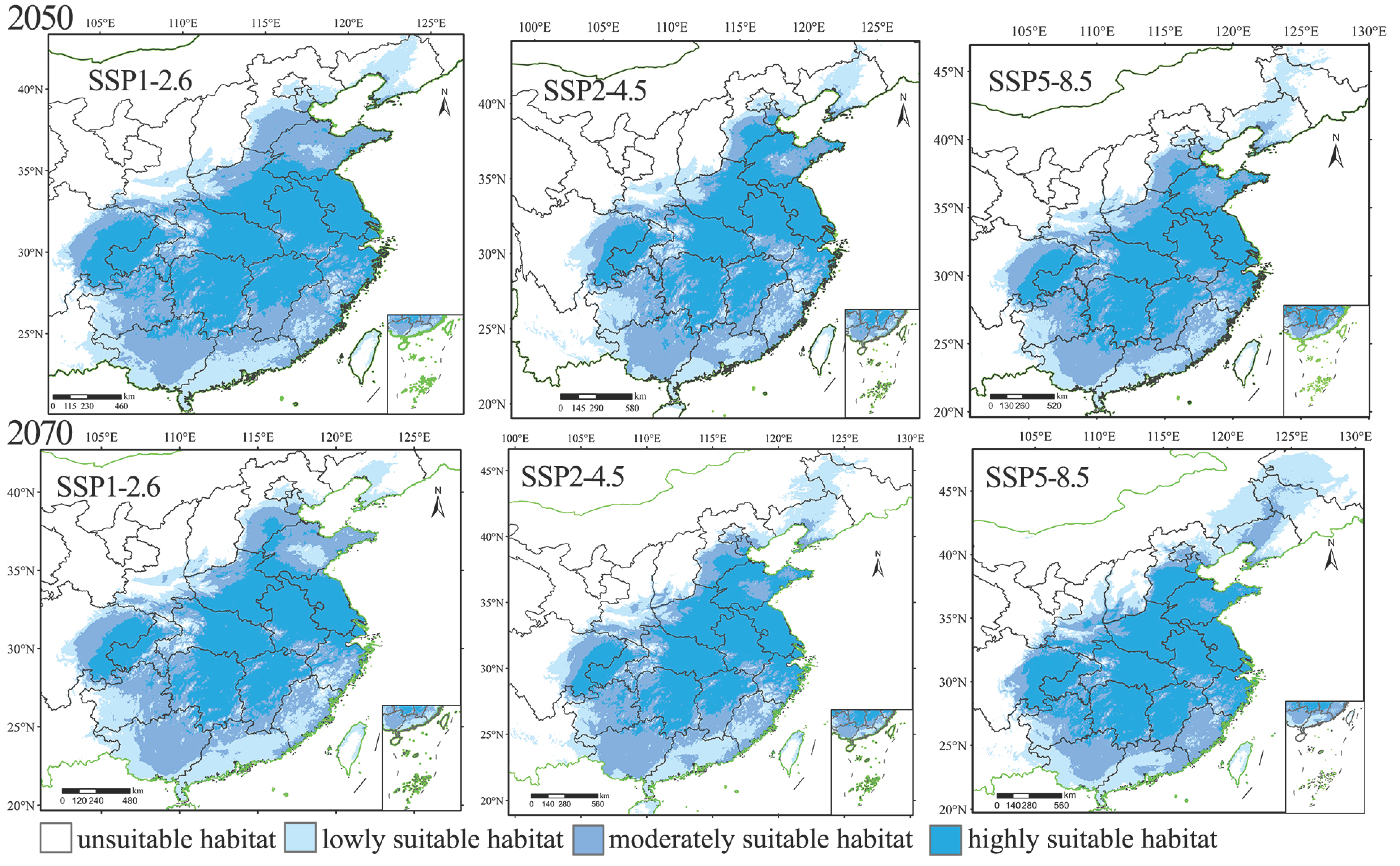
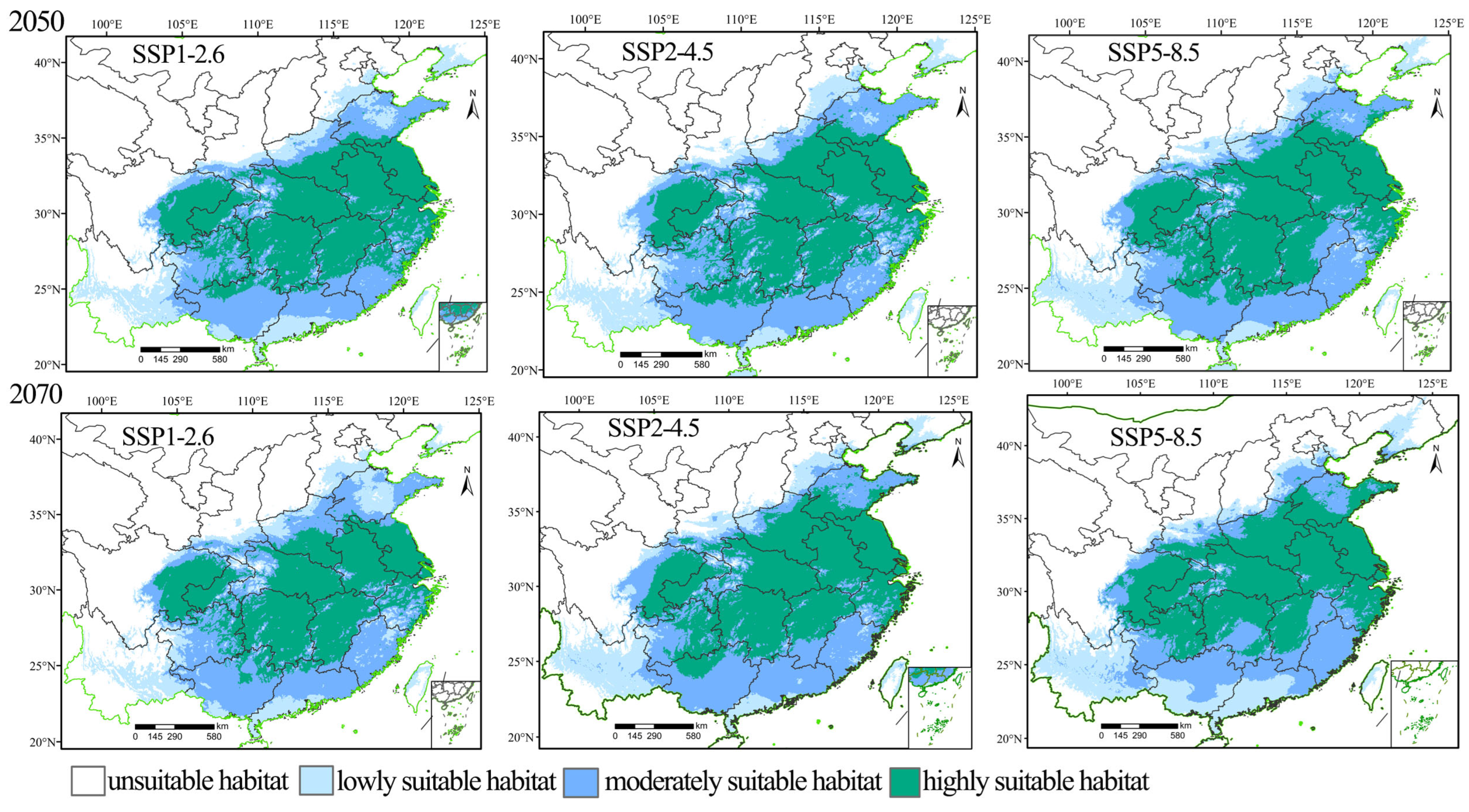
3.3. Prediction of the Distribution of the Suitable Habitats for H. rhodope and B. polycarpa in the Future
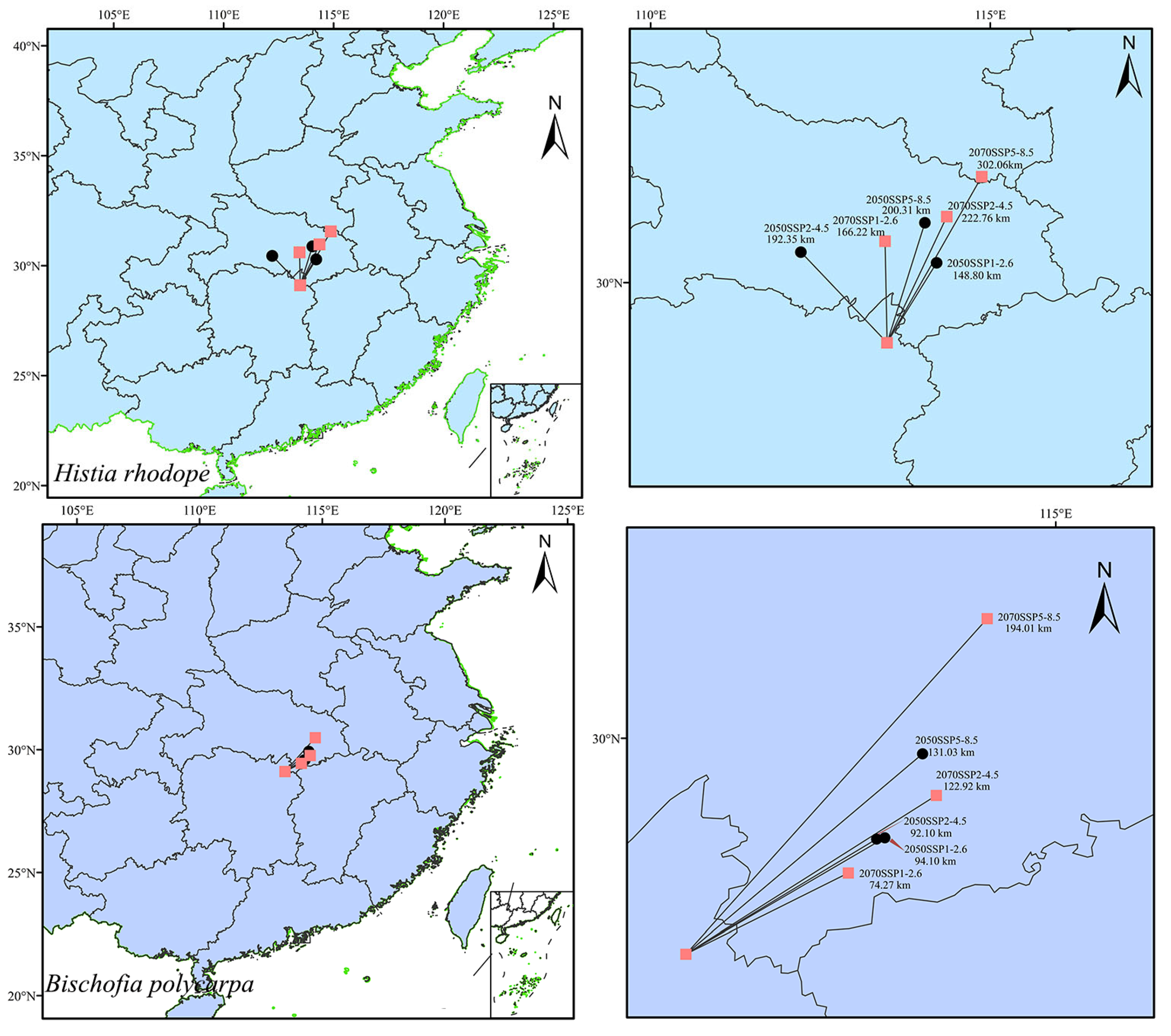
3.4. Migration of the Centroids of Suitable Habitats
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, R.T.; Chi, D.F. Effects of pest and disease disturbance on forest carbon sink a review. Sci. Silvae Sin. 2025, 61, 1–11. [Google Scholar]
- Derek, M.J.; Kyle, J.H. Spatiotemporal dynamics of forest insect populations under climate change. Curr. Opin. Insect Sci. 2023, 56, 101020. [Google Scholar]
- Bradshaw, C.J.A.; Leroy, B.; Bellard, C.; Roiz, D.; Albert, C.; Fournier, A.; Barbet-Massin, M.; Salles, J.-M.; Simard, F.; Courchamp, F. Massive yet grossly underestimated global costs of invasive insects. Nat. Commun. 2016, 7, 12986. [Google Scholar] [CrossRef]
- Lehmann, P.; Ammunét, T.; Barton, M.; Battisti, A.; Eigenbrode, S.D.; Jepsen, J.U.; Kalinkat, G.; Neuvonen, S.; Niemelä, P.; Terblanche, J.S. Complex responses of global insect pests to climate warming. Front. Ecol. Environ. 2020, 18, 141–150. [Google Scholar] [CrossRef]
- Howarth, C.; Viner, D. Intergrating adaptation practice in assessments of climate change science: The case of IPCC Working Group II reports. Environ. Sci. Policy 2022, 135, 1–5. [Google Scholar] [CrossRef]
- Jorda, G.; Marba, N.; Bennett, S.; Santana-Garcon, J.; Agusti, S.; Duarte, C.M. Ocean warming compresses the three-dimensional habitat of marine life. Nat. Ecol. Evol. 2019, 4, 109–114. [Google Scholar] [CrossRef]
- Cudmore, T.J.; Bjorklund, N.; Carroll, A.L.; Lindgren, B.S. Climate change and range expansion of an aggressive bark beetle: Evidence of higher beetle reproduction in native host tree populations. Ecol. Appl. 2010, 47, 1036–1043. [Google Scholar] [CrossRef]
- Giroday, H.M.C.; Carroll, A.L.; Aukema, B.H. Breach of the northern Rocky Mountain geoclimatic barrier: Initiation of range expansion by the mountain pine beetle. J. Biogeogr. 2012, 39, 1112–1123. [Google Scholar] [CrossRef]
