Distribution Pattern and Change Prediction of Luprops orientalis (Coleoptera: Tenebrionidae) Suitable Area in East Asia Under Climate Change
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
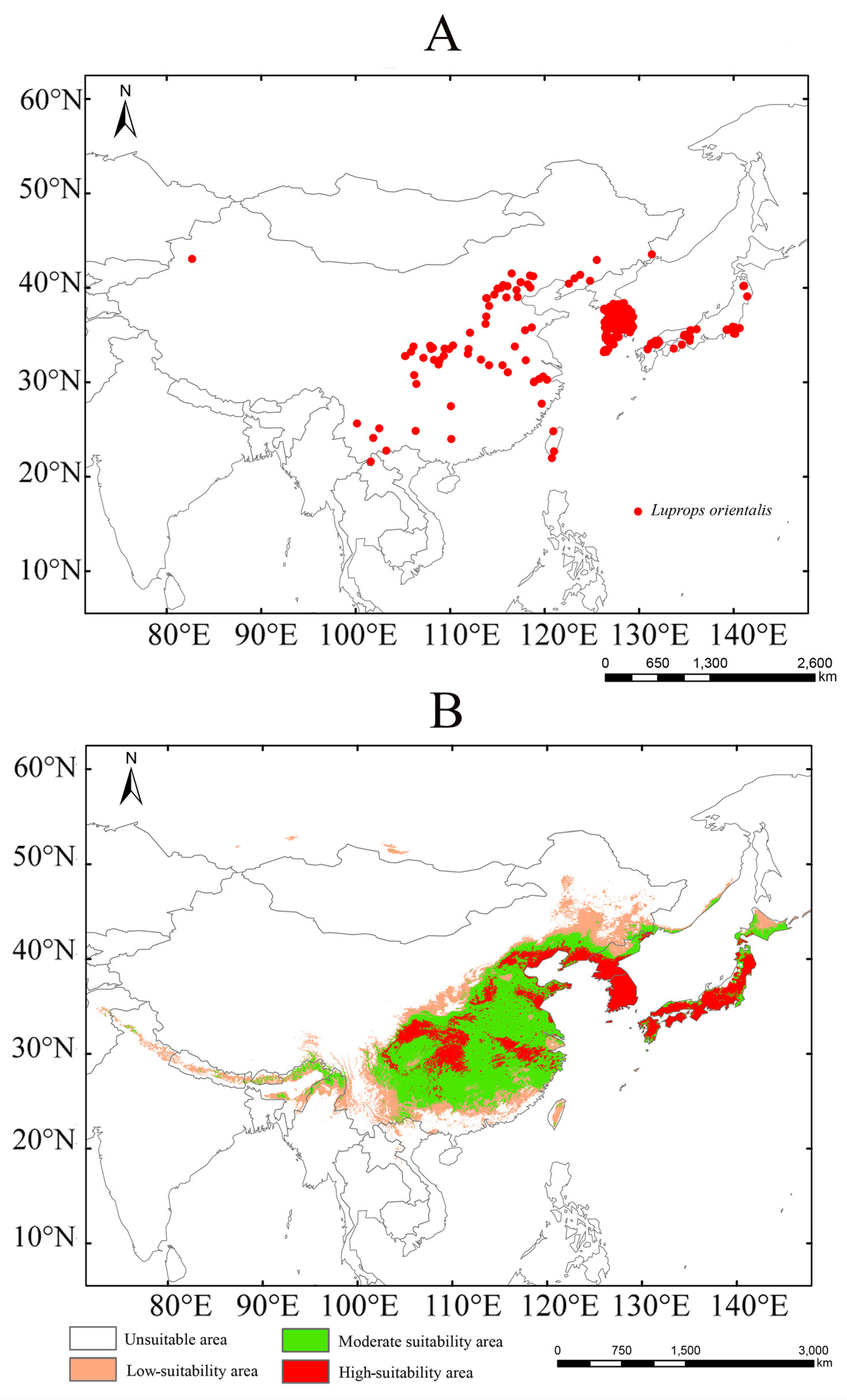
2.1. Species Occurrence Data
2.2. Environmental Variables
2.3. Modeling Methods
3. Results
3.1. Model Optimization and Accuracy Evaluation
3.2. Dominant Environmental Variables and Their Response Curves
3.3. Near-Current Suitable Areas for Luprops orientalis
3.4. Changes in Suitable Areas for Luprops orientalis Under Future Climate Conditions
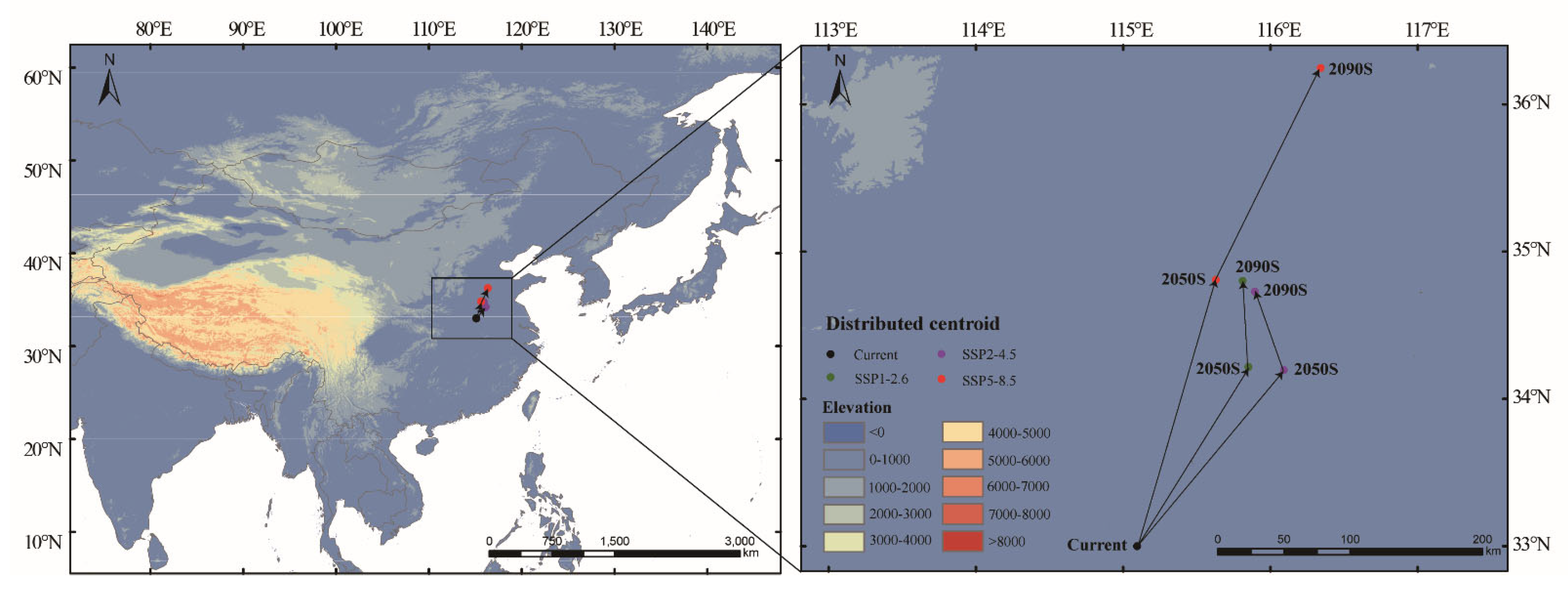
3.5. Shift in the Centroids Within the Suitable Area
4. Discussion
4.1. Restriction of Environmental Variables
4.2. Distribution Pattern of Luprops orientalis Under Near-Current Climate Conditions
4.3. The Impact of Climate Change on Suitable Habitats of Luprops orientalis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| maximum entropy | MaxEnt |
| area under the curve | AUC |
| receiver operating characteristic | ROC |
References
- Bellard, C.; Bertelsmeier, C.; Leadley, P.; Thuiller, W.; Courchamp, F. Impacts of climate change on the future of biodiversity. Ecol. Let. 2012, 15, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ge, D.; Ericson, P.G.P.; Song, G.; Wen, Z.X.; Yang, Q.S.; Lei, F.M.; Qu, Y.H. Alpine burrow-sharing mammals and birds show similar population-level climate change risks. Nat. Clim. Change 2023, 13, 990–996. [Google Scholar] [CrossRef]
- Pecl, G.T.; Araújo, M.B.; Bell, J.D.; Blanchard, J.; Bonebrake, T.C.; Chen, I.C.; Clark, T.D.; Colwell, R.K.; Danielsen, F.; Evengård, B.; et al. Biodiversity redistribution under climate change: Impacts on ecosystems and human well-being. Science 2017, 355, eaai9214. [Google Scholar] [CrossRef]
- Kearney, M.; Porter, W. Mechanistic niche modelling: Combining physiological and spatial data to predict species’ ranges. Ecol. Lett. 2009, 12, 334–350. [Google Scholar] [CrossRef]
- Anibaba, Q.A.; Dyderski, M.K.; Jagodzinski, A.M. Predicted range shifts of invasive giant hogweed (Heracleum mantegazzianum) in Europe. Sci. Total Environ. 2022, 825, 154053. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.Z.; He, S.Y.; Zhu, C.Y.; Wang, T.; Xu, Z.C.; Zong, S.X. Projecting the current and future potential global distribution of Hyphantria cunea (Lepidoptera: Arctiidae) using CLIMEX. Pest Manag. Sci. 2019, 75, 160–169. [Google Scholar] [CrossRef]
