Simple Summary
Organic farming can contribute to counteract the negative effects of agriculture on biodiversity, but its impact—especially on genetic diversity—is not well understood. This study examines how organic and conventional farming influence the population dynamics and genetic diversity of the earwig Euborellia annulipes, a beneficial insect, in banana groves. We found more earwigs in organic orchards, and particularly more females, possibly because they are more sensitive to pesticides due to their sedentary behaviour associated with parental care. Males, being more mobile, showed no difference between management regimes. Genetic analyses revealed that, although both systems exhibited similar levels, conventional groves had a greater nucleotide diversity. This may suggest that pesticide exposure is inducing mutations related to detoxification and resistance, or that increased gene flow—driven by greater mobility in response to disturbances—is enhancing genetic mixing. Our findings illustrate that combining ecological and genetic information is crucial for better understanding how agricultural practices affect invertebrates.
Abstract
Organic farming can help mitigate the negative impacts of agriculture on biodiversity, but its effects remain controversial and poorly understood for many taxa, especially from a genetic perspective, where major knowledge gaps persist. This study investigates how the organic and conventional management of banana groves influences population dynamics (i.e., total abundance and abundance by sex and developmental stage) and mitochondrial genetic diversity (cytochrome b gene) of the earwig Euborellia annulipes, a natural biological control agent. The results revealed higher overall abundance, particularly of females, in organic groves. This could be due to females’ more sedentary behaviour linked to parental care, increasing their vulnerability to local disturbances such as pesticide application. In contrast, males, being more mobile, did not respond to the farming system. Genetic analyses revealed similar haplotypic diversity across systems but higher nucleotide diversity in conventional orchards. This may suggest either pesticide-induced mutations associated with detoxification and resistance or increased gene flow driven by greater mobility in response to disturbance. Although the high genetic diversity observed raises questions about the introduced status of E. annulipes, its origin remains unresolved. These findings highlight the relevance of integrating ecological and genetic data when assessing the impacts of agricultural practices on beneficial arthropods.
1. Introduction
Agricultural biodiversity is currently facing multiple threats, including habitat loss, the homogenisation of agricultural systems, or the intensive application of pesticides and fertilisers [1]. The decline in agrobiodiversity may have significant implications for food security, as it diminishes critical ecological services like natural pest control, consequently increasing the vulnerability of crops to pest outbreaks, diseases, and climate-related shocks [2,3,4]. Nevertheless, agriculture itself is not solely responsible for the significant loss of biodiversity but rather expansion and intensification processes [5,6,7]. In fact, certain forms of agriculture and management strategies, such as traditional farming, agroforestry, low-intensity management, crop rotation, or the preservation and promotion of habitat heterogeneity, make agroecosystems sustainable environments capable of supporting high levels of biodiversity [8,9,10,11,12].
Another strategy that enables the establishment of ecologically balanced (i.e., resilient, diverse) and productive ecosystems is organic farming. This low-intensity system adheres to rigorous guidelines and regulations that, among the key provisions, prohibit the application of synthetic pesticides and fertilisers, the use of genetically modified organisms (GMOs), and boost the promotion of functional biodiversity for enhancing ecosystem services (Regulation UE 2018/848). From a biodiversity perspective, there is some controversy about the effects of organic farming on biodiversity [11]. Overall, it is considered that wildlife-friendly practices developed under organic management can help to counteract the negative impacts of agriculture on associated biodiversity [7,13,14,15,16,17,18,19]. However, it is also emphasised that the benefits of organic farming can be contingent upon factors such as geographical region, crop type, management practices, or the composition of the species or taxa involved, thus resulting in heterogeneous impacts on biodiversity [1,14,15,20,21,22,23].
Agrobiodiversity comprises different components [24]. In fact, agricultural zones often constitute the habitat of non-native species that have been introduced and spread across cropped areas worldwide by changing plant or soil material [25,26,27], a process that is currently being magnified by the increase in global trade [28,29]. Although most non-native insects stand out for their global negative impacts [30,31], other have been deliberately introduced due to their functional role in order to enhance ecosystem services such as biological control [32,33].
Ecosystem homogenisation tends to simplify the genetic diversity of species that inhabit them, as the reduction in ecological niche variety simultaneously reduces the range of selective pressures promoting genetic diversity within populations [34,35]. Agricultural intensification, which generally involves a homogenisation of agricultural systems at both local and landscape levels, may lead to a reduction in the genetic diversity of genotypes [36,37]. It is noteworthy that, although genetic diversity determines the evolutionary potential of a species, the extent to which agricultural practices influence such diversity remains poorly understood. This genetic footprint, shaped by agricultural practices, may also interact with that left by species introductions. Populations introduced to new environments, typically derived from a small number of individuals (founder effect), tend to experience a reduction in genetic diversity compared to their native counterparts [38,39].
Among the multiple examples of alien species, earwigs, considered to be significant biocontrol agents [1,40,41,42], have been frequently introduced worldwide through the international trade of plants and goods [25,43,44,45,46,47,48,49,50]. The introduction of species of the genus Euborellia Burr, 1910 has been reported in many European, American, and Australasian countries, sometimes as a biological control agent [25,44,46,50,51,52,53,54]. Occasionally, some species were described from greenhouses where they were already introduced, rendering the origin of these species doubtful, as is the case of E. arcanum Matzke and Kočárek, 2015 or E. annulipes (Lucas, 1847). Euborellia annulipes (Figure 1), possibly native to the Mediterranean or East Africa [50,54], is considered a “probable introduced species” by the Canary Islands Government [55], where it is extensively present in cropped areas.

Figure 1.
Female Euborellia annulipes (Lucas, 1847) from Puntallana, La Palma, Canary Islands. Photograph by M. G.-P.
In the Canary Islands, agriculture plays an important role in shaping the islands’ landscape, especially the banana production [56,57,58]. Banana crops hold substantial global significance, ranking as the world’s 12th largest crop in terms of production [59]. In the Canary Islands, banana production constitutes approximately 30% of the total agricultural output [57,60], making it the primary agricultural crop in the region and an essential part of the economy and culture of the archipelago. Although numerous farmers have transitioned to organic farming practices, in global terms, there is limited available information on the impact of organic banana cultivation on biodiversity, even considering that biodiversity responses to organic farming can vary widely among crop types [14].
In this study, we examine populations of E. annulipes in banana crops on the island of La Palma from an ecological and genetic perspective in order to (i) evaluate the extent to which farming system (organic vs. conventional) determines the overall abundance of the species, (ii) analyse whether potential sensitivity to farming system differs between sexes and/or instars, (iii) assess whether the genetic diversity of the cytochrome b mitochondrial gene (cytb) of E. annulipes is influenced by farming system, and (iv) explore the ecological or historical factors that may have shaped these patterns of genetic diversity in E. annulipes.
2. Materials and Methods
2.1. Study Area
This study was carried out in 2021 in Breña Baja and Puntallana on the island of La Palma (28°38′36″ N 17°46′7″ W; 28°45′38″ N 17°44′47″ W) (Canary Islands, Spain). La Palma is located in the northwestern area of the Canaries, with an area of 728 km2 and a maximum altitude of 2426 m a.s.l. [61]. The climate is predominantly subtropical–Mediterranean, with humid winters and dry summers. However, climatic conditions differ considerably within the island. Annual precipitation ranges from about 170 mm to almost 1400 mm, although fog drip can locally lead to an increase in precipitation, particularly during the summer. The north and east slopes of the island are especially exposed to the trade winds below 1500 m, which make them extremely humid. The annual temperature ranges from about 9 °C on the island summit to around 22 °C at the leeward southwestern coast [62].
The zonal vegetation reflects the climatic conditions of the island, including halophytic communities in arid coastal areas, succulent scrub and thermophilic woodlands in semi-arid lower elevations, endemic Canary Pine Forest in mid-elevations, or high-elevation summit scrub [62].
Lowlands are dominated by traditional banana crops (Musa acuminata Colla) that, due to the rough topography of the island, consist of small fields arranged on terraces. However, other crops such as avocado (Persea americana Mill) and vineyard (Vitis vinifera L.) are also well represented in the area [63]. Traditional orchards are interspersed with remnants of natural vegetation, shaping a mosaic agricultural landscape. These remnants of natural vegetation are formed mainly by sub-desert shrubby vegetation, typically present in the low-elevation parts of the island, and composed of singular species of Euphorbia L., Senecio L., and Aeonium Webb & Berthel, among others [64].