- Raffa, K.F.; Powell, E.N.; Townsend, P.A. Temperature-driven range expansion of an irruptive insect heightened by weakly coevolved plant defenses. Proc. Natl. Acad. Sci. USA 2013, 110, 2193–2219. [Google Scholar] [CrossRef]
- Weed, A.S.; Ayres, M.P.; Hicke, J.A. Consequences of climate change for biotic disturbances in North American forests. Ecol. Monogr. 2013, 83, 441–470. [Google Scholar] [CrossRef]
- Chapman, T.B.; Veblen, T.T.; Schoennagel, T. Spatiotemporal patterns of mountain pine beetle activity in the southern Rocky Mountains. Ecology 2012, 93, 2175–2185. [Google Scholar] [CrossRef] [PubMed]
- Hart, S.J.; Veblen, T.T.; Eisenhart, K.S.; Jarvis, D.; Kulakowski, D. Drought induces spruce beetle (Dendroctonus rufipennis) outbreaks across north western Colorado. Ecology 2013, 95, 930–939. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Dang, Y.Q.; Wang, X.Y. Risk Assessment of the Worldwide Expansion and Outbreak of Massicus raddei (Blessig) (Coleoptera: Cerambycidae) Based on Host Plant and Climatic Factors. Insects 2022, 13, 730. [Google Scholar] [CrossRef]
- Yu, Y.L.; Li, Z.Q. Predicting the Potential Distribution of Cheirotonus jansoni (Coleoptera: Scarabaeidae) Under Climate Change. Insects 2024, 15, 1012. [Google Scholar] [CrossRef]
- Huang, B.K. A study on the bionomic characteristics and control of the Bischofia burnet Histia rhodope Cramer. J. Fujian Agric. Coll. 1980, 1, 61–79. [Google Scholar]
- Yang, H.B.; Dong, J.F.; Sun, Y.L.; Hu, Z.J.; Lv, Q.H.; Li, D.X. Antennal transcriptome analysis and expression profiles of putative chemosensory soluble proteins in Histia rhodope Cramer (Lepidoptera: Zygaenidae). Comp. Biochem. Physiol. Part D Genom. Proteom. 2020, 33, 100654. [Google Scholar] [CrossRef]
- Yang, H.B.; Hu, Z.J.; Dong, J.F.; Zhu, P.H.; Li, D.X. Changes in the cold hardiness of overwintering larvae of Histia rhodope (Lepidoptera: Zygaenidae). Acta. Entomol. Sin. 2019, 62, 979–986. [Google Scholar]