- Mondanaro, A.; Di Febbraro, M.; Castiglione, S.; Melchionna, M.; Serio, C.; Girardi, G.; Belfiore, A.M.; Raia, P. ENphylo: A new method to model the distribution of extremely rare species. Methods Ecol. Evol. 2023, 14, 911–922. [Google Scholar] [CrossRef]
- González-Miguéns, R.; Cano, E.; Guillén-Oterino, A.; Quesada, A.; Lahr, D.J.G.; Tenorio-Rodríguez, D.; de Salvador-Velasco, D.; Velázquez, D.; Carrasco-Braganza, M.I.; Patterson, R.T.; et al. A needle in a haystack: A new metabarcoding approach to survey diversity at the species level of Arcellinida (Amoebozoa: Tubulinea). Mol. Ecol. Resour. 2023, 23, 1034–1049. [Google Scholar] [CrossRef]
- Booth, T.H.; Nix, H.A.; Busby, J.R.; Hutchinson, M.F. BIOCLIM: The first species distribution modelling package, its early applications and relevance to most current MaxEnt studies. Divers. Distrib. 2014, 20, 1–9. [Google Scholar] [CrossRef]
- Wainwright, B.J.; Leon, J.; Vilela, E.; Hickman, K.J.E.; Caldwell, J.; Aimone, B.; Bischoff, P.; Ohran, M.; Morelli, M.W.; Arlyza, I.S.; et al. Wallace’s line structures seagrass microbiota and is a potential barrier to the dispersal of marine bacteria. Environ. Microbiome 2024, 19, 23. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, H.X.; Wang, H. Potential geographical distribution of Populus euphratica in China under future climate change scenarios based on Maxent model. Acta Ecol. Sin. 2020, 40, 6552–6563. [Google Scholar]
- Bouchard, P.; Bousquet, Y.; Aalbu, R.L.; Alonso-Zarazaga, M.A.; Merkl, O.; Davies, A.E. Review of genus-group names in the family Tenebrionidae (Insecta, Coleoptera). ZooKeys 2021, 1050, 1–633. [Google Scholar]
- Kamiński, M.J.; Lumen, R.; Kanda, K.; Iwan, D.; Johnston, M.A.; Kergoat, G.J.; Bouchard, P.; Bai, X.L.; Li, X.M.; Ren, G.D.; et al. Reevaluation of Blapimorpha and Opatrinae: Addressing a major phylogeny-classification gap in darkling beetles (Coleoptera: Tenebrionidae: Blaptinae). Syst. Entomol. 2021, 46, 140–156. [Google Scholar] [CrossRef]
- Hagstrum, D.W.; Subramanyam, B. Stored-Product Insect Resource; AACC International: Eagan, MN, USA, 2009. [Google Scholar]