2.2. Sampling Design
Across a study area of 170,000 and 500,000 m2, we sampled banana groves under different management regimes (three conventional and three organic groves) in an elevation range of 75 to 200 m. Organic and conventional groves were not clustered in separate zones but were interspersed throughout the landscape. The mean field area was 2017 ± 303 m2, and the mean nearest-neighbour distance was 647.82 ± 191.7 m. Organic banana groves were certified for organic production (EU Parliament Regulation 2018/848) [65], which implies that no synthetic fertilisers and pesticides are applied. Among the management strategies implemented in organic farming is the application of fertilisers made from organic plant and animal matter and manure, such as enriched liquid manure and compost [66,67]. Banana exhibits vegetative propagation and is mostly grown as a perennial crop. Propagation traditionally consists of selecting plant sprouts from the mother plants growing in the field. Banana plants are periodically renewed by cutting old plants, which are replaced by newly selected plants [68,69]. Once cut, old stems are typically left in the soil within the plot.
Earwigs, Euborellia annulipes, were sampled inside the cut banana stems of the six groves in June over five consecutive days. In each banana grove, we randomly selected a total of eight cut stems of similar size (approximately 20 cm in diameter) and at an advanced stage of decomposition in order to ensure comparable microhabitat conditions. Furthermore, all stems were shaded similarly, avoiding differences in temperature and humidity. All sampling was conducted during the same daytime interval (12:00–17:00), a period when earwigs shelter in the stems. The stems were thoroughly inspected for E. annulipes by shredding the rotten tissue; all specimens detected were taken directly by hand and transferred to individual 2 mL cryotubes with absolute ethanol for preservation. Sex and instar were determined for all the collected individuals.
2.3. Statistical Analysis
We applied separate generalised linear models (GLMs) to analyse the effects of farming system (organic vs. conventional farming) on (i) total earwig abundance and on the number of (ii) adults (female plus male), (iii) nymphs, (iv) females, and (v) males. Models were fitted with a Poisson distribution and log link function and were inspected for overdispersion. When overdispersion was detected, the models were adjusted with a negative binomial distribution. All models were validated by inspecting the residuals both graphically and with the DHARMa package. Analyses were performed using R 4.3.1 software [70].
2.4. DNA Extraction, Amplification, and Sequencing
We obtained total DNA from 12 specimens of E. annulipes (2 specimens from each banana grove) (Table 1). DNA was extracted from one leg, using the DNeasy Blood and Tissue Isolation Kit (Qiagen, Hilden, Germany), according to the manufacturer’s instructions, and then stored at 4 °C until further processing. The polymerase chain reaction (PCR) was used to amplify fragments (385 bp, 373 bp after end trimming) of the cytochrome b gene (cytb) using the set of primers CB-J-10933 (TATGTACTACCATGAGGACAAATATC) [71] and CB4 (AAAAGAAARTATCATTCAGGTTGAAT) [72]. PCR amplifications consisted of 18.8 μL of distilled water, 2.5 μL of 10× PCR buffer, 1 μL of dNTP mix (10 mM), 0.5 μL of MgCl2 (50 mM), 0.5 μL of each primer (10 μM), 0.2 μL of DNA polymerase (5 U/μL), and 1 μL of DNA template, consisting of a final reaction volume of 25 μL. The thermocycling conditions consisted of an initial denaturation at 95 °C for 5 min, followed by 40 cycles of denaturation at 94 °C for 1 min, annealing at 40 °C for 1 min and extension at 72 °C for 1 min, and a final elongation step at 72 °C for 5 min. After the amplification, 3 μL of the reaction was analysed by electrophoresis for 40 min at 90 V on a 1% agarose gel. Samples with single bands were sent to the company Macrogen Inc. (Macrogen Europe, Madrid, Spain) for sequencing both strands.

Table 1.
Specimens of Euborellia annulipes (Lucas, 1847) used for DNA analyses with their corresponding voucher numbers and GenBank accession number and haplotype codes.
Sequences were checked, edited, and aligned using Geneious Prime 21.1.1 software.
2.5. Genetic Analyses
To identify potential patterns of cytb variation driven by the farming system (conventional vs. organic), haplotype networks were generated with Population Analysis with Reticulated Trees (PopART), using a TCS algorithm to shape the relationships between haplotypes. Haplotype networks were edited with Adobe Photoshop CS5 version 12.0 (Adobe Systems Incorporated). Uncorrected (p) pairwise genetic distances were estimated using PAUP * v.4.0a [73].
Genetic diversity in populations of E. annulipes from both organic and conventional groves was analysed by calculating the haplotype diversity (Hd) and nucleotide diversity (π) of cytb sequences. Both metrics were computed using R 4.3.1 software [72]. Sequences were categorised into two groups based on the farming system (organic and conventional), and diversity analyses were conducted using the ‘pegas’ [74] and ‘poppr’ packages [75]. Haplotype diversity reflects the probability that two randomly chosen sequences are different, i.e., it represents the variety of haplotypes present. Nucleotide diversity estimates the average number of nucleotide differences between pairs of sequences, i.e., it considers how different the sequences are from each other.
3. Results
3.1. Effects of Farming System on the Abundance of Euborellia annulipes
A total of 218 specimens of E. annulipes were collected from banana stems, of which 121 were adults, 97 were nymphs, 84 were females, and 37 were males.
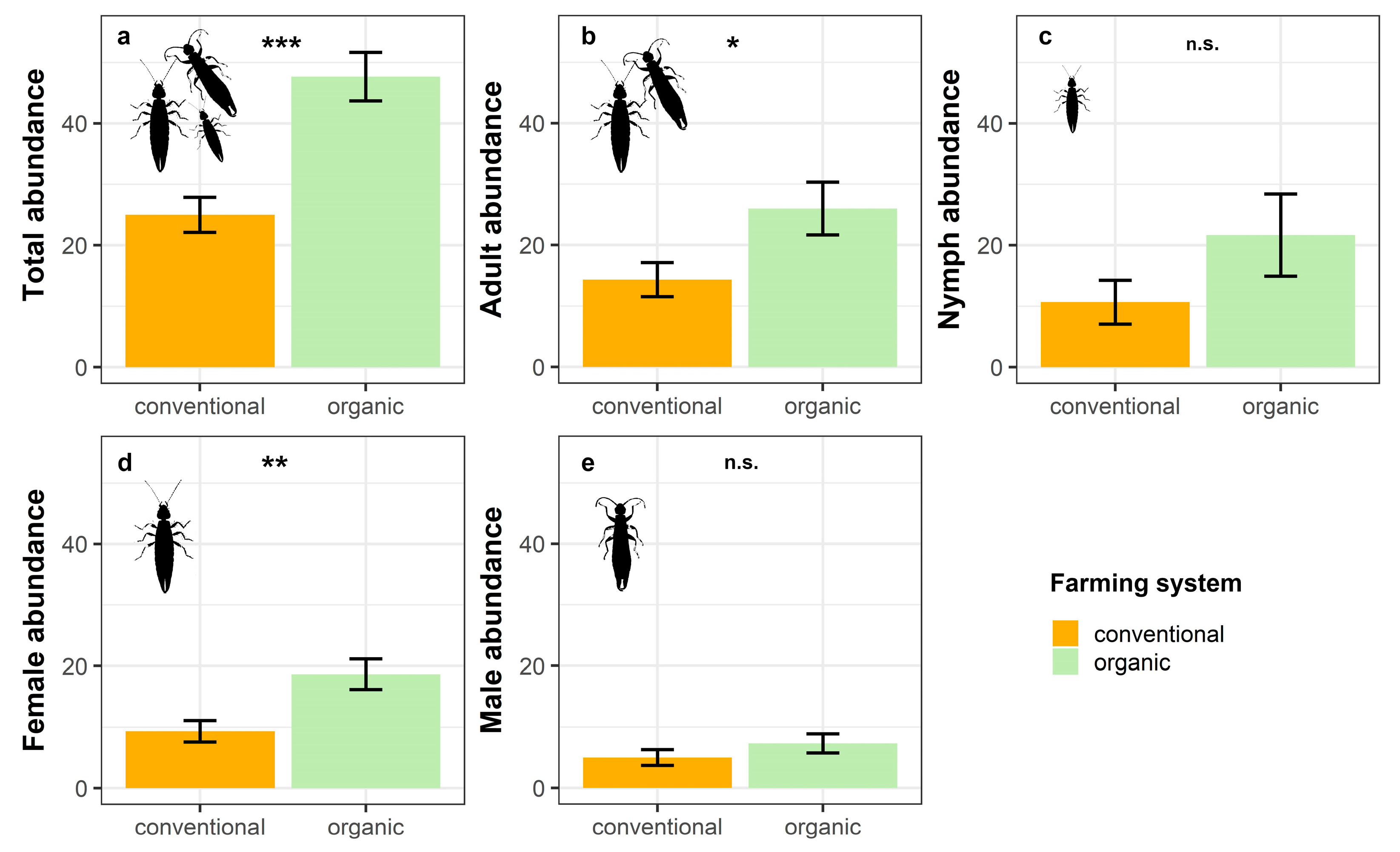
The results from the GLMs showed that total earwig abundance was significantly affected by the farming system, with organic banana groves harbouring larger numbers of E. annullipes than conventional ones (Table 2; Figure 2a). However, the benefits of organic farming differed between sexes and also varied depending on the development stage.