- Yang, H.; Dong, J.; Sun, Y.L.; Hu, Z.; Lu, Q.H.; Li, D. Identification and expression profiles of candidate chemosensory receptors in Histia rhodope (Lepidoptera: Zygaenidae). PeerJ 2020, 8, e10035. [Google Scholar] [CrossRef]
- Qin, Z.P.; Song, G.L.; Liu, P.; Li, G.P.; Liang, X.; Wang, H.T. Experimental study on pesticides control of Histia rhodope Cramer. J. Shandong For. Sci. Tech. 2018, 238, 78–80. [Google Scholar]
- Yang, H.B.; Chen, Z.H.; Zhu, P.H.; Guo, S.S.; Wang, Y.; Li, D.X.; Ji, S.Y.; Zhang, G. Cold tolerance and prediction of northern distribution of Histia rhodope (Lepidoptera: Zygaenidae) in China. Environ. Entomol. 2025, 54, 174–183. [Google Scholar] [CrossRef]
- Booth, T.H.; Nix, H.A.; Busby, J.R.; Huchinson, M.F. Bioclim: The first species distribution modeling package, its early applicationsand relevance to most current MaxEnt studies. Divers. Distrib. 2014, 20, 1–9. [Google Scholar] [CrossRef]
- Li, Y.; Johnson, A.J.; Gao, L.; Wu, C.X.; Hulcr, J. Two new invasive Ips bark beetles (Coleoptera: Curculiionidae) in mainland China and their potential distribution in Asia. Pest Manag. Sci. 2021, 77, 4000–4008. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Zhao, R.; Zhou, X.Y.; Zhang, X.L.; Zhao, G.H.; Zhang, F.G. Agastache rugosa Prediction of potential distribution areas and priority protected areas of based on Maxent model and Marxan model. Front. Plant Sci. 2023, 14, 1200796. [Google Scholar] [CrossRef] [PubMed]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudík, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.; et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Phillips, S.J.; Miroslav, D. Modeling of species distributions with Maxent: New extensions a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Waldock, C.; Stuart-Smith, R.D.; Albouy, C.; Cheung, W.L.; Edgar, G.J.; Mouillot, D.; Tjiputra, J.; Pellissier, L. A quantitative review of abundance-based species distribution models. Ecography 2022, 1, 2022. [Google Scholar] [CrossRef]