- Ren, G.D.; Li, X. Advances in classification of the storage tenebrionid beetles in the world. J. Hebei Univ. (Nat. Sci.) 2020, 40, 49–56. [Google Scholar]
- Yan, X.; Zhou, H.; Shen, Z.; Li, W.; Guo, D.; Song, Y.; Lan, S.; Zhang, J. National investigations of stored grain arthropods in China. In Proceedings of the 10th International Working Conference on Stored Product Protection, Estoril, Portugal, 27 June–2 July 2010. [Google Scholar]
- Wang, D.X.; Wang, X.; Bai, C.Q.; Zhang, H.; Shao, X.L.; Shen, F.; Song, W.; He, Y.P.; Xie, L.D. Survey on community insect species of Tenebroides mauritanicus (Linnaeus) and their habitat character in grain storge environment. J. Henan Univ. Technol. (Nat. Sci.) 2017, 38, 96–100. [Google Scholar]
- Kaszab, Z. Synonymie Indoaustralischer und Neotropisher Tenebrioniden (Coleoptera). Acta Zool. Acad. Sci. Hung. 1983, 29, 129–138. [Google Scholar]
- Wei, Z.H.; Ren, G.D. Taxonomy of the genus Luprops from China, with the description of larva of Luprops horni (Coleoptera: Tenebrionidae). Entomotaxonomia 2019, 41, 299–312. [Google Scholar]
- Leung, B.; Lodge, D.M.; Finnoff, D.; Shogren, J.F.; Lewis, M.A.; Lamberti, G. An ounce of prevention or a pound of cure: Bioeconomic risk analysis of invasive species. Proc. R. Soc. B-Biol. Sci. 2002, 269, 2407–2413. [Google Scholar] [CrossRef]
- Fournier, A.; Penone, C.; Pennino, M.G.; Courchamp, F. Predicting future invaders and future invasions. Proc. Natl. Acad. Sci. USA 2019, 116, 7905–7910. [Google Scholar] [CrossRef]
- Verbruggen, H.; Tyberghein, L.; Belton, G.S.; Mineur, F.; Jueterbock, A.; Hoarau, G.; Gurgel, C.F.; Clerk, O.D. Improving transferability of introduced species’ distribution models: New tools to forecast the spread of a highly invasive seaweed. PLoS ONE 2013, 8, e63337. [Google Scholar] [CrossRef]
- Kobayashi, T.; Tateishi, R.; Alsaaideh, B.; Sharma, R.C.; Wakaizumi, T.; Miyamoto, D.; Bai, X.; Long, B.D.; Gegentana, G.; Maitiniyazi, A. Production of global land cover data–GLCNMO2013. J. Geogr. Geol. 2017, 9, 1–15. [Google Scholar] [CrossRef]
- Heikkinen, R.K.; Araújo, M.B.; Virkkala, R.; Thuiller, W.; Sykes, M.T. Methods and uncertainties in bioclimatic envelop modeling under climate change. Prog. Phys. Geogr. 2006, 30, 751–777. [Google Scholar] [CrossRef]