Table 2.
Parameter estimates for the generalised linear models (GLMs) assessing the effect of the farming system (organic vs. conventional) on earwig total abundance and on the number of adults, nymphs, females, and males. Reference coefficient is system (conventional) (* p < 0.05, ** p < 0.01, *** p < 0.001).
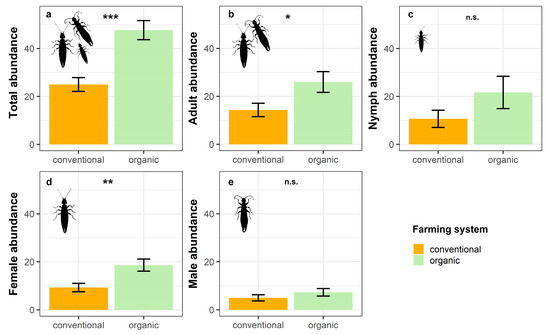
Figure 2.
Estimated mean ± SE of (a) total abundance, (b) adult abundance, (c) nymph abundance, (d) female abundance, and (e) male abundance of E. annulipes in conventional and organic banana groves (n.s. not significant, * p < 0.05, ** p < 0.01, *** p < 0.001).
Thus, adults were benefited by organic farming, whereas nymphs did not show any significant response to this factor (Table 2; Figure 2b,c). Regarding sexes, females reached significantly higher abundances in organic orchards compared to conventional orchards (Table 2; Figure 2d). Conversely, we did not find any effect of farming system on males, with their densities being similar under both management regimes (Table 2; Figure 2e).
3.2. Genetic Diversity
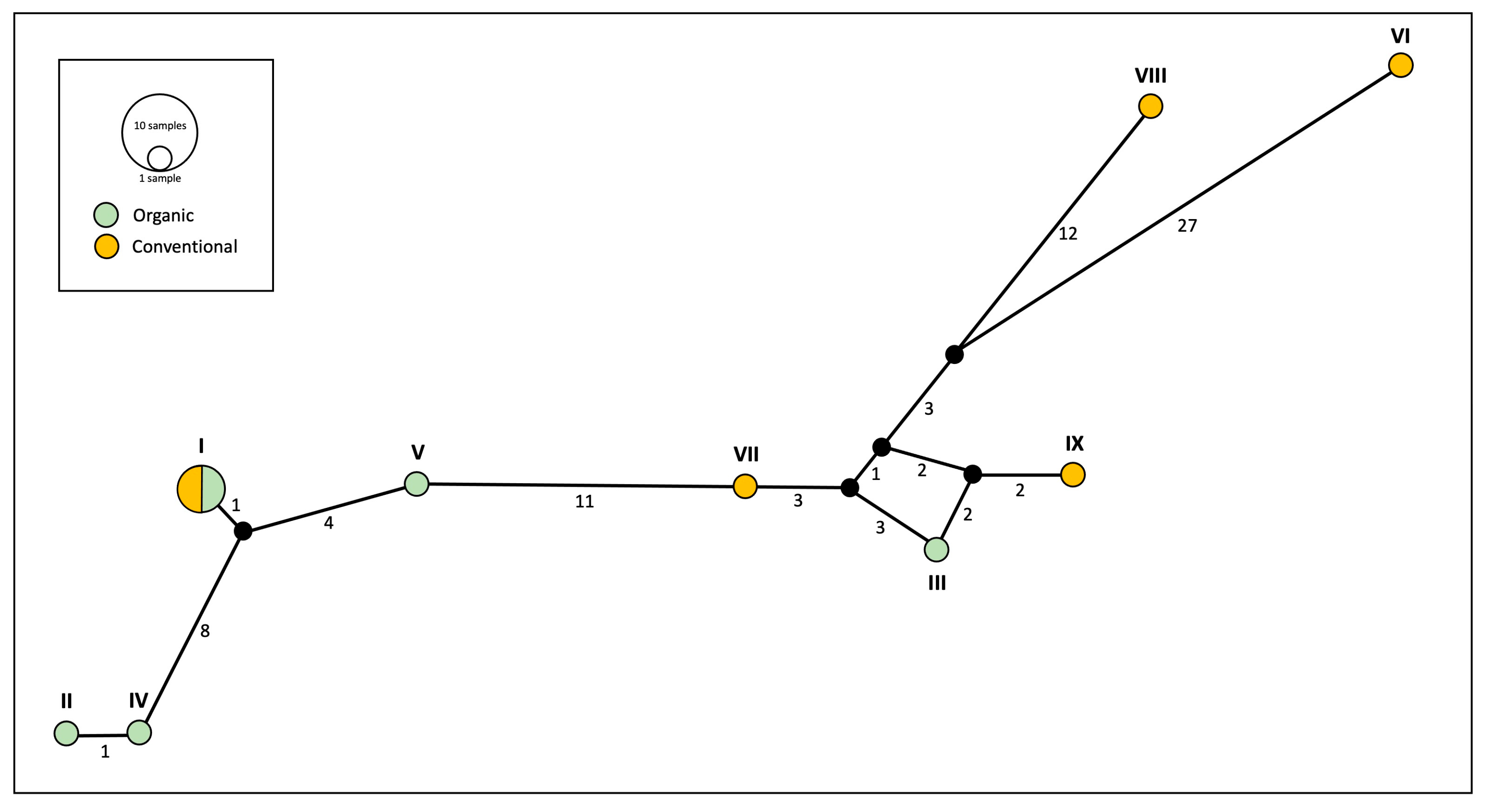
The phylogeographic network based on the mitochondrial gene cytb (Figure 3) revealed nine distinct haplotypes among the 12 specimens of E. annulipes analysed. One haplotype was shared between the two farming systems, occurring in two individuals from organic groves and two others from conventional orchards. The remaining eight haplotypes were evenly distributed, with four exclusively present in organic farming and the other four unique to conventional management.
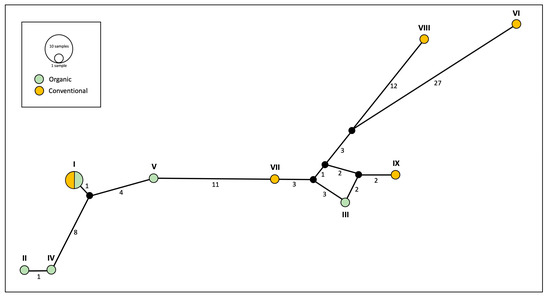
Figure 3.
Mitochondrial (cytb) network analyses of specimens of Euborellia annulipes from organic and conventional banana groves. Numbers below the lines connecting haplotypes represent the number of mutations from one point to another. Roman numerals (I–IX) refer to the haplotype assignment for each specimen (see Table 1).
Sequence divergence within mtDNA from organic banana groves was relatively low (uncorrected p distance = 0.0000–0.0643). In comparison, genetic distances within conventional (p distance = 0.0000–0.1206) and across samples from conventional and organic orchards (p distance = 0.0000–0.1260) were considerably large. Genetic diversity analyses of cytb sequences highlighted differences between the two farming systems in terms of nucleotide diversity (π). Sequences from conventional banana groves exhibited significantly higher nucleotide diversity (π = 0.070) compared to those from organic ones (π = 0.033). This is consistent with the results from the genetic distance matrix, where we observed that genetic distance between sequences within the conventional orchards was significantly higher than the distance between sequences within organic groves. In contrast, haplotype diversity was identical across both management regimes (Hd = 0.933), consistent with the results from the haplotype network (Figure 3), where an equal number of unique haplotypes was identified in each system.
