Growth Performance and Nutritional Content of Tropical House Cricket (Gryllodes sigillatus (Walker, 1969)) Reared on Diets Formulated from Weeds and Agro By-Products
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Gryllodes sigillatus Colony
2.2. Cricket Feeding Experiments
2.3. Diets and Their Preparation
2.4. Impact of Developed Diets and Chicken Feed on the Development and Survival Rate of Gryllodes sigillatus
2.5. Impact of Developed Diets and Chicken Diet on Wet Body Weight, Body Size and Yield of Gryllodes sigillatus
2.6. Impact of Developed Diets and Chicken Diet on Feed Conversion Ratio and Yield of Gryllodes sigillatus on a Simulated Small-Scale Farm
2.7. Gryllodes sigillatus Cricket Samples and Their Preparation
2.8. Proximate Content of Developed Diets, Chicken Feed and Gryllodes sigillatus Cricket Powders
2.9. Mineral Element Analysis
2.10. Fatty Acid Profile
2.11. Statistical Analysis
3. Results
3.1. Diet Composition
3.2. Impact of Tested Diets and Chicken Feed on the Development Time of Gryllodes sigillatus
3.3. Impact of Developed Diets and Chicken Feed on Survivorship of Gryllodes sigillatus
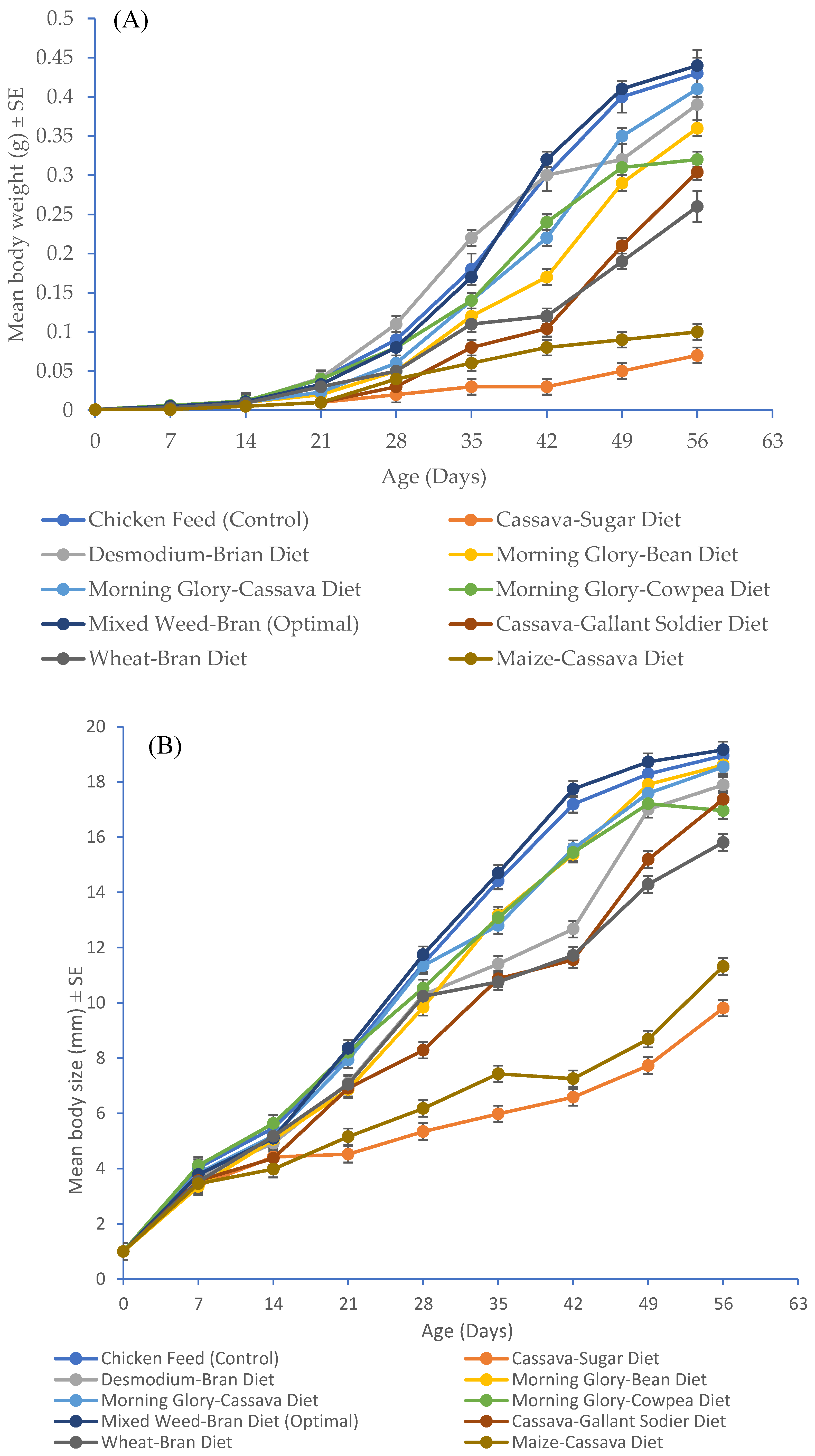
3.4. Impact of Developed Diets and Chicken Feed on Wet Body Weight and Body Size of Gryllodes sigillatus
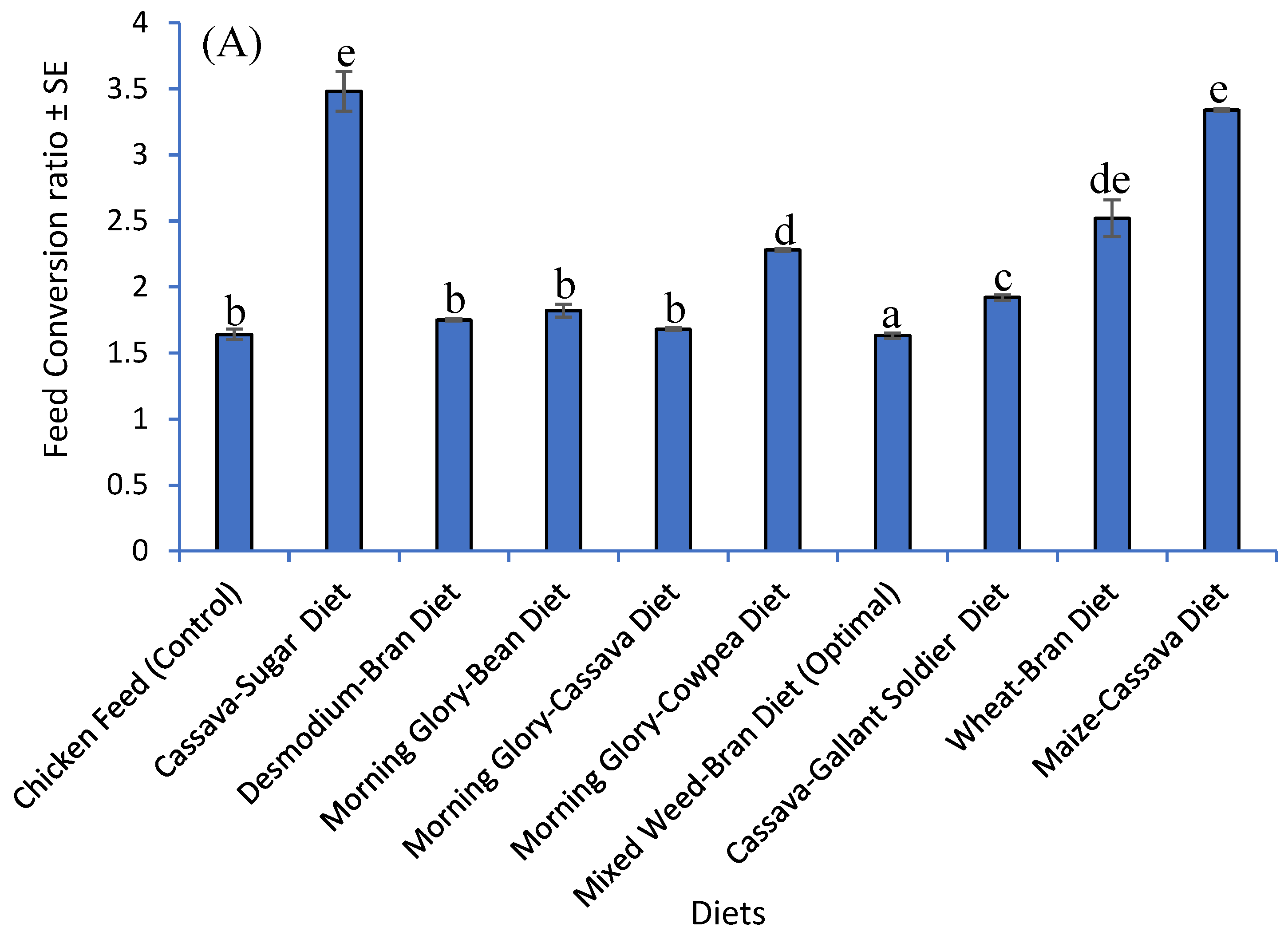
3.5. Impact of Developed Diets and Chicken Diet on Feed Conversion Ratio and Yield of G. sigillatus on a Simulated Small-Scale Farm
3.6. Multivariate Analysis of Effect of Tested Diets and Chicken Feed Nutrition Content on Growth Performance Traits of Gryllodes sigillatus
3.7. Proximate Composition