- Lin, S.L.; Yao, D.D.; Jiang, H.X.; Qin, J.; Feng, Z. Predicting current and future potential distributions of the greater bandicoot rat (Bandicota indica) under climate change conditions. Pest Manag. Sci. 2024, 80, 734–743. [Google Scholar] [CrossRef]
- Zhang, G.L.; Liu, S.; Xu, C.Q.; Wei, H.S.; Guo, K.; Xu, R.; Qiao, H.L.; Lu, P.F. Prediction of Potential Distribution of Carposina coreana in China Under the Current and Future Climate Change. Insect 2024, 15, 411. [Google Scholar] [CrossRef]
- Pellissier, L.; Rohr, R.P.; Ndiribe, C.; Pradervand, J.N.; Salamin, N.; Guisan, A.; Wisz, M. Combining food web and species distribution models for improved community projections. Ecol. Evol. 2013, 3, 4572–4583. [Google Scholar] [CrossRef]
- Ji, W.; Gao, G.; Wei, J.F. Potential global distribution of Daktulosphaira vitifoliae under climate change based on MaxEnt. Insects 2021, 12, 347. [Google Scholar] [CrossRef]
- Khadka, D.; Babel, M.S.; Abatan, A.A. An evaluation of CMIP5 and CMIP6 climate models in simulating summer rainfall in the Southeast Asian monsoon domain. Int. J. Climatol. 2022, 42, 1181–1202. [Google Scholar] [CrossRef]
- Ridder, N.N.; Pitman, A.J.; Ukkola, A.M. Do CMIP6 climate models simulate global or regional compound events skillfully? Geophys. Res. Lett. 2021, 48, e2020GL091152. [Google Scholar] [CrossRef]
- Muscarella, R.; Galante, P.J.; Soley-Guardia, M.; Boria, R.A.; Kass, J.M.; Uriarte, M.; ENMeval, R.A. An R package for conducting spatially independent evaluations and estimating optimal model complexity for MAXENT ecological niche models. Methods Ecol. Evol. 2014, 12261, 1198–1205. [Google Scholar] [CrossRef]
- Wang, S.Y.; Lu, Y.Y.; Han, M.Y.; Li, L.L.; He, P.; Shi, A.; Bai, M. Using MaxEnt model to predict the potential distribution of three potentially invasive scarab beetles in China. Insects 2023, 14, 239. [Google Scholar] [CrossRef]