4. Discussion
The discussion surrounding the impact of agricultural systems on organisms’ population structure continues to advance. Organic farming, in particular, appears to create conditions that benefit certain species, though their responses can vary significantly. Consistent with previous studies on diverse earwigs [76,77,78], the total abundance of E. annulipes was higher in organic groves compared to conventional ones. Studies on Forficula auricularia Linnaeus, 1758 have revealed that more complex and less intensive agricultural systems, such as organic farming, tend to support higher populations, likely as a result of increased food resources and shelter availability [79,80]. In addition, pesticide application inevitably involves the exposure of non-target organisms, with consequent effects on species, communities, or ecosystems [81]. Among that, earwigs are sensitive to pesticide application and can also be influenced by different agricultural practices and by crop characteristics [22,81,82,83]. Therefore, the ban of pesticide use in organic farming can promote higher survival rates of beneficial fauna, including earwigs [76,80], which could explain the observed greater abundance of E. annulipes in these systems. However, earwig responses to organic farming are not always consistent [22,78,82], as we detected when diving deeper into how the different sexes and life stages are influenced by the management regime.
Aligning with previous studies that demonstrate sex-specific reactions to agricultural management [80,82,83], we found that males and females exhibited contrasting patterns, with only females being favoured by organic farming. This disparity may be linked to the differing behaviours of both sexes and their distinct biological roles. Females of E. annulipes provide intensive parental care to the eggs, but males provide no care and frequently prey upon them [84]. During offspring development, females take care of the eggs, licking and cleaning them to prevent fungal infections [85,86], and also care for nymphs during the first days of development [87]. This behaviour suggests that females exhibit a high fidelity to a specific microhabitat, being largely conditioned by the local characteristics of that habitat since they remain close to the nest. In the specific case of conventional banana groves, this situation could lead to greater exposure to chemicals, and thus to a reduced abundance in such systems. This hypothesis is consistent with previous studies demonstrating that females can be more sensitive to pesticides than males [88]. In contrast to the females, males of E. annulipes are likely to be generally more mobile, spending more time searching for mates or food [84,85,86]. Their increased mobility may render them less susceptible to local habitat characteristics, enabling them to seek refuge in areas with reduced pesticide exposure. Our results suggest that male biology or behaviour may confer resilience to certain environmental pressures, which could explain their consistent abundance across both organic and conventional orchards.
These findings could have implications for key ecosystem services, such as crop pest control. Most earwigs are known as generalist predators that capture a wide variety of prey [53,89] and may play an important role in controlling orchard pests in the absence of chemical pesticides [77,90,91], as occurs in organic farming. In the specific case of our focus species, due to its high voracity preying upon several insect orders, such as Diptera [92,93], Hemiptera [94], Lepidoptera [95,96], and Coleoptera [85], E. annulipes has been considered a promising natural enemy [97,98,99,100]. In addition, Coelho et al. [97] found that females can consume larger numbers of prey than males. In view of the above considerations, the situation depicted by our results could imply that pest control may be to some extent reinforced in organic banana groves, in which not only higher densities of E. annulipes but also larger numbers of females were recorded compared to conventional ones.
In relation to life stages, although adults were overall favoured by organic farming, we did not detect any significant differences in nymph preferences. This observation is striking considering that in experiments testing pesticide impacts where different instars have been used, adult earwigs were found to be less sensitive than nymphs [88,98,101,102]. In addition, Meunier et al. [103] reported that pesticides exert sublethal effects on F. auricularia females, particularly affecting maternal care behaviour; females exposed to pesticides tended to abandon their nests for prolonged periods, with significant reductions in their grooming and egg-clumping activities. As previously discussed, females may be somewhat more sensitive to pesticides than males and, at first glance, one might expect these potential effects on maternal care to have a greater impact on egg or nymph mortality. However, this expectation does not align with our findings, which showed no significant differences in nymph abundance between organic and conventional groves. Although some studies suggest that nymphs can be more susceptible to pesticide exposure due to the higher permeability of their cuticles, as noted by [82], it is important to mention that previous research has mainly focused on F. auricularia, and specific data on the cuticle permeability of E. annulipes are currently lacking.
One potential explanation for the absence of differences in nymph abundance between farming systems could be that, overall, organic farming may promote both biodiversity and predator richness [23,104,105,106], resulting in increased predation pressures. This situation may buffer the potential higher survival rates of nymphs in the absence of pesticides due to a higher predation risk. This hypothesis is consistent with the findings of Sinclair et al. [107], who showed that predation pressure tends to be higher in more biodiverse environments.
In addition to population abundance, genetic diversity provides relevant insights for understanding how populations adapt and maintain resilience to environmental changes [108]. Organic and conventional farming exert different pressures, potentially shaping genetic variation within populations. Habitat heterogeneity and population size are key factors influencing genetic diversity [109], which in turn affects how species like Euborellia annulipes respond to agricultural management. In organic farming, one might expect increased species diversity [13,19,110] to be reflected in greater genetic diversity within populations. However, our findings revealed a contrasting pattern: although haplotypic diversity is similar in both systems, conventional groves exhibited higher nucleotide diversity than those under organic management. Furthermore, the genetic distance between conventional orchards was comparable to that observed across different farming systems (0–0.1206 vs. 0–0.1260, respectively). One possible explanation for the larger genetic diversity observed in conventional farming could be related to the selective pressure exerted by pesticides. Previous research has shown that invertebrates exposed to pesticides can develop genetic resistance mechanisms [111]. In the case of F. auricularia, for example, pesticides have been linked to mutations in genes associated with detoxification and resistance to these compounds [111]. This adaptive process, driven by a continuous exposure to pesticides, could be increasing genetic variability in E. annulipes populations in conventional systems. In addition, conventional banana plantations also tend to be exposed to greater landscape fragmentation and soil changes, which, combined with the presence of chemicals, would result in the invertebrate populations that inhabit them being subjected to a more dynamic and stressful environment. This context could favour a larger divergence between haplotypes. In contrast, being free of harsh pesticides, organic groves may represent more stable environments, in which reduced rates of persistence of new genetic variants may occur due to lower selective pressures. While overall biodiversity (i.e., species diversity) tends to be higher in organic farming [7,16,20,104], this does not have to translate into larger genetic diversity within populations, as we observed in this study.
Another factor that may explain the high genetic diversity in conventional farming is increased gene flow, reflecting a greater contribution of genetic material. This could result from the movement or introduction of specimens, potentially associated with higher mobility in intensively managed systems. A history of agricultural practices that facilitated the introduction of populations from diverse sources may have further contributed to the observed genetic variation.
Genetic variability often plays a crucial role in determining whether a species is native to a region or has been introduced. In native species, genetic diversity tends to reflect long-term evolutionary processes such as local adaptation, isolation by distance, and historical populations’ connectivity. In contrast, non-native species may show signs of recent bottlenecks, founder effects, or genetic homogeneity, particularly when introductions are recent or derived from a limited number of individuals [112,113]. When a limited number of species are studied or in a limited geographic area, high genetic diversity is often associated with native species or species that have been established for a long period of time in the territory. This is because they have had enough time to evolve and accumulate haplotypic diversity. According to our results, we observed high genetic diversity comparable to that reported for other long-established species of earwigs using the same molecular marker (cytb) [114]. Specifically, populations of Pseudochelidura cantabrica Cuesta-Segura, Jurado-Angulo & García-París, 2023, from Burgos and León populations of the Iberian Peninsula that are about 100 km apart and separated by mountains of the Cantabrian Mountains), presented an uncorrected mitochondrial (p) pairwise genetic distance of 0.112–0.123, comparable to the 0–0.1206 distance presented across populations of E. annulipes in this study. These results could suggest that E. annulipes has been established for a long time, at least on the island of La Palma. However, E. annulipes is currently considered an introduced species by the Canary Islands government [115]. If the species responds to this last hypothesis, the high haplotypic diversity observed may result from a large genetic pool generated through repeated introductions due to the transfer of materials or soil for cultivation. Nevertheless, large populations also tend to exhibit greater genetic variation due to a combination of factors, including higher potential for mutation, a greater probability of retaining new alleles, and a reduced influence of genetic drift. Nevertheless, in this study we have focused on the overall abundance of E. annulipes, which serves as a proxy for general population size, rather than examining the effective population size (Ne). It is the effective population size that more directly affects genetic diversity, as it represents the number of individuals that actually genetically contribute to the next generation.
The problem of the origin of E. annulipes is far from solved. The native or introduced status in the Canary Islands and other Atlantic Islands is totally unclear. The species was described originally from the greenhouses of the Jardin des Plantes of Paris, where according to Lucas [116], the species was introduced in exotic plants brought to the greenhouses, maybe from tropical America. If this was so, the plants were likely stationed in the Canary Islands for some time before reaching the European continent, thus making possible the landing of Euborellia in the Islands, or alternatively the boarding of the native earwigs while watering the plants on the ships, if they were native to the Canaries. No molecular studies have been undertaken to study E. annulipes populations from the Mediterranean basin, confined to mild-winter sea shores, and compare their genetic structure and diversity with that of populations from the Atlantic Islands and even from the known American populations, which include, for example, high elevation urban areas in inland Mexico (own data). The relatively limited range of the species in Southern Europe and Northwestern Africa might question a Mediterranean origin for the species, while the large diversity shown in our study in cropped areas from a single Atlantic Island (La Palma) does not support, a priori, the introduced status for the species. Even the taxonomy of E. annulipes as a whole is questionable, since no direct comparisons of the specimens used in the original description (certainly introduced in Paris) have been established with Mediterranean, Macaronesian, or continental American populations. And the problem is becoming even more complex with the recent discovery of exotic species of Euborellia in Europe that can be confused superficially with E. annulipes [25,50].