- Aguirre-Gutiérrez, J.; Carvalheiro, L.G.; Polce, C.; Emiel, V.E.E.; Raes, N.; Reemer, M.; Biesmeijer, J.C. Fit-for-purpose: Species distribution model performance depends on evaluation criteria–Dutch hoverflies as a case study. PLoS ONE 2013, 8, e63708. [Google Scholar] [CrossRef]
- Dormann, C.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Leitão, P. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Liu, L.; Wang, R.L.; Zhang, Y.Y.; Mou, Q.Y.; Gou, Y.S.; Liu, K.; Huang, N.; Ouyang, C.L.; Hu, J.Y.; Du, B.G. Simulation of potential suitable distribution of Alnus cremastogyne Burk. In China under climate change scenarios. Ecol. Indic. 2021, 133, 108396. [Google Scholar] [CrossRef]
- Chen, B.G.; Zou, H.; Zhang, B.Y.; Zhang, X.Y.; Jin, X.X.; Wang, C.; Zhang, X.X. Distribution pattern and change prediction of Saposhnikovia divaricata suitable area in China under climate change. Ecol. Indic. 2022, 143, 109311. [Google Scholar] [CrossRef]
- Zhu, G.P.; Jiao, H.J. Effect of the MaxEnt model’s complexity on the prediction of species potential distributions. Biodivers. Sci. 2016, 24, 1189–1196. [Google Scholar] [CrossRef]
- He, Y.L.; Ma, J.M.; Chen, G.S. Potential geographical distribution and its multi-factor analysis of Pinus massoniana in China based on the MaxEnt model. Ecol. Indic. 2023, 154, 110790. [Google Scholar] [CrossRef]
- Wei, B.; Wang, R.L.; Hou, K.; Wang, X.Y.; Wu, W. Predicting the current and future cultivation regions of Carthamus tinctorius L. using MaxEnt model under climate change in China. Glob. Ecol. Conserv. 2018, 16, e00477. [Google Scholar] [CrossRef]
- Mamun, M.; Kim, S.; An, K.G. Distribution pattern prediction of an invasive alien species largemouth bass using a maximum entropy model (MaxEnt) in the Korean peninsula. J. Asia-Pac. Biodivers. 2018, 11, 516–524. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapirer, E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Kim, M.J.; Baek, S.; Lee, S.B.; Lee, S.K.; Park, B.; Lee, Y.S.; Ahn, K.S.; Choi, Y.S.; Seo, H.Y.; Lee, J.H. Current and future distribution of Metcalfa pruinosa (Say) (Hemiptera: Flatidae) in Korea: Reasoning of fast spreading. J. Asia-Pac. Entomol. 2019, 22, 933–940. [Google Scholar] [CrossRef]