3.8. Mineral Elements in Gryllodes sigillatus Fed on Different Developed Diets and Chicken Feed
3.9. Fatty Acid Content in Gryllodes sigillatus Crickets Reared on Different Developed Diets and a Control Diet
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| G. sigillatus Reared on | Minerals (mg/100 g DM) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mg | Fe | Ca | Cu | P | Zn | K | Mn | Na | |
| Chicken Feed (Control) | 126.04 ± 4.81 f | 18.03 ± 0.76 b | 407.35 ± 18.98 c | 0.21 ± 0.04 f | 1151.82 ± 37.71 bc | 27.86 ± 1.57 b | 1575.41 ± 52.02 a | 4.83 ± 016 h | 369.89 ± 7.67 a |
| Cassava–Sugar Diet | 172.11 ± 0.96 a | 14.62 ± 0.31 c | 369.48 ± 18.92 d | 4.47 ± 0.03 a | 1127.05 ± 36.85 bcd | 24.01 ± 0.34 c | 1282.59 ± 9.42 ab | 10.01 ± 0.19 a | 214.93 ± 28.13 b |
| Desmodium–Bran Diet | 142.40 ± 5.13 d | 10.53 ± 1.56 d | 436.47 ± 9.06 c | 2.58 ± 0.05 e | 967.70 ± 45.04 e | 19.44 ± 0.72 d | 1413.74 ± 23.83 a | 4.86 ± 0.20 h | 221.02 ± 29.47 b |
| Morning Glory–Bean Diet | 144.58 ± 7.52 d | 12.86 ± 1.28 cd | 320.15 ± 20.40 e | 4.05 ± 0.11 b | 1026.38 ± 6.11 cde | 24.09 ± 0.48 c | 1328.03 ± 3.05 ab | 7.22 ± 0.31 d | 187.64 ± 1.56 bc |
| Morning Glory–Cassava Diet | 134.57 ± 3.37 e | 12.74 ± 0.61 cd | 534.41 ± 10.00 b | 3.55 ± 0.00 c | 1166.03 ± 21.58 b | 23.52 ± 0.12 c | 1580.65 ± 5.39 a | 5.45 ± 0.04 g | 187.93 ± 8.26 bc |
| Morning Glory–Cowpea Diet | 151.17 ± 1.16 c | 13.49 ± 3.45 cd | 373.52 ± 6.48 d | 2.83 ± 0.03 d | 1004.47 ± 10.59 de | 23.49 ± 0.66 c | 1230.80 ± 3.20 ab | 7.71 ± 0.27 c | 210.52 ± 28.12 b |