Author Contributions
Conceptualisation, P.J.-A., M.G.-P., and N.R.-R.; field sampling, P.J.-A.; methodology, P.J.-A. and N.R.-R.; formal analysis, P.J.-A. and N.R.-R.; investigation, P.J.-A. and N.R.-R.; data curation, P.J.-A.; writing—original draft preparation, P.J.-A. and N.R.-R.; writing—review and editing, P.J.-A., M.G.-P., and N.R.-R.; funding acquisition, M.G.-P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Spanish Government project grant PID2019-110243GB-100/AEI/10.13039/501100011033 (Ministry of Science and Innovation) to M.G.-P.
Data Availability Statement
The genetic data supporting the findings of this study are freely available in GenBank (PV737866- PV737877).
Acknowledgments
We thank Javier Ortiz Rivero, Ana Hernández Martinez de la Riva, Juliana Cruz Montilla, Noelia Guerra González, and Victor Pérez Calle for their help during the sampling. We also thank R. Piñero Hernández, J. Rodríguez González, J. Henriquez, M. Ediseo, M.A. García Ferraz, and A. Castro for allowing us to conduct this study in their banana groves. Thanks to Javier Diéguez Uribeondo, director of the “Master’s degree in Biodiversity and conservation in tropical regions, UIMP-CSIC”, for all his support and the organisation of the trip to the Canary Islands as part of the master’s practices. P.J.-A. was supported by an FCT (Fundação para a Ciência e a Tecnologia, I.P) PhD grant (2022.14742.BD; financed by the European Social Fund and the national program ‘Portugal 2030’).
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
References
- Reiff, J.M.; Kolb, S.; Entling, M.H.; Herndl, T.; Möth, S.; Walzer, A.; Kropf, M.; Hoddmann, C.; Winter, S. Organic farming and cover-crop management reduce pest predation in Austrian vineyards. Insects 2021, 12, 220. [Google Scholar] [CrossRef] [PubMed]
- Mburu, S.W.; Koskey, G.; Kimiti, J.M.; Ombori, O.; Maingi, J.M.; Njeru, E.M. Agrobiodiversity conservation enhances food security in subsistence-based farming systems of Eastern Kenya. Agric. Food Secur. 2016, 5, 19. [Google Scholar] [CrossRef]
- FAO—Food and Agriculture Organization of the United Nations. Risk Reduction and Mainstreaming Biodiversity in Agriculture. 2024. Available online: https://www.fao.org/pest-and-pesticide-management/pesticide-risk-reduction/risk-reduction-mainstreaming-biodiversity/en/ (accessed on 5 May 2025).
- Salem, K.F.; Ibrahim, A.A. Plant Biodiversity in the Context of Food Security Under Climate Change. In Sustainable Utilization and Conservation of Plant Genetic Diversity; Al-Khayri, J.M., Mohan Jain, S., Suprasanna, P., Eds.; Springer Nature: Singapore, 2024; pp. 3–30. [Google Scholar]
- Uchida, K.; Ushimaru, A. Biodiversity declines due to abandonment and intensification of agricultural lands: Patterns and mechanisms. Ecol. Monogr. 2014, 84, 637–658. [Google Scholar] [CrossRef]
- Habel, J.C.; Ulrich, W.; Biburger, N.; Seibold, S.; Schmitt, T. Agricultural intensification drives butterfly decline. Insect Conserv. Divers. 2019, 12, 289–295. [Google Scholar] [CrossRef]
- Stein-Bachinger, K.; Gottwald, F.; Haub, A.; Schmidt, E. To what extent does organic farming promote species richness and abundance in temperate climates? A review. Org. Agric. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Stein-Bachinger, K.; Fuchs, S. Protection strategies for farmland birds in legume-grass leys as trade-offs between nature conservation and farmers’ needs. Org. Agric. 2012, 2, 145–162. [Google Scholar] [CrossRef]
- Gottwald, F.; Stein-Bachinger, K. Landwirtschaft für Artenvielfalt–Ein Naturschutzmodul für Ökologisch Bewirtschaftete Betriebe. 2016. 208p. Available online: www.landwirtschaft-artenvielfalt.de (accessed on 5 May 2025).
- Landis, D.A. Designing agricultural landscapes for biodiversity-based ecosystem services. Basic Appl. Ecol. 2017, 18, 1–12. [Google Scholar] [CrossRef]
- Tscharntke, T.; Grass, I.; Wanger, T.C.; Westphal, C.; Batáry, P. Beyond organic farming–harnessing biodiversity-friendly landscapes. Trends Ecol. Evol. 2021, 36, 919–930. [Google Scholar] [CrossRef]
- Rosas-Ramos, N.; Asís, J.D.; Tobajas, E.; de Paz, V.; Baños-Picón, L. Disentangling the Benefits of Organic Farming for Beetle Communities (Insecta: Coleoptera) in Traditional Fruit Orchards. Agriculture 2022, 12, 243. [Google Scholar] [CrossRef]
- Mondelaers, K.; Aertsens, J.; Van Huylenbroeck, G. A meta—analysis of the differences in environmental impacts between organic and conventional farming. Br. Food J. 2009, 111, 1098–1119. [Google Scholar] [CrossRef]
- Tuck, S.L.; Winqvist, C.; Mota, F.; Ahnström, J.; Turnbull, L.A.; Bengtsson, J. Land-use intensity and the effects of organic farming on biodiversity: A hierarchical meta-analysis. J. Appl. Ecol. 2014, 51, 746–755. [Google Scholar] [CrossRef] [PubMed]
- Feber, R.E.; Johnson, P.J.; Bell, J.R.; Chamberlain, D.E.; Firbank, L.G.; Fuller, R.J.; Manley, W.; Mathews, F.; Norton, L.R.; Townsend, M.; et al. Organic farming: Biodiversity impacts can depend on dispersal characteristics and landscape context. PLoS ONE 2015, 10, e0135921. [Google Scholar] [CrossRef] [PubMed]
- Froidevaux, J.S.; Louboutin, B.; Jones, G. Does organic farming enhance biodiversity in Mediterranean vineyards? A case study with bats and arachnids. Agric. Ecosyst. Environ. 2017, 249, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Porcel, M.; Andersson, G.K.S.; Pålsson, J.; Tasin, M. Organic management in apple orchards: Higher impacts on biological control than on pollination. J. Appl. Ecol. 2018, 55, 2779–2789. [Google Scholar] [CrossRef]
- Gamage, A.; Gangahagedara, R.; Gamage, J.; Jayasinghe, N.; Kodikara, N.; Suraweera, P.; Merah, O. Role of organic farming for achieving sustainability in agriculture. Farming Syst. 2023, 1, 100005. [Google Scholar] [CrossRef]
- Sidemo-Holm, W.; Brady, M.V.; Carrié, R.; Ekroos, J.; Smith, H.G. Cost-effective biodiversity conservation with organic farming-spatial allocation is key. Biol. Conserv. 2024, 294, 110624. [Google Scholar] [CrossRef]
- Bengtsson, J.; Ahnström, J.; Weibull, A.C. The effects of organic agriculture on biodiversity and abundance: A meta-analysis. J. Appl. Ecol. 2005, 42, 261–269. [Google Scholar] [CrossRef]
- Kehinde, T.; von Wehrden, H.; Samways, M.; Klein, A.M.; Brittain, C. Organic farming promotes bee abundance in vineyards in Italy but not in South Africa. J. Insect Conserv. 2018, 22, 61–67. [Google Scholar] [CrossRef]
- Happe, A.K.; Roquer-Beni, L.; Bosch, J.; Alins, G.; Mody, K. Earwigs and woolly apple aphids in integrated and organic apple orchards: Responses of a generalist predator and a pest prey to local and landscape factors. Agric. Ecosyst. Environ. 2018, 268, 44–51. [Google Scholar] [CrossRef]
- Rosas-Ramos, N.; Banos-Picon, L.; Tormos, J.; Asis, J.D. Natural enemies and pollinators in traditional cherry orchards: Functionally important taxa respond differently to farming system. Agric. Ecosyst. Environ. 2020, 295, 106920. [Google Scholar] [CrossRef]
- Altieri, M.A.; Nicholls, C.I. Biodiveristy and Pest Management in Agroecosystems; Haworth Press: New York, NY, USA, 2004; 252p. [Google Scholar]