- Wei, J.; Gao, G.; Wei, J.F. Potential global distribution of Daktulosphaira vitifoliae under climate change based on MaxEnt. Insects 2021, 12, 347. [Google Scholar] [CrossRef]
- Ramasamy, M.; Das, B.; Ramesh, R. Predicting climate change impacts on potential worldwide distribution of fall armyworm based on CMIP6 projections. J. Pest Sci. 2021, 95, 841–854. [Google Scholar] [CrossRef]
- Zhao, Z.X.; Yang, L.; Long, J.K.; Chang, Z.M.; Chen, X.S. Predicting suitable areas for Metcalfa pruinose (Hemiptera: Flatidae) under climate change and implications for management. J. Insect Sci. 2024, 24, 7. [Google Scholar] [CrossRef]
- Wang, S.Y.; Lu, Y.Y.; Han, M.Y.; Li, L.L.; He, P.; Shi, A.M.; Bai, M. Using MaxEnt model to predict the potential distribution of three potentially invasive scarab beetles in China. Insects 2023, 14, 239. [Google Scholar] [CrossRef]
- Yu, Y.L.; Li, Z.Q. Predicting the potential distribution of Cheirotonus jansoni (Coleoptera: Scarabaeidae) under climate change. Insects 2024, 15, 1012. [Google Scholar] [CrossRef]
- Zhang, G.L.; Liu, S.; Xu, C.Q.; Wei, H.S.; Guo, K.; Xu, R.; Qiao, H.L.; Liu, P.F. Prediction of potential distribution of Carposina coreana in China under the current and future climate change. Insects 2024, 15, 411. [Google Scholar] [CrossRef]
- Wisz, M.S.; Hijmans, R.J.; Li, J.; Peterson, A.T.; Graham, C.H.; Guisan, A. Effects of sample size on the performance of species distribution models. Divers. Distrib. 2008, 14, 763–773. [Google Scholar] [CrossRef]
- Chen, X.M.; Lei, Y.C.; Zhang, X.Q.; Jia, H.Y. Effects of sample sizes on accuracy and stability of maximum entropy model in predicting species distribution. Sci. Silv. Sin. 2012, 48, 53–59. [Google Scholar]
- Ji, Q.Z.; Wang, R.X.; Huang, Z.P.; Yuan, J.H.; Ren, G.P.; Xiao, W. Effects of sample size and study range on accuracy of MaxEnt in predicting species distribution: A case study of the black-and-white snub-nosed monkey. Acta Theriol. Sin. 2019, 39, 126–133. [Google Scholar]
- Mota, J.D.; Barbosa, L.R.; Marchioro, C.A. Suitable areas for invasive insect pests in Brazil and the potential impacts for eucalyptus forestry. Pest Manag. Sci. 2022, 78, 2596–2606. [Google Scholar] [CrossRef]