| Mixed Weed–Bran Diet (Optimal) | 143.33 ± 1.37 d | 23.86 ± 1.28 a | 839.05 ± 47.02 a | 4.49 ± 0.17 a | 1495.67 ± 159.81 a | 34.36 ± 1.31 a | 1607.36 ± 6.09 a | 6.82 ± 0.17 e | 370.48 ± 4.64 a |
| Cassava–Gallant Soldier Diet | 164.80 ± 2.48 b | 15.61 ± 0.05 bc | 370.54 ± 0.70 d | 4.27 ± 0.04 a | 1050.08 ± 3.20 bcde | 26.72 ± 0.59 b | 1207.10 ± 5.27 ab | 8.85 ± 0.02 b | 194.30 ± 26.95 bc |
| Wheat–Bran Diet | 144.55 ± 2.71 d | 10.81 ± 0.12 d | 183.59 ± 3.11 g | 3.55 ± 0.15 c | 934.73 ± 12.46 e | 22.81 ± 0.37 c | 1098.59 ± 14.40 ab | 6.19 ± 0.09 f | 114.89 ± 11.67 d |
| Maize–Cassava Diet | 95.06 ± 2.11 g | 15.85 ± 1.22 bc | 266.53 ± 10.10 f | 3.04 ± 0.30 d | 724.68 ± 25.27 f | 19.59 ± 0.20 d | 894.23 ± 0.05 b | 6.62 ± 0.45 e | 159.68 ± 4.79 c |
| F9,20 | 96.50 | 24.79 | 257.70 | 309.8 | 37.22 | 93.30 | 40.70 | 163.40 | 59.79 |
| p | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Fatty Acid | Chicket Feed (Control) | Cassava–Sugar Diet | Desmodium–Bran Diet | Morning Glory–Bean Bran Diet | Morning Glory–Cassava Diet | Morning Glory–Cowpea Diet | Mixed Weed–Bran Diet (Optimal) | Cassava–Gallant Soldier Diet | Wheat–Bran Diet | Maize–Cassava Diet | F9,20 | p-Value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Saturated fatty acids (SFAs) | ||||||||||||
| Lauric acid (C12:0) | 0.11 ± 0.01 cd | 0.22 ± 0.01 bc | 0.13 ± 0.02 cd | 0.90 ± 0.13 a | 0.11 ± 0.01 cd | 0.25 ± 0.02 b | 0.19 ± 0.01 bc | 0.07 ± 0.01 d | 0.11 ± 0.01 cd | 0.22 ± 0.01 bc | 92.86 | 0.001 |
| Myristic acid (C14:00 | 1.79 ± 0.04 c | 1.49 ± 0.11 cd | 1.52 ± 0.14 cd | 1.32 ± 0.09 d | 1.23 ± 0.07 d | 3.96 ± 0.28 a | 1.81 ± 0.15 c | 1.22 ± 0.02 d | 1.54 ± 0.19 cd | 2.60 ± 0.06 b | 57.37 | 0.001 |