- Matzke, D.; Kočárek, P. Description and biology of Euborellia arcanum sp. nov., an alien earwig occupying greenhouses in Germany and Austria (Dermaptera: Anisolabididae). Zootaxa 2015, 3956, 131–139. [Google Scholar] [CrossRef]
- Fried, G.; Chauvel, B.; Reynaud, P.; Sache, I. Decreases in crop production by non-native weeds, pests, and pathogens. In Impact of Biological Invasions on Ecosystem Services; Vilà, M., Hulme, P.E., Eds.; Springer: Cham, Switzerland, 2017; pp. 83–101. [Google Scholar]
- Quarrell, S.R.; Arabi, J.; Suwalski, A.; Veuille, M.; Wirth, T.; Allen, G.R. The invasion biology of the invasive earwig, Forficula auricularia in Australasian ecosystems. Biol. Invasions 2018, 20, 1553–1565. [Google Scholar] [CrossRef]
- Hulme, P.E. Trade, transport and trouble: Managing invasive species pathways in an era of globalization. J. Appl. Ecol. 2009, 46, 10–18. [Google Scholar] [CrossRef]
- Pyšek, P.; Hulme, P.E.; Simberloff, D.; Bacher, S.; Blackburn, T.M.; Carlton, J.T.; Dawson, W.; Essl, F.; Foxcroft, L.C.; Genovesi, P.; et al. Scientists’ warning on invasive alien species. Biol. Rev. 2020, 95, 1511–1534. [Google Scholar] [CrossRef] [PubMed]
- Kenis, M.; Auger-Rozenberg, M.A.; Roques, A.; Timms, L.; Péré, C.; Cock, M.J.; Settele, J.; Augustin, S.; Lopez-Vaamonde, C. Ecological effects of invasive alien insects. Biol. Invasions 2009, 11, 21–45. [Google Scholar] [CrossRef]
- Bradshaw, C.J.; Leroy, B.; Bellard, C.; Roiz, D.; Albert, C.; Fournier, A.; Barbet-Massin, M.; Saller, J.; Simard, F.; Courchamp, F. Massive yet grossly underestimated global costs of invasive insects. Nat. Commun. 2016, 7, 12986. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, P.; Cox, C.; Coombs, E. Successful biological control of ragwort, Senecio jacobaea, by introduced insects in Oregon. Ecol. Appl. 1991, 1, 430–442. [Google Scholar] [CrossRef]
- Baker, B.P.; Green, T.A.; Loker, A.J. Biological control and integrated pest management in organic and conventional systems. Biol. Control 2020, 140, 104095. [Google Scholar] [CrossRef]
- Olden, J.D.; Poff, N.L.; Douglas, M.R.; Douglas, M.E.; Fausch, K.D. Ecological and evolutionary consequences of biotic homogenization. Trends Ecol. Evol. 2004, 19, 18–24. [Google Scholar] [CrossRef]
- Holl, K.D.; Luong, J.C.; Brancalion, P.H. Overcoming biotic homogenization in ecological restoration. Trends Ecol. Evol. 2022, 37, 777–788. [Google Scholar] [CrossRef]
- Gauffre, B.; Boissinot, A.; Quiquempois, V.; Leblois, R.; Grillet, P.; Morin, S.; Picard, D.; Ribout, C.; Lourdais, O. Agricultural intensification alters marbled newt genetic diversity and gene flow through density and dispersal reduction. Mol. Ecol. 2022, 31, 119–133. [Google Scholar] [CrossRef] [PubMed]
- Nolen, Z.J.; Jamelska, P.K.; Lara, A.S.T.; Wahlberg, N.; Runemark, A. Species-specific loss of genetic diversity and accumulation of genetic load following agricultural intensification. bioRxiv 2024. [Google Scholar] [CrossRef]
- Grapputo, A.; Boman, S.; Lindstroem, L.; Lyytinen, A.; Mappes, J. The voyage of an invasive species across continents: Genetic diversity of North American and European Colorado potato beetle populations. Mol. Ecol. 2005, 14, 4207–4219. [Google Scholar] [CrossRef]
- Lambea-Camblor, A.; Morcillo, F.; Muñoz, J.; Perdices, A. Genetic and ecological approaches to introduced populations of pumpkinseed sunfish (Lepomis gibbosus) in Southwestern Europe. Diversity 2023, 15, 1059. [Google Scholar] [CrossRef]
- Mollot, G.; Duyck, P.F.; Lefeuvre, P.; Lescourret, F.; Martin, J.F.; Piry, S.; Canard, E.; Tixier, P. Cover cropping alters the diet of arthropods in a banana plantation: A metabarcoding approach. PLoS ONE 2014, 9, e93740. [Google Scholar] [CrossRef] [PubMed]
- Carval, D.; Resmond, R.; Achard, R.; Tixier, P. Cover cropping reduces the abundance of the banana weevil Cosmopolites sordidus but does not reduce its damage to the banana plants. Biol. Control 2016, 99, 14–18. [Google Scholar] [CrossRef]
- Orpet, R.J.; Crowder, D.W.; Jones, V.P. Biology and management of European earwig in orchards and vineyards. J. Integr. Pest. Manag. 2019, 10, 21. [Google Scholar] [CrossRef]
- Weidner, H. Einschleppung von Ohrwürmern nach Deutschland (Dermaptera). Anz. Für Schädlingskunde Pflanz. -Und Umweltschutz 1974, 47, 145–148. [Google Scholar] [CrossRef]
- Albouy, V.; Caussanel, C. Dermaptères ou Perce-oreilles. Faune de France 75; Fédération Française des Societés de Sciences Naturelles: Paris, France, 1990; 245p. [Google Scholar]
- Guillet, S.; Josselin, N.; Vancassel, M. Multiple introductions of the Forficula auricularia species complex (Dermaptera: Forficulidae) in eastern North America. Can. Entomol. 2000, 132, 49–57. [Google Scholar] [CrossRef]
- Vigna Taglianti, A. Insecta Dermaptera. In Checklist e Distribuzione Della Fauna Italiana; Memorie del Museo Civico di Storia naturale di Verona 2. Serie, Sezione Scienze della Vita; Ruffo, S., Stoch, F., Eds.; Ministero Dell’ambiente e Della Tutela del Territorio: Verona, Italy, 2005; pp. 141–142. [Google Scholar]
- Matzke, D. Aktuell synanthrop lebende Ohrwürmer in Deutschland. Dtsch. Ges. Für Allg. Angew. Entomol. Nachrichten 2018, 32, 6–9. [Google Scholar]
- González-Miguéns, R.; Muñoz-Nozal, E.; Jiménez-Ruiz, Y.; Mas-Peinado, P.; Ghanavi, H.R.; García-París, M. Speciation patterns in the Forficula auricularia species complex: Cryptic and not so cryptic taxa across the western Palaearctic region. Zool. J. Linn. Soc. 2020, 190, 788–823. [Google Scholar] [CrossRef]
- Zafeiriou, S.; Kočárek, P.; Kalaentzis, K. First record of the desert earwig Forficula lucasi Dohrn, 1865 (Dermaptera: Forficulidae) in Greece: A hitchhiker among the refugees or a seldom encounter? J. Insect Biodivers. 2021, 21, 15–17. [Google Scholar] [CrossRef]
- Kalaentzis, K.; Kazilas, C.; Agapakis, G.; Kocarek, P. Hidden in plain sight: First records of the alien earwig Euborellia femoralis (Dohrn, 1863) in Europe. BioInvasions Rec. 2021, 10, 1022–1031. [Google Scholar] [CrossRef]
- Nishikawa, M.; Kusui, Y. Earwigs (Dermaptera) collected in airplanes and ships called at ports in Japan. Tettigonia 2008, 9, 7–11. [Google Scholar]
- Rasplus, J.Y.; Roques, A. Dictyoptera (Blattodea, Isoptera), Orthoptera, Phasmatodea and Dermaptera. Chapter 13.3. BioRisk 2010, 4, 807–831. [Google Scholar] [CrossRef]
- Kocarek, P.; Dvorak, L.; Kirstova, M. Euborellia annulipes (Dermaptera: Anisolabididae), a new alien earwig in Central European greenhouses: Potential pest or beneficial inhabitant? Appl. Entomol. Zool. 2015, 50, 201–206. [Google Scholar] [CrossRef]
- Murányi, D.; Puskás, G. Hungarian occurrence of a potential horticultural pest earwig, Euborellia annulipes (Lucas, 1847) (Dermaptera: Anisolabididae). Növényvédelem 2018, 54, 513–517. [Google Scholar]
- Báez, M.; Relación de Las Categorías de Origen de Las Especies de Artrópodos de Canarias. Banco de Datos de Biodiversidad de Canarias. 2002, 73p. Available online: https://www.biodiversidadcanarias.es/biota/documento/A02090 (accessed on 6 November 2024).