- Zhang, Y.; Hughes, A.C.; Zhao, Z.H.; Li, Z.H.; Qin, Y.J. Including climate change to predict the global suitable area of an invasive pest: Bactrocera correcta (Diptera: Tephritidae). Glob. Ecol. Conserv. 2022, 34, e02021. [Google Scholar] [CrossRef]
- Santos, R.C.; Batista, C.V.; Santos, A.A.; da Silva, R.S.; Picanç, M.C. Predicting suitable areas for the invasive species Erthesina fullo (Hemiptera: Pentatomidae). Crop Prot. 2023, 172, 106297. [Google Scholar] [CrossRef]
- Yang, F.; Liu, Q.; Yang, J.; Liu, B.; Deng, X.; Gan, T.; Liao, X.; Li, X.; Xu, D.; Zhuo, Z. Evaluating the impact of climate change on the Asia habitat suitability of Troides helena using the MaxEnt model. Insects 2025, 16, 79. [Google Scholar] [CrossRef]
- Guo, Y.L.; Wei, H.Y.; Lu, C.Y.; Zhang, H.L.; Gu, W. Predictions of potential geographical distribution of Sinopodophyllum hexandrum under climate change. Chin. J. Plant Ecol. 2014, 38, 249–261. [Google Scholar]
- Ji, L.T.; Zheng, T.Y.; Chen, Q.; Zhong, J.J.; Kang, B. Responses of potential suitable area of Paris verticillata to climate change and its dominant climate factors. Chin. J. Appl. Ecol. 2020, 31, 89–96. [Google Scholar]
- Tan, Y.H.; Zhang, X.J.; Yuan, S.S.; Yu, J.H. Prediction of the ecological suitability of Gentiana macrophylla Pall. under scenarios of global climate change. Chin. J. Ecol. 2020, 39, 3766–3773. [Google Scholar]
- Chen, S.; Holyoak, M.; Liu, H.; Bao, H.; Ma, Y.; Dou, H.; Jiang, G. Effects of spatially heterogeneous warming on gut microbiota, nutrition and gene flow of a heat-sensitive ungulate population. Sci. Total Environ. 2022, 806, 150537. [Google Scholar] [CrossRef]
- Miao, G.T.; Zhao, Y.J.; Wang, Y.J.; Yu, C.J.; Xiong, F.; Sun, Y.K.; Cao, Y. Suitable habitat prediction and analysis of Dendrolimus houi and its host Cupressus funebris in the Chinese region. Forests 2024, 15, 162. [Google Scholar] [CrossRef]
- Kwon, T.-S.; Lee, C.M.; Kim, S.-S. Prediction of abundance of beetles according to climate warming in South Korea. J. Asia-Pac. Biodivers. 2015, 8, 7–30. [Google Scholar] [CrossRef]
- Wang, Y.H.; Ma, D.Y.; Deng, H.Y.; Wu, S.H. Climate change risk assessment of ecosystem productivity in the arid/humid transition zone of northern China. Acta Geogr. Sin. 2021, 76, 1605–1617. [Google Scholar]