- Guo, Y.; Li, X.; Zhao, Z.; Nawaz, Z. Predicting the impacts of climate change, soils and vegetation types on the geographic distribution of Polyporus umbellatus in China. Sci. Total Environ. 2019, 648, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Deng, C.; Duan, G.; Wang, Z.; Zhang, Y.; Fan, G. Potentially suitable habitats of Daodi goji berry in China under climate change. Front. Plant Sci. 2024, 14, 1279019. [Google Scholar] [CrossRef]
- Steven, J.P.; Robert, P.; Anderson, M.D.; Robert, E.S.; Mary, B. Opening the black box: An open-source release of Maxent. Ecography 2017, 40, 887–893. [Google Scholar]
- Peterson, A.; Soberón, J.; Pearson, R.; Anderson, R.; Martínezmeyer, E.; Nakamura, M.; Araújo, M. Ecological Niches and Geographic Distributions; Princeton University Press: Princeton, NJ, USA, 2011. [Google Scholar]
- Lin, W.; Xu, M.F.; Quan, Y.B.; Liao, L.; Gao, L. Potential geographic distribution of Spodoptera frugiperda in China based on MaxEnt model. Plant Quar. 2019, 33, 69–73. [Google Scholar]
- Tang, X.G.; Yuan, Y.D.; Li, X.M.; Zhang, J.C. Maximum Entropy Modeling to Predict the Impact of Climate Change on Pine Wilt Disease in China. Front. Plant Sci. 2021, 12, 764. [Google Scholar] [CrossRef]
- Tang, X.; Yuan, Y.; Liu, X.; Zhang, J.J.E.E. Potential range expansion and niche shift of the invasive Hyphantria cunea between native and invasive countries. Ecol. Entomol. 2021, 46, 910–925. [Google Scholar] [CrossRef]
- Yuan, Y.D.; Tang, X.G.; Liu, M.Y.; Liu, X.F.; Tao, J. Species Distribution Models of the Spartina alterniflora Loisel in Its Origin and Invasive Country Reveal an Ecological Niche Shift. Front. Plant Sci. 2021, 12, 738769. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.K.; Fan, G.H.; Deng, C.R.; Duan, G.Z.; Li, J.L. Predicting the Distribution of Neoceratitis asiatica (Diptera: Tephritidae), a Primary Pest of Goji Berry in China, Under Climate Change. Insects 2024, 15, 558. [Google Scholar] [CrossRef]