| Pentadecanoic acid (C15:0) | 0.19 ± 0.01 e | 0.66 ± 0.04 a | 0.38 ± 0.04 bc | 0.33 ± 0.01 cd | 0.26 ± 0.02 de | 0.69 ± 0.07 a | 0.39 ± 0.05 bc | 0.34 ± 0.01 cd | 0.34 ± 0.09 cd | 0.46 ± 0.03 b | 37.07 | 0.001 |
| Palmitic acid (C16:0) | 26.59 ± 0.70 c | 21.16 ± 1.00 de | 20.24 ± 1.29 e | 0.49 ± 0.13 g | 20.34 ± 0.20 e | 37.06 ± 1.34 a | 23.86 ± 2.74 d | 15.73 ± 0.78 f | 27.38 ± 2.19 c | 32.93 ± 0.79 b | 166.70 | 0.001 |
| Margaric acid (C17:0) | 0.65 ± 0.04 d | 1.79 ± 0.17 b | 0.97 ± 0.20 d | 0.74 ± 0.18 d | 0.66 ± 0.06 d | 1.45 ± 0.15 c | 0.86 ± 0.18 d | 0.79 ± 0.01 d | 0.85 ± 0.21 d | 2.10 ± 0.06 a | 37.39 | 0.001 |
| Stearic acid (C18:0) | 16.25 ± 0.52 bc | 15.47 ± 1.14 bc | 8.90 ± 0.28 d | 13.10 ± 1.97 c | 9.89 ± 0.84 d | 18.48 ± 1.79 a | 10.73 ± 0.92 d | 10.76 ± 0.82 d | 13.42 ± 1.40 c | 16.66 ± 0.91 ab | 22.01 | 0.001 |
| Arachidic acid (C20:0) | 2.01 ± 0.26 bcd | 2.12 ± 0.22 bcde | 1.33 ± 0.31 cd | 3.53 ± 0.31 a | 1.81 ± 0.19 bcd | 2.56 ± 0.24 b | 1.11 ± 0.08 d | 1.14 ± 0.30 d | 2.33 ± 0.88 bc | 2.60 ± 0.78 b | 9.09 | 0.001 |
| Behenic acid (C22:0) | 0.26 ± 0.02 d | 0.96 ± 0.25 b | 0.54 ± 0.08 cd | 1.47 ± 0.20 a | 0.45 ± 0.04 cd | 1.04 ± 0.15 b | 0.61 ± 0.12 c | 0.38 ± 0.02 cd | 0.33 ± 0.08 cd | 0.34 ± 0.08 cd | 28.36 | 0.001 |
| Lignoceric acid (C24:0) | nd | nd | nd | nd | nd | nd | nd | 0.35 ± 0.00 | nd | nd | nd | 0.001 |
| Polyunsaturated fatty acid (PUFAs) | ||||||||||||
| Alpha-linolenic acid (C 18:3(n-3)) | 0.82 ± 0.01 e | 1.24 ± 0.08 c | 1.25 ± 0.14 c | 0.84 ± 0.10 e | 1.44 ± 0.06 ab | 1.35 ± 0.05 abc | 1.30 ± 0.09 bc | 1.50 ± 0.04 a | 1.02 ± 0.07 d | 0.46 ± 0.01 f | 57.89 | 0.001 |
| Linoleic acid (C18:2(n-6)) | 16.38 ± 0.10 e | 24.73 ± 1.58 c | 25.11 ± 2.85 c | 16.96 ± 2.07 e | 29.01 ± 1.22 ab | 27.19 ± 1.02 abc | 26.12 ± 1.71 bc | 30.11 ± 0.64 a | 20.55 ± 1.36 d | 9.23 ± 0.21 f | 58.02 | 0.001 |
| Monounsaturated fatty acids (MUFAs) | ||||||||||||
| Oleic acid (C18:1(n-9)) | 34.95 ± 1.45 bcd | 30.16 ± 2.00 d | 39.63 ± 4.52 b | 60.32 ± 0.13 a | 34.80 ± 2.29 bcd | 5.97 ± 0.39 e | 33.02 ± 3.08 cd | 37.58 ± 2.48 bc | 32.13 ± 2.30 cd | 32.40 ± 0.58 cd | 96.75 | 0.001 |
| MUFAs | 34.95 ± 1.45 bcd | 30.15 ± 2.00 d | 39.63 ± 4.52 b | 60.32 ± 0.13 a | 34.80 ± 2.29 bcd | 5.97 ± 0.39 e | 33.02 ± 3.08 cd | 37.58 ± 2.48 bc | 32.13 ± 2.30 cd | 32.40 ± 0.58 cd | 96.75 | 0.001 |
| PUFAs | 17.20 ± 0.11 e | 25.97 ± 1.66 c | 26.36 ± 2.99 c | 17.81 ± 2.18 e | 30.45 ± 1.28 ab | 28.54 ± 1.07 abc | 27.42 ± 1.80 bc | 31.61 ± 0.68 a | 21.57 ± 1.43 d | 9.69 ± 0.22 f | 58.01 | 0.001 |
| SFAs | 47.85 ± 1.37 c | 43.88 ± 0.51 c | 34.01 ± 1.69 e | 21.88 ± 2.07 f | 34.75 ± 1.20 e | 65.49 ± 1.37 a | 39.56 ± 3.19 d | 30.81 ± 1.84 e | 46.30 ± 3.72 c | 57.91 ± 0.76 b | 124.30 | 0.001 |
| EFAs | 17.20 ± 0.11 e | 25.97 ± 1.66 c | 26.36 ± 2.99 c | 17.80 ± 2.18 e | 30.45 ± 1.28 ab | 28.54 ± 1.07 abc | 27.42 ± 1.80 bc | 31.61 ± 0.68 a | 21.57 ± 1.43 d | 9.69 ± 0.22 f | 58.01 | 0.001 |
| PUFA/SFA | 0.36 | 0.59 | 0.78 | 0.81 | 0.88 | 0.44 | 0.69 | 1.02 | 0.47 | 0.17 | ||
References
- United Nations. World Population Prospects. Summary of Results; United Nations: New York, NY, USA, 2023; Available online: https://www.worldometers.info/world-population/madagascar-population/ (accessed on 10 March 2025).
- Behixhe, A.; Irene, B.; Gloriana, C.; Silvia, I.; Anna, A.; Federico, G.; Matteo, F.; Francesca, T.; Tommaso, P.; Cristina, T. Modulating the nutritional value of Acheta domesticus (house cricket) through the eco-sustainable Ascophyllum nodosum dietary supplementation. J. Food Compos. Anal. 2025, 140, 107263. [Google Scholar] [CrossRef]
- Tomlinson, I. Doubling food production to feed the 9 billion: A critical perspective on a key discourse of food security in the UK. J. Rural Stud. 2013, 29, 81–90. [Google Scholar] [CrossRef]
- FAO; Global Forum for food and agriculture (GFFA). Food Systems for Our Future: Joining Forces for a Zero Hunger World, Berlin, Germany, 17-20/01/2024; FAO regional office for Europe and Central Asia: Budapest, Hungary, 2024; pp. 1–3. [Google Scholar]
- Nasir, S.Q.; Palupi, E.; Nasution, Z.; Ploeger, A.; Susanto, I.; Setiawan, B.; Rimbawan, R.; Jayanegara, A. Edible insects as a sustainable protein source: A meta-analysis. J. Insects Food Feed 2024, 10, 1841–1853. [Google Scholar] [CrossRef]
- O’Sullivan, J.N. The social and environmental influences of population growth rate and demographic pressure deserve greater attention in ecological economics. Ecol. Econ. 2020, 172, 106648. [Google Scholar] [CrossRef]
- Zandi-Sohani, N.; Tomberlin, J.K. Comparison of growth and composition of black soldier fly (Hermetia illucens L.) larvae reared on sugarcane by-products and other substrates. Insects 2024, 15, 771. [Google Scholar] [CrossRef]
- Magara, H.J.O.; Solofondranohatra, C.L.; Hugel, S.; Fisher, B.L. Weeds and agro byproducts for sustainable farming of edible field cricket, Gryllus madagascarensis (Orthoptera: Gryllidae). PLoS ONE 2025, 20, e0313083. [Google Scholar] [CrossRef]
- Lange, K.W.; Nakamura, Y. Edible insects as future food: Chances and challenges. J. Future Foods 2021, 1, 38–46. [Google Scholar] [CrossRef]
- Murugu, D.K.; Onyango, A.N.; Ndiritu, A.K.; Osuga, I.M.; Xavier, C.; Nakimbugwe, D.; Tanga, C.M. From farm to fork: Crickets as alternative source of protein, minerals, and vitamins. Front. Nutr. 2021, 8, 704002. [Google Scholar] [CrossRef] [PubMed]
- Magara, H.J.O.; Niassy, S.; Ayieko, M.A.; Mukundamago, M.; Egonyu, J.P.; Tanga, C.M.; Kimathi, E.K.; Ongere, J.O.; Fiaboe, K.K.M.; Hugel, S.; et al. Edible Crickets (Orthoptera) Around the world: Distribution, nutritional value, and other benefits—A review. Front. Nutr. 2021, 7, 537915. [Google Scholar] [CrossRef]
- Magara, H.J.O.; Hugel, S.; Fisher, B.L. Effect of feed on the growth performance, nutrition content and cost of raising the field cricket (Gryllus madagascarensis) as a sustainable nutrient source in Madagascar. Foods 2024, 13, 3139. [Google Scholar] [CrossRef]
- Oonincx, D.G.; van Broekhoven, S.; van Huis, A.; van Loon, J.J. Feed conversion, survival and development, and composition of four insect species on diets composed of food by-products. PLoS ONE 2015, 10, e0144601. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.; Fisher, B.L. A literature review of the use of weeds and agricultural and food industry by-products to feed farmed crickets (Insecta; Orthoptera; Gryllidae). Front. Sustain. Food Syst. 2022, 5, 810421. [Google Scholar] [CrossRef]
- Orinda, M.A.; Oloo, J.; Magara, H.J.O.; Ekesi, S.; Nanna, R.; Ayieko, M. Cricket Rearing Handbook; Services for science and education: Birmingham, UK, 2021; pp. 1–78. [Google Scholar] [CrossRef]
- Magara, H.J.O.; Tanga, C.M.; Fisher, B.L.; Azrag, A.G.A.; Niassy, S.; Egonyu, J.P.; Hugel, S.; Roos, N.; Ayieko, M.A.; Sevgan, S.; et al. Impact of temperature on the bionomics and geographical range margins of the two-spotted field cricket Gryllus bimaculatus in the world: Implications for its mass farming. PLoS ONE 2024, 19, e0300438. [Google Scholar] [CrossRef] [PubMed]
- Andrianorosoa Ony, C.; Solofondranohatra, C.L.; Ramiadantsoa, T.; Ravelomanana, A.; Ramanampamonjy, R.N.; Hugel, S. Effect of cricket frass fertilizer on growth and pod production of green beans (Phaseolus vulgaris L.). PLoS ONE 2024, 19, e0303080. [Google Scholar] [CrossRef]
- Baiano, A. Edible insects: An overview on nutritional characteristics, safety, farming, production technologies, regulatory framework, and socio-economic and ethical implications. Trends Food Sci. Technol. 2020, 100, 35–50. [Google Scholar] [CrossRef]
- Magara, H.J.O. Assessment of Cricket Species Composition, Feed Substrates, Optimal Temperature and Nutritional Content of Edible Cricket Scapsipedus icipe. Doctoral Dissertation, Jaramogi Oginga Odinga University of Science and Technology, Bondo, Kenya, 2020. [Google Scholar]
- Fisher, B.; Hugel, S. Edible insects traditions and uses on Madagascar. In The New Natural History of Madagascar; Princeton University Press: Princeton, NJ, USA, 2022; pp. 218–230+252–264. [Google Scholar] [CrossRef]
- Jongema, Y. List of Edible Insects of the World; Wageningen University and Research: Wageningen, The Netherlands, 2017; pp. 1–75. [Google Scholar]
- Govorushko, S. Global status of insects as food and feed source: A review. Trends Food Sci. Technol. 2019, 91, 436–445. [Google Scholar] [CrossRef]
- Tanga, C.M.; Egonyu, J.P.; Beesigamukama, D.; Niassy, S.; Emily, K.; Magara, H.J.O.; Omuse, E.R.; Subramanian, S.; Ekesi, S. Edible insect farming as an emerging and profitable enterprise in East Africa. Curr. Opin. Insect. Sci. 2021, 8, 64–71. [Google Scholar] [CrossRef]
- Van Itterbeeck, J.; Pelozuelo, L. How many edible insect species are there? A not so simple question. Diversity 2022, 14, 143. [Google Scholar] [CrossRef]
- Rowe, E.; López, K.Y.; Robinson, K.M.; Baudier, K.M.; Barrett, M. Farmed cricket (Acheta domesticus, Gryllus assimilis, and Gryllodes sigillatus; Orthoptera) welfare considerations: Recommendations for improving global practice. J. Insects Food Feed. 2024, 10, 1–59. [Google Scholar] [CrossRef]
- Walker, T.J. Tropical house cricket, Gryllodes sigillatus (F. Walker 1869). In Singing Insects of North America; University of Florida: Gainesville, Florida, USA, 2011. [Google Scholar]
- Bertola, M.; Mutinelli, F. A Systematic Review on Viruses in Mass-Reared Edible Insect Species. Viruses 2021, 13, 2280. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Krönauer, T.; Döring, T.F.; Röder, G. Advances in production of crickets as food and animal feed: A case study of five wild European plants as cricket feed. Sustain. Agric. Res. 2025, 14, 4559. [Google Scholar]
- Yuzer, A.; Dizhi, X.; Retno, T.A.; Joey, W.; Le, W. Insects as a feed ingredient for fish culture: Status and trends. Aquac. Fish. 2022, 7, 166–178. [Google Scholar] [CrossRef]
- Muzzatti, M.J.; McConnell, E.; Neave, S.; MacMillan, H.A.; Bertram, S.M. Fruitful female fecundity after feeding Gryllodes sigillatus (Orthoptera: Gryllidae) royal jelly. Can. Entomol. 2022, 154, e50. [Google Scholar] [CrossRef]
- Kasdorf, S.Y.; Muzzatti, M.J.; Haider, F.; Bertram, S.M.; MacMillan, H.A. Brewery waste as a sustainable protein source for the banded cricket (Gryllodes sigillatus). J. Insects Food Feed 2025, 11, 1417–1429. [Google Scholar] [CrossRef]
- Comunian, T.A.; Silva, M.P.; Souza, C.J.F. The use of food by-products as a novel for functional foods: Their use as ingredients and for the encapsulation process. Trends Food Sci. Technol. 2021, 108, 269–280. [Google Scholar] [CrossRef]
- Magara, H.J.O.; Tanga, C.M.; Ayieko, M.A.; Hugel, S.; Mohamed, S.A.; Khamis, F.M.; Salifu, D.; Niassy, S.; Sevgan, S.; Fiaboe, K.K.M.; et al. Performance of newly described native edible cricket Scapsipedus icipe (Orthoptera: Gryllidae) on various diets of relevance for farming. J. Econ. Entomol. 2019, 112, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Vastolo, A.; Calabrò, S.; Cutrignelli, M.I. A review on the use of agro-industrial CO-products in animals’ diets. Ital. J. Anim. Sci. 2022, 21, 577–594. [Google Scholar] [CrossRef]
- Otieno, M.H.J.; Ayieko, M.A.; Niassy, S.; Salifu, D.; Abdelmutalab, A.G.; Fathiya, K.M.; Subramanian, S.; Fiaboe, K.K.M.; Roos, N.; Ekesi, S.; et al. Integrating temperature-dependent life table data into Insect Life Cycle Model for predicting the potential distribution of Scapsipedus icipe Hugel & Tanga. PLoS ONE 2019, 14, e0222941. [Google Scholar] [CrossRef]
- Sorjonen, J.M.; Valtonen, A.; Hirvisalo, E.; Karhapää, M.; Lehtovaara, V.J.; Lindgren, J.; Marnila, P.; Mooney, P.; Mäki, M.; Siljander-Rasi, H.; et al. The plant-based by-product diets for the mass-rearing of Acheta domesticus and Gryllus bimaculatus. PLoS ONE 2019, 14, e0218830. [Google Scholar] [CrossRef]
- Bawa, M.; Songsermpong, S.; Kaewtapee, C.; Chanput, W. Effect of diet on the growth performance, feed conversion, and nutrient content of the house cricket. J. Insect Sci. 2020, 20, 10. [Google Scholar] [CrossRef]
- Sorjonen, J.M.; Karhapa, M.; Valtonen, A.; Holm, A.; Roininen, H. Performance of the house cricket (Acheta domesticus) on by-product diets in small-scale production. J. Insects Food Feed 2021, 8, 1–6. [Google Scholar] [CrossRef]
- Finke, M.D.; DeFoliart, G.R.; Benevenga, N.J. Use of a four-parameter logistic model to evaluate the quality of the protein from three insect species when fed to rats. J. Nutr. 1989, 119, 864–871. [Google Scholar] [CrossRef] [PubMed]
- AOAC. International Official Methods of Analysis, 20th ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2016. [Google Scholar]
- Ajdini, B.; Biancarosa, I.; Cardinaletti, G.; Illuminati, S.; Annibaldi, A.; Girolametti, F.; Truzzi, C. The use of seaweed as sustainable feed ingredient for the house cricket (Acheta domesticus): Investigating cricket performance and nutritional composition. J. Insects Food Feed 2024, 10, 1313–1330. [Google Scholar] [CrossRef]
- AOAC. International Official Methods of Analysis, 19th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2010. [Google Scholar]
- Galland-Irmouli, A.V.; Fleurence, J.; Lamghari, R.; Luçon, M.; Rouxel, C.; Barbaroux, O.; Guéant, J.L. Nutritional value of proteins from edible seaweed Palmaria palmata (dulse). J. Nutr. Biochem. 1999, 10, 353–359. [Google Scholar] [CrossRef]
- Ritvanen, T.; Pastell, H.; Welling, A.; Raatikainen, M. The nitrogen-to-protein conversion factor of two cricket species—Acheta domesticus and Gryllus bimaculatus. Agric. Food Sci. 2020, 29, 1–5. [Google Scholar] [CrossRef]
- Biancarosa, I.; Espe, M.; Bruckner, C.G.; Heesch, S.; Liland, N.; Waagbø, R.; Lock, E.J. Amino acid composition, protein content, and nitrogen-to-protein conversion factors of 21 seaweed species from Norwegian waters. J. Appl. Phycol. 2017, 29, 1001–1009. [Google Scholar] [CrossRef]
- AOAC (Association of Official Analytical Chemists). Official Methods of Analysis (15th ed.). Off. Methods Anal. J. Assoc. Off. Anal. Chem. 1990, 62, 2742–2744. [Google Scholar]
- Fairulnizal, M.M.; Vimala, B.; Rathi, D.N.; Naeem, M.M. Atomic