- González-Concepción, C.; Gil-Fariña, M.C.; Pestano-Gabino, C. Multivariate modelling of the Canary Islands banana output. The role of farmer income expectation. J. Soc. Sci. 2008, 4, 88–97. [Google Scholar]
- Fuentes, E.G.; Hernández-Suárez, E.; Simón, O.; Williams, T.; Caballero, P. Chrysodeixis chalcites, a pest of banana crops on the Canary Islands: Incidence, economic losses and current control measures. Crop Prot. 2018, 108, 137–145. [Google Scholar] [CrossRef]
- Petrovan, S.; Aldridge, D.; Smith, R.; White, T.; Sutherland, W. Halyomorpha halys invasion front jumps 1500 kilometres to reach the Canary Islands; a framework for rapid response, identification of urgent questions, and assessment of potential impacts. ARPHA Prepr. 2022, 3, e84924. [Google Scholar]
- FAOSTAT. Food and Agriculture Data [WWW Document]. Available online: http://www.fao.org/faostat/en/#home (accessed on 11 February 2025).
- Robinson, J.C.; Galán-Saúco, V. Bananas and Plantains; CAB International: Wallingford, UK, 2010; Volume 19, 297p. [Google Scholar]
- Medina, F.M.; Nogales, M.; Farnworth, M.J.; Bonnaud, E. Human-cat relationship in an oceanic biosphere reserve: The case of La Palma Island, Canary archipelago. J. Nat. Conserv. 2016, 34, 8–14. [Google Scholar] [CrossRef]
- Irl, S.D.; Harter, D.E.; Steinbauer, M.J.; Gallego Puyol, D.; Fernández-Palacios, J.M.; Jentsch, A.; Beierkuhnlein, C. Climate vs. topography–spatial patterns of plant species diversity and endemism on a high-elevation island. J. Ecol. 2015, 103, 1621–1633. [Google Scholar] [CrossRef]
- Confederación Canaria de Empresarios. 8. Actividad del Sector Primario. In Informe Anual de la Economía Canaria; Confederación Canaria de Empresarios: Las Palmas, Spain, 2020; pp. 171–188. Available online: https://www.ccelpa.org/informe-anual/IA2020/pdf/indicadores/08-2020.pdf (accessed on 2 May 2025).
- Hernández-Hernández, R.; Kluge, J.; Ah-Peng, C.; González-Mancebo, J.M. Natural and human-impacted diversity of bryophytes along an elevational gradient on an oceanic island (La Palma, Canarias). PLoS ONE 2019, 14, e0213823. [Google Scholar] [CrossRef]
- Regulation (EU) 2018/848 of the European Parliament and of the Council of 30 May 2018 on organic production and labelling of organic products and repealing Council Regulation (EC) No 834/2007. Off. J. Eur. Union 2018, 150, 1–92.
- Purnomo, B.; Fahrurrozi, F.; Sariasih, Y.; Muktamar, Z.; Efendi, Z. Determination of potential bacteria from five different types of green biomass enriched liquid organic fertilizer for developing Bio-decomposer. Int. J. Agric. Technol. 2017, 13, 1175–1182. [Google Scholar]
- Excmo. Cabildo Insular de La Palma. Estudio de la Sostenibilidad del Cultivo de Platanera en la Isla de la Palma, Tratados Con Purines Enriquecidos y Compost. 2018. Available online: https://www.ecofincanogales.com/documentos/memoria-proyecto-platanera-ipna-cabildo-de-la-palma.pdf (accessed on 15 March 2025).
- D’hont, A.; Denoeud, F.; Aury, J.M.; Baurens, F.C.; Carreel, F.; Garsmeur, O.; Noel, B.; Bocs, S.; Droc, G.; Rouard, M.; et al. The banana (Musa acuminata) genome and the evolution of monocotyledonous plants. Nature 2012, 488, 213–217. [Google Scholar] [CrossRef]
- Méndez Hernández, C.; Rodríguez Serrano, M. Deshijado de la Platanera. AgroCabildo, Excmo. Cabildo Insular de Tenerife. 2016. 20p. Available online: https://www.agrocabildo.org/publica/Publicaciones/subt_596_platanera.pdf (accessed on 21 November 2024).
- R Core Team. R: A Language and Environment for Statistical Computing (Version 4.5.0); R Foundation for Statistical Computing: Vienna, Austria, 2025; 3940p, Available online: https://cran.r-project.org (accessed on 5 May 2025).
- Simon, C.; Frati, F.; Beckenbach, A.; Crespi, B.; Liu, H.; Flook, P. Evolution, weighting and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Ann. Entomol. Soc. Am. 1994, 87, 651–701. [Google Scholar] [CrossRef]
- Pons, J. DNA-based identification of preys from non-destructive, total DNA extractions of predators using arthropod universal primers. Mol. Ecol. Notes 2006, 6, 623–626. [Google Scholar] [CrossRef]
- Swofford, D.L. PAUP*: Phylogenetic Analysis Using Parsimony (*and Other Methods) Version 4.0b10; Sinauer Associates: Sunderland, MA, USA, 2002. [Google Scholar]
- Paradis, E. pegas: An R package for population genetics with an integrated–modular approach. Bioinformatics 2010, 26, 419–420. [Google Scholar] [CrossRef]
- Kamvar, Z.N.; Tabima, J.F.; Grünwald, N.J. Poppr: An R package for genetic analysis of populations with clonal, partially clonal, and/or sexual reproduction. PeerJ 2014, 2, e281. [Google Scholar] [CrossRef]
- Dib, H.; Sauphanor, B.; Capowiez, Y. Effect of management strategies on arthropod communities in the colonies of rosy apple aphid, Dysaphis plantaginea Passerini (Hemiptera: Aphididae) in south-eastern France. Agric. Ecosyst. Environ. 2016, 216, 203–206. [Google Scholar] [CrossRef]
- Dib, H.; Sauphanor, B.; Capowiez, Y. Report on the life history traits of the generalist predator Forficula auricularia (Dermaptera Forficulidae) in organic apple orchards in southeastern France. Can. Entomol. 2017, 149, 56–72. [Google Scholar] [CrossRef]
- Simon, S.; Riotord, D.; Morel, K.; Fleury, A.; Capowiez, Y. A shift towards softer pest management differently benefits earwig and spider communities in a multiyear experiment in apple orchards. Pest Manag. Sci. 2024, 80, 5088–5098. [Google Scholar] [CrossRef]
- Moerkens, R.; Leirs, H.; Peusens, G.; Gobin, B. Are populations of European earwigs, Forficula auricularia, density dependent? Entomol. Exp. Appl. 2009, 130, 198–206. [Google Scholar] [CrossRef]
- Niedobová, J.; Skalský, S.; Ouředníčková, J.; Michalko, R. Forficula auricularia (Dermaptera) in orchards: Monitoring seasonal activity, the effect of pesticides, and the perception of European fruit growers on its role as a predator or pest. Pest Manag. Sci. 2021, 77, 1694–1704. [Google Scholar]
- Malagnoux, L.; Marliac, G.; Simon, S.; Rault, M.; Capowiez, Y. Management strategies in apple orchards influence earwig community. Chemosphere 2015, 124, 156–162. [Google Scholar] [CrossRef]
- Malagnoux, L.; Capowiez, Y.; Rault, M. Impact of insecticide exposure on the predation activity of the European earwig Forficula auricularia. Environ. Sci. Pollut. Res. 2015, 22, 14116–14126. [Google Scholar] [CrossRef]
- Le Navenant, A.; Brouchoud, C.; Capowiez, Y.; Rault, M.; Suchail, S. How lasting are the effects of pesticides on earwigs? A study based on energy metabolism, body weight and morphometry in two generations of Forficula auricularia from apple orchards. Sci. Total Environ. 2021, 758, 143604. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, A.C.; Stigall, T. Paternity and egg cannibalism in the ringlegged earwig Euborellia annulipes (Dermaptera: Anisolabididae). Entomol. Sci. 2019, 22, 250–257. [Google Scholar] [CrossRef]