- Wang, R.L.; Li, Q.; Feng, C.H.; Shi, C.P. Predicting potential ecological distribution of Locusta migratoria tibetensis in China using MaxEnt ecological niche modeling. Acta Ecol. Sin. 2017, 37, 8556–8566. [Google Scholar]
- Shi, W.; Zhu, E.J.; Wang, W.C.; Ma, F.Z.; He, Q.J.; Yi, C.H. Prediction of potentially suitable distribution area of Propomacrus davidi Deyrolle in China based on MaxEnt model. Chinese J. Ecol. 2021, 40, 2936–2944. [Google Scholar]
- Bale, J.S. Insect cold hardiness: A matter of life and death. Eur. J. Entomol. 1996, 33, 899–908. [Google Scholar]
- Bale, J.S. Insects and low temperatures: From molecular biology to distributions and abundance. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2002, 357, 849–862. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.Z.; Gong, W.C.; Ma, Q.Y.; Niu, Y.; Mei, W.L.; Chen, G.; Zhao, Y.X. Isolation and identification of cyanogenic glycosides from larval secretions of Achelura yunnanensis (Lepidoptera: Zygaenidae) and the bioactivity against Tapinoma melanocephalum (Hymenoptera: Fromicidae). Acta Entomol. Sin. 2013, 56, 207–211. [Google Scholar]
- Zhao, H.X.; Xian, X.Q.; Zhao, Z.H.; Zhang, G.F.; Liu, W.X.; Wan, F.H. Climate Change Increases the Expansion Risk of Helicoverpa zea in China According to Potential Geographical Distribution Estimation. Insects 2021, 13, 79. [Google Scholar] [CrossRef]
- Zhang, Q.C.; Wang, J.G.; Lei, Y.H. Predicting Distribution of the Asian Longhorned Beetle, Anoplophora glabripennis (Coleoptera: Cerambycidae) and Its Natural Enemies in China. Insects 2022, 13, 687. [Google Scholar] [CrossRef]
- Skendžić, S.; Zovko, M.; Živković, I.P.; Lešić, V.; Lemić, D. The impact of climate change on agricultural insect pests. Insects 2021, 12, 440. [Google Scholar] [CrossRef]
- Gómez, S.R.; Gil-Tapetado, D.; García-Gila, J.; Blasco-Aróstegui, J.; Polidori, C. The leaf beetle Labidostomis lusitanica (Coleoptera: Chrysomelidae) as an Iberian pistachio pest: Projecting risky areas. Pest Manag. Sci. 2021, 78, 217–229. [Google Scholar] [CrossRef]