- Zhang, Q.; Zhu, B.; Yang, J.H.; Ma, P.L.; Liu, X.Y.; Lu, G.Y.; Wang, Y.H.; Yu, H.P.; Liu, W.P.; Wang, D.W. New characteristics about the climate humidification trend in Northwest China. Chin. Sci. Bull. 2021, 66, 3757–3771. [Google Scholar] [CrossRef]
- Flower, A.; Murdock, T.Q.; Taylor, S.W.; Zwiers, F.W. Using an ensemble of downscaled climate model projections to assess impacts of climate change on the potential distribution of spruce and Douglas-fir forests in British Columbia. Environ. Sci. Policy 2013, 26, 63–74. [Google Scholar] [CrossRef]

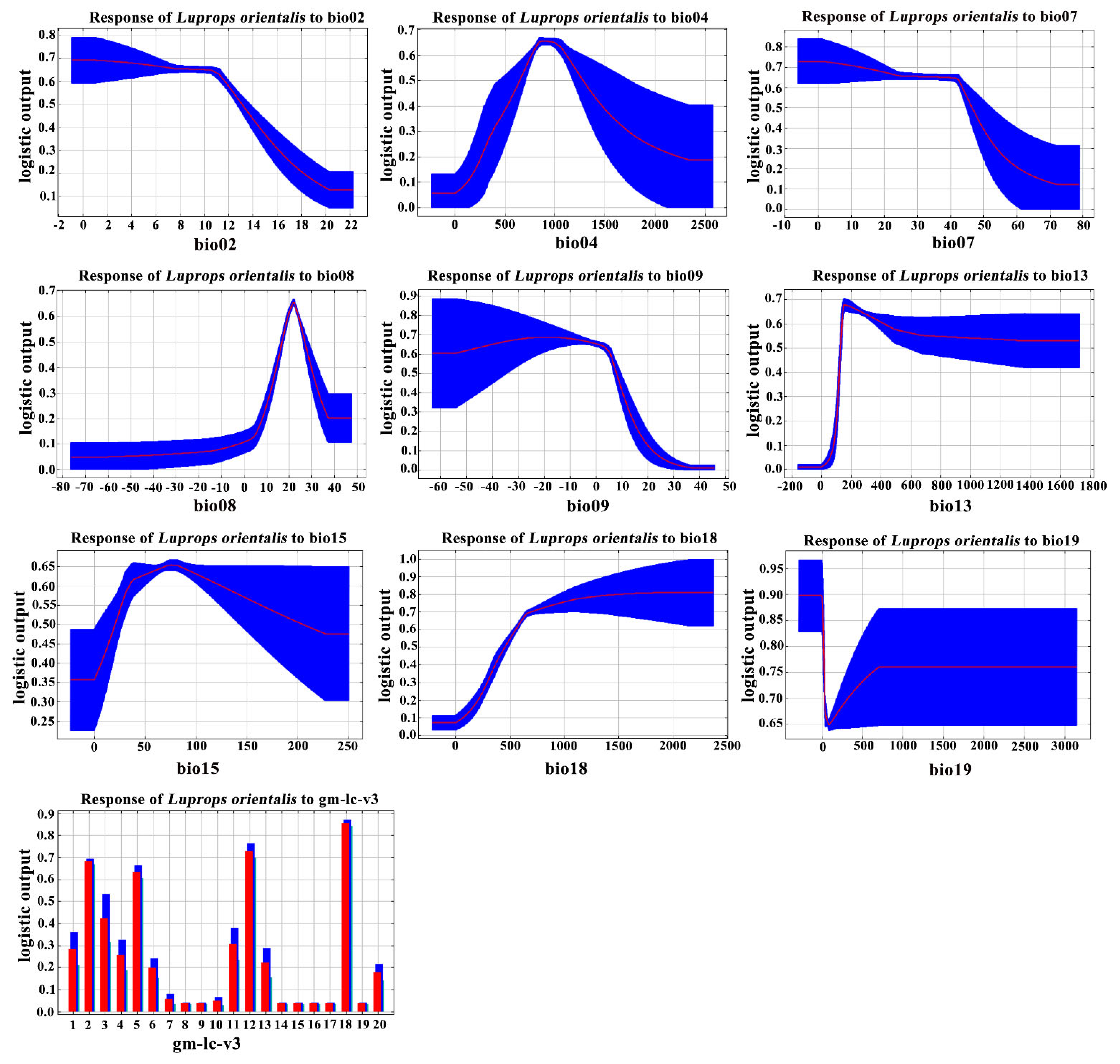


| Variable | Variable Description | Contribution (%) |
|---|---|---|
| Bio02 | Mean daily temperature range | 0.2 |
| Bio04 | Temperature seasonality | 22.2 |
| Bio07 | Average annual temperature range | 2.3 |
| Bio08 | Mean temperature of the wettest quarter | 0.8 |
| Bio09 | Mean temperature of the driest quarter | 2.4 |
| Bio13 | Precipitation of the wettest month | 9 |
| Bio15 | Precipitation seasonality | 0.4 |
| Bio18 | Precipitation of the warmest quarter | 61.3 |
| Bio19 | Precipitation of the coldest quarter | 0.7 |
| gm-lc-v3 | Land cover type | 0.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Wang, S.; Li, Y.; Ding, S.; Wei, Z.; Shi, A.; Yang, D. Distribution Pattern and Change Prediction of Luprops orientalis (Coleoptera: Tenebrionidae) Suitable Area in East Asia Under Climate Change. Insects 2025, 16, 626. https://doi.org/10.3390/insects16060626
Wang J, Wang S, Li Y, Ding S, Wei Z, Shi A, Yang D. Distribution Pattern and Change Prediction of Luprops orientalis (Coleoptera: Tenebrionidae) Suitable Area in East Asia Under Climate Change. Insects. 2025; 16(6):626. https://doi.org/10.3390/insects16060626
Chicago/Turabian StyleWang, Jieqiong, Shuangyi Wang, Yunchun Li, Shuangmei Ding, Zhonghua Wei, Aimin Shi, and Ding Yang. 2025. "Distribution Pattern and Change Prediction of Luprops orientalis (Coleoptera: Tenebrionidae) Suitable Area in East Asia Under Climate Change" Insects 16, no. 6: 626. https://doi.org/10.3390/insects16060626
APA StyleWang, J., Wang, S., Li, Y., Ding, S., Wei, Z., Shi, A., & Yang, D. (2025). Distribution Pattern and Change Prediction of Luprops orientalis (Coleoptera: Tenebrionidae) Suitable Area in East Asia Under Climate Change. Insects, 16(6), 626. https://doi.org/10.3390/insects16060626