- Lemos, W.P.; Ramalho, F.S.; Zanuncio, J.C. Age-dependent fecundity and life-fertility tables for Euborellia annulipes (Lucas) (Dermaptera: Anisolabididae) a cotton boll weevil predator in laboratory studies with an artificial diet. Environ. Entomol. 2003, 32, 592–601. [Google Scholar] [CrossRef]
- Núñez-Pascual, V.; Calleja, F.; Pardo, R.V.; Sarrazin, A.F.; Irles, P. The ring-legged earwig Euborellia annulipes as a new model for oogenesis and development studies in insects. J. Exp. Zool. Part B Mol. Dev. Evol. 2022, 340, 18–33. [Google Scholar] [CrossRef]
- Jamet, C.; Caussanel, C. Données biologiques, fonctionnement des appareils génitaux, comportements sexuels et maternels chez Euborellia annulipes (Lucas) (Dermaptère, Carcinophoridae). Bull. Société Entomol. Fr. 1995, 100, 37–58. [Google Scholar] [CrossRef]
- Fountain, M.T.; Harris, A.L. Non-target consequences of insecticides used in apple and pear orchards on Forficula auricularia L. (Dermaptera: Forficulidae). Biol. Control 2015, 91, 27–33. [Google Scholar] [CrossRef]
- Burton, M.; Burton, R. Earwigs. In International Wildlife Encyclopedia, 3rd ed.; Burton, M., Burton, R., Eds.; Marshall Cavendish Corporation: New York, NY, USA, 2002; pp. 738–740. [Google Scholar]
- Cross, J.; Fountain, M.; Marko, V.; Nagy, C. Arthropod ecosystem services in apple orchards and their economic benefits. Ecol. Entomol. 2015, 40, 82–96. [Google Scholar] [CrossRef]
- Jiang, Z.R.; Kajimura, H. Earwig preying on ambrosia beetle: Evaluating predatory process and prey preference. J. Appl. Entomol. 2020, 144, 743–750. [Google Scholar] [CrossRef]
- Byttebier, B.; Fischer, S. Predation on eggs of Aedes aegypti (Diptera: Culicidae): Temporal dynamics and identification of potential predators during the winter season in a temperate region. J. Med. Entomol. 2019, 56, 737–743. [Google Scholar] [CrossRef]
- Tangkawanit, U.; Seehavet, S.; Siri, N. The potential of Labidura riparia and Euborellia annulipes (Dermaptera) as predators of house fly in livestock. Songklanakarin J. Sci. Technol. 2021, 43, 603–607. [Google Scholar]
- Oliveira, L.V.Q.; Oliveria, R.; Nascimento Júnior, J.L.; Silva, I.T.F.A.; Barbosa, V.O.; Batista, J.L. Capacidade de busca da tesourinha Euborellia annulipes sobre o pulgão Brevicoryne brassicae (Hemiptera: Aphididae). PesquisAgro 2019, 2, 3–10. [Google Scholar] [CrossRef]
- Nunes, G.S.; Dantas, T.A.V.; Souza, M.S.; Nascimento, I.N.; Batista, J.L.; Malaquias, J.B. Life stage and population density of Plutella xylostella affect the predation behavior of Euborellia annulipes. Entomol. Exp. Appl. 2019, 167, 544–552. [Google Scholar] [CrossRef]
- Silva, A.B.; Batista, J.L.; de Brito, C.H. Capacidade predatória de Euborellia annulipes (Lucas, 1847) sobre Spodoptera frugiperda (Smith, 1797). Acta Sci. -Agron. 2009, 31, 7–11. [Google Scholar] [CrossRef]
- Coelho, R.S.; Pec, M.; Silva, A.L.R.; Peñaflor, M.F.; Marucci, R.C. Predation potential of the earwig Euborellia annulipes on fruit fly larvae and trophic interactions with the parasitoid Diachasmimorpha longicaudata. J. Appl. Entomol. 2023, 147, 147–156. [Google Scholar] [CrossRef]
- Marin Arroyo, R.; Mendoça De Souza, J.; Da Silva Nunes, G.; Gomes Ramalho, D.; De Bortoli, S.A. Euborellia annulipes mortality and predation on Diatraea saccharalis eggs after application of chemical and biological insecticides. Agric. Sci. 2023, 14, 11–22. [Google Scholar]
- Morato, R.P.; Cutler, G.C.; Torres, J.B. Insecticide compatibility with the predatory ring-legged earwig Euborelia annulipes increases mortality of diamondback moth. Biocontrol Sci. Technol. 2023, 33, 327–343. [Google Scholar] [CrossRef]
- Morato, R.P.; Nascimento, D.V.D.; Oliveira, G.M.; Bermúdez, N.C.; Lira, R.; Torres, J.B. Indoxacarb, cyantraniliprole, and Euborellia annulipes as options for integrated control of diamondback moth. J. Appl. Entomol. 2024, 148, 1300–1310. [Google Scholar] [CrossRef]
- Peusens, G.; Moerkens, R.; Beliën, T.; Gobin, B. Side effects of plant protection products and biological interactions on the European earwig Forficula auricularia L. Commun. Agric. Appl. Biol. Sci. 2009, 74, 411–417. [Google Scholar]
- Peusens, G.; Belien, T.; Gobin, B. Comparing different test methods for evaluating lethal side effects of some insecticides on the European earwig Forficula auricularia L. IOBC-WPRS Bull. 2010, 55, 95–100. [Google Scholar]
- Meunier, J.; Dufour, J.; Van Meyel, S.; Rault, M.; Lécureuil, C. Sublethal exposure to deltamethrin impairs maternal egg care in the European earwig Forficula auricularia. Chemosphere 2020, 258, 127383. [Google Scholar] [CrossRef]
- Hole, D.G.; Perkins, A.J.; Wilson, J.D.; Alexander, I.H.; Grice, P.V.; Evans, A.D. Does organic farming benefit biodiversity? Biol. Conserv. 2005, 122, 113–130. [Google Scholar] [CrossRef]
- Jacobsen, S.K.; Moraes, G.J.; Sørensen, H.; Sigsgaard, L. Organic cropping practice decreases pest abundance and positively influences predator-prey interactions. Agric. Ecosyst. Environ. 2019, 272, 1–9. [Google Scholar] [CrossRef]
- Galloway, A.D.; Seymour, C.L.; Gaigher, R.; Pryke, J.S. Organic farming promotes arthropod predators, but this depends on neighbouring patches of natural vegetation. Agric. Ecosyst. Environ. 2021, 310, 107295. [Google Scholar] [CrossRef]
- Sinclair, A.R.; Mduma, S.; Brashares, J.S. Patterns of predation in a diverse predator–prey system. Nature 2003, 425, 288–290. [Google Scholar] [CrossRef]
- Allendorf, F.W.; Luikart, G.; Aitken, S.N. Conservation and the Genetics of Populations, 2nd ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2012; 624p. [Google Scholar]
- Frankham, R. Genetics and extinction. Biol. Conserv. 2005, 126, 131–140. [Google Scholar] [CrossRef]
- Chateil, C.; Goldringer, I.; Tarallo, L.; Kerbiriou, C.; Le Viol, I.; Ponge, J.F.; Salmon, S.; Gachet, S.; Porcher, E. Crop genetic diversity benefits farmland biodiversity in cultivated fields. Agric. Ecosyst. Environ. 2013, 171, 25–32. [Google Scholar] [CrossRef]
- Fricaux, T.; Le Navenant, A.; Siegwart, M.; Rault, M.; Coustau, C.; Le Goff, G. The molecular resistance mechanisms of European earwigs from apple orchards subjected to different management strategies. Insects 2023, 14, 944. [Google Scholar] [CrossRef]
- Avise, J.C. Phylogeography: The History and Formation of Species; Harvard University Press: Cambridge, MA, USA, 2000; 447p. [Google Scholar]
- Jurado-Angulo, P.; García-París, M. Historical demographic determinants complement climate model predictions of co-occurring cryptic species. Divers. Distrib. 2025, 31, e70007. [Google Scholar] [CrossRef]
- Cuesta-Segura, A.D.; Jurado-Angulo, P.; Jiménez-Ruiz, Y.; García-París, M. Taxonomy of the Iberian species of Pseudochelidura (Dermaptera: Forficulidae). Eur. J. Taxon. 2023, 860, 81–115. [Google Scholar] [CrossRef]
- Biocan—Banco del Inventario Natural de Canarias. Available online: https://www.biodiversidadcanarias.es (accessed on 5 May 2025).
- Lucas, H. Description de cette nouvelle espèce. Ann. Société Entomol. Fr. 1847, 5, LXXXIV. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).