- Stockwell, D.R.B.; Beach, J.H.; Stewart, A.; Vorontsov, G.; Vieglais, D.; Pereira, R.S. The use of the GARP genetic algorithm and internet grid computing in the Lifemapper world atlas of species biodiversity. Ecol. Model. 2006, 195, 139–145. [Google Scholar] [CrossRef]
- Wang, X.Y.; Huang, X.L.; Jiang, L.Y.; Qiao, G.X. Predicting potential distribution of Chestnut phylloxerid (Hemiptera: Phylloxeridae) based on GARP and Maxent ecological niche models. J. Appl. Entomol. 2010, 134, 45–54. [Google Scholar] [CrossRef]
- Du, H.C.; Fang, J.X.; Shi, X.; Yu, C.M.; Deng, M.; Zhang, S.F.; Liu, F.; Zhang, Z.; Han, F.Z.; Kong, X.B. Insights into the Divergence of Chinese Ips Bark Beetles During Evolutionary Adaptation. Biology 2022, 11, 384. [Google Scholar] [CrossRef]
- Aidoo, O.F.; Souza, P.G.C.; Silva, R.S.; Santana, P.A.; Picano, M.C.; Kyerematen, R.; Sètamou, M.; Ekesi, S.; Borgemeister, C. Climate-induced range shifts of invasive species (Diaphorina citri Kuwayama). Pest. Manag. Sci. 2022, 78, 2534–2549. [Google Scholar] [CrossRef]
- Berzitis, E.A.; Minigan, J.N.; Hallett, R.H.; Newman, J.A. Climate and host plant availability impact the future distribution of the bean leaf beetle (Cerotoma trifurcata). Glob. Change Biol. 2014, 20, 2778–2792. [Google Scholar] [CrossRef]
- Wang, F.; Xu, Y.; Zhao, Z.Y.; Li, Y.Z.; Ju, R.T. Toxicity test and control efficacy of six insecticides against Histia rhodope. For. Pest Dis. 2012, 31, 39–42. [Google Scholar]
- Liu, N.J.; Zhao, X.Q.; Luo, F.Y. Types and application techniques of truck coating of kiwifruit. J. Fruit Resour. 2024, 5, 91–93. [Google Scholar]
- Fan, M. Life history and population dynamics of Histia rhodope in Shanghai. Acta Agric. Shanghai 2013, 29, 122–124. [Google Scholar]
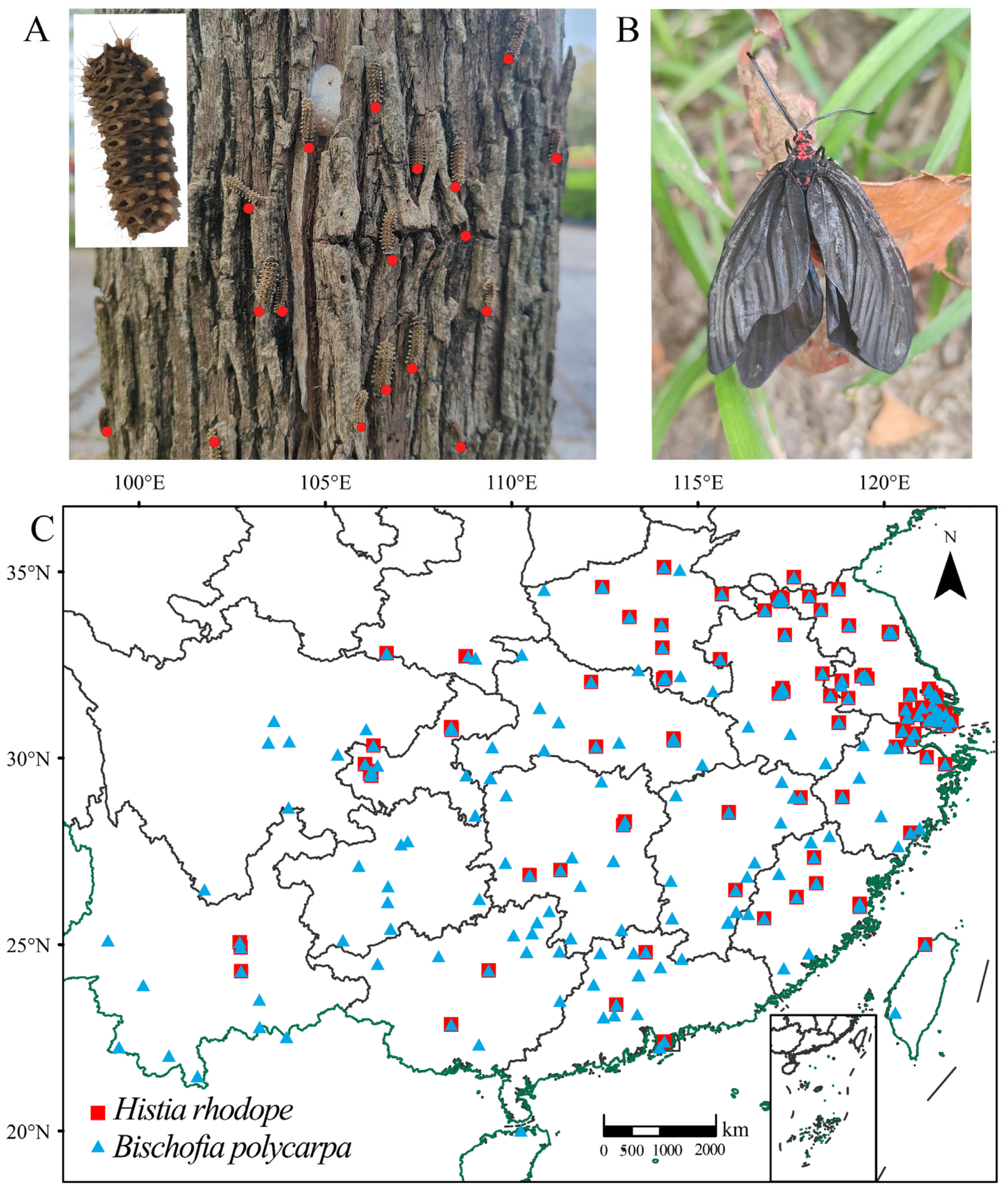
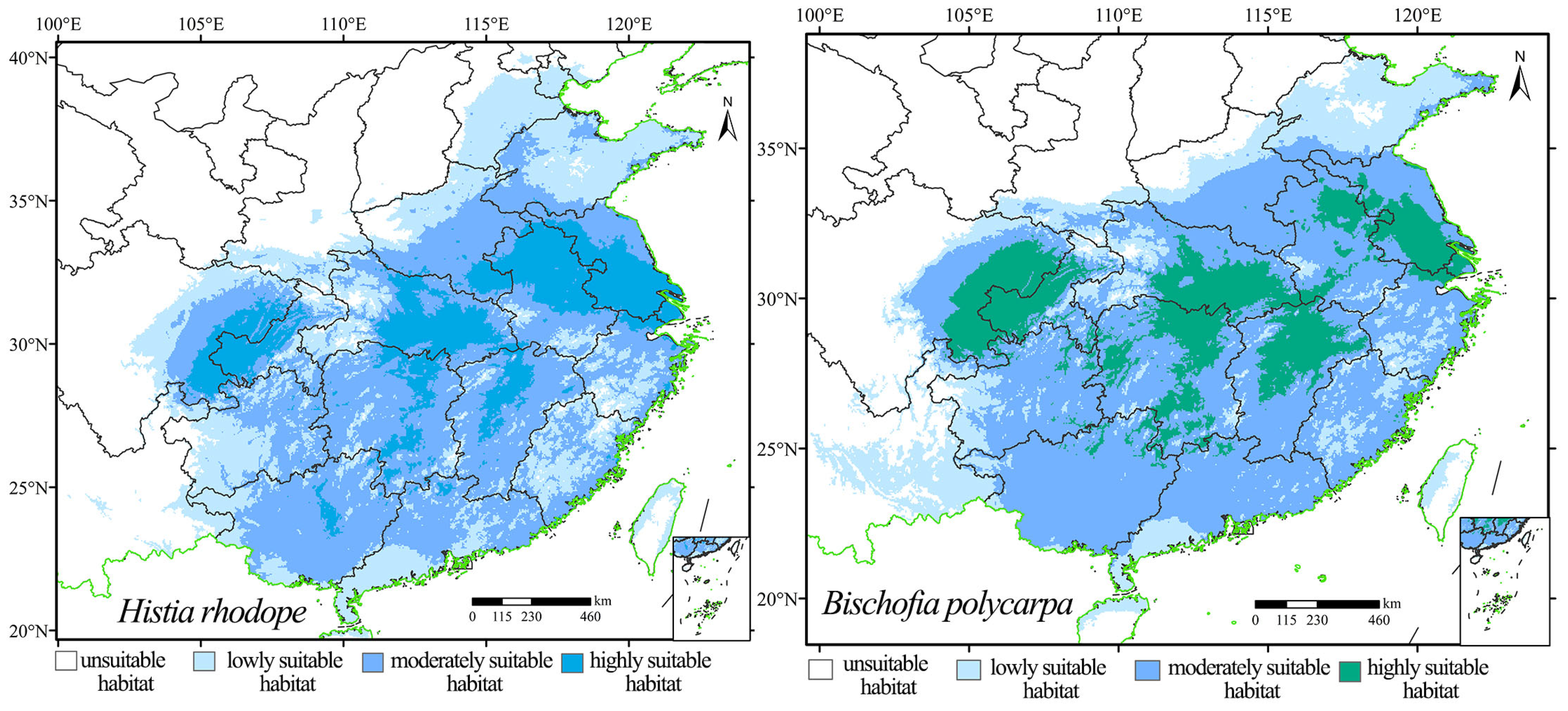
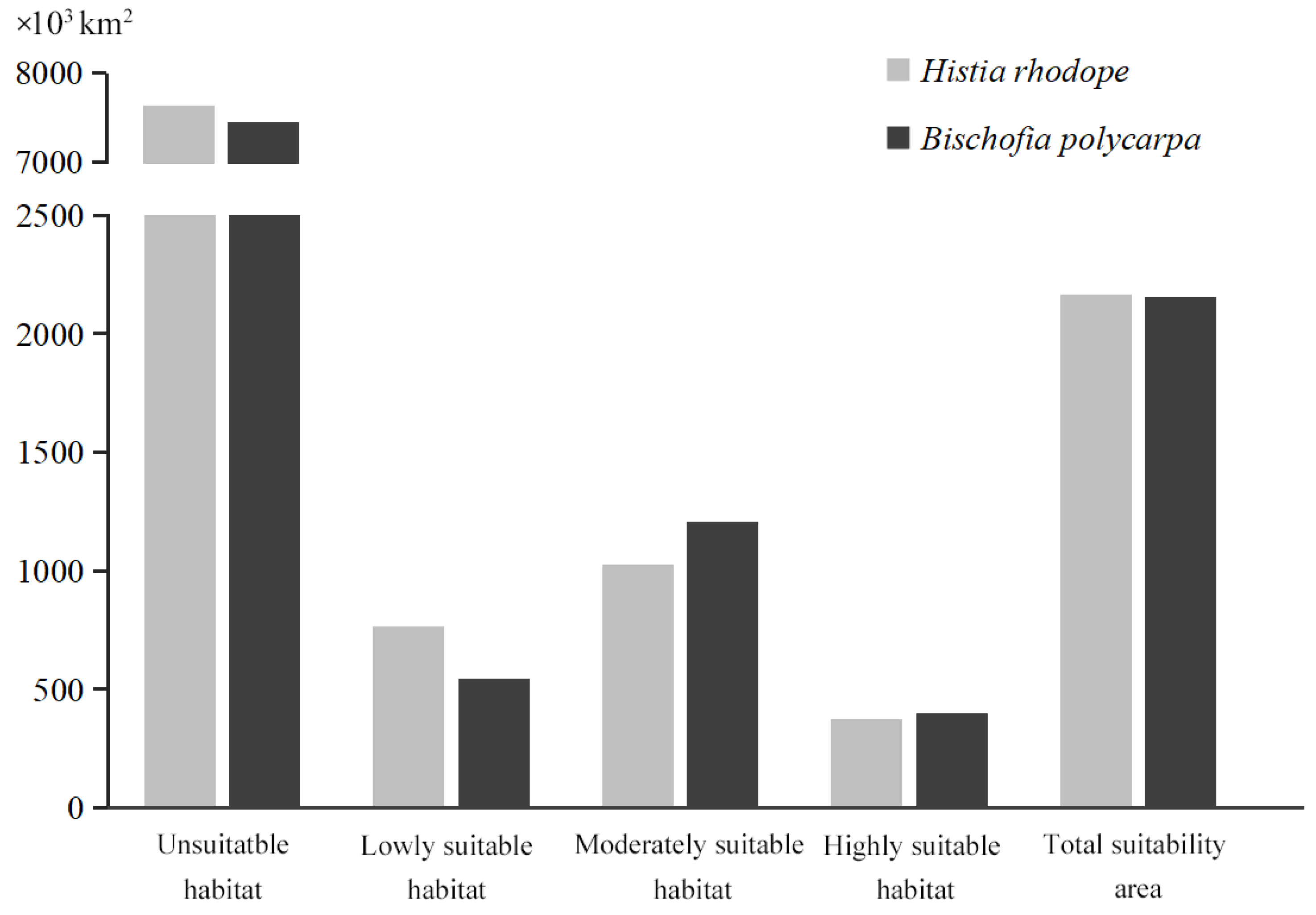
| Species | Unsuitable Habitat | Lowly Suitable Habitat | Moderately Suitable Habitat | Highly Suitable Habitat |
|---|---|---|---|---|
| Histia rhodope | k < 0.08 | 0.08 ≤ k < 0.27 | 0.27 ≤ k < 0.55 | k > 0.55 |
| Bischofia polycarpa | k < 0.20 | 0.20 ≤ k < 0.40 | 0.40 ≤ k < 0.60 | k > 0.60 |
| Environmental Factors and Description | Percent Contribution | |
|---|---|---|
| (H. rhodope)/% | (B. polycarpa)/% | |
| Bio18: Precipitation of Warmest Quarter | 52.1 | 58.2 |
| Bio4: Temperature Seasonality | 20 | 19 |
| Bio15: Precipitation Seasonality | 4.8 | 6.6 |
| Bio14: Precipitation of Driest Month | 4.5 | 3.9 |
| Bio13: Precipitation of Wettest Month | 3.9 | 3.7 |
| Bio10: Mean Temperature of Warmest Quarter | 2.2 | 1.4 |
| Bio8: Mean Temperature of Wettest Quarter | 5.7 | 0.5 |
| Bio1: Annual Mean Temperature | 0.6 | 0.4 |
| Bio2: Mean Diurnal Range | 0.8 | 0.1 |
| Bio6: Min Temperature of Coldest Month. | - | 2 |
| Bio3: Isothermality (Bio2/Bio7) (×100). | 0.8 | - |
| Total percent contribution | 95.4 | 95.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, H.; Shen, J.; Luo, W.; Yang, Z.; Zhang, D.; Kong, X. Potentially Suitable Habitat for the Pest Histia rhodope Based on Its Host Plant Bischofia polycarpa and Climatic Factors in China. Insects 2025, 16, 627. https://doi.org/10.3390/insects16060627
Du H, Shen J, Luo W, Yang Z, Zhang D, Kong X. Potentially Suitable Habitat for the Pest Histia rhodope Based on Its Host Plant Bischofia polycarpa and Climatic Factors in China. Insects. 2025; 16(6):627. https://doi.org/10.3390/insects16060627
Chicago/Turabian StyleDu, Huicong, Jingxin Shen, Wenping Luo, Zi Yang, Daizhen Zhang, and Xiangbo Kong. 2025. "Potentially Suitable Habitat for the Pest Histia rhodope Based on Its Host Plant Bischofia polycarpa and Climatic Factors in China" Insects 16, no. 6: 627. https://doi.org/10.3390/insects16060627
APA StyleDu, H., Shen, J., Luo, W., Yang, Z., Zhang, D., & Kong, X. (2025). Potentially Suitable Habitat for the Pest Histia rhodope Based on Its Host Plant Bischofia polycarpa and Climatic Factors in China. Insects, 16(6), 627. https://doi.org/10.3390/insects16060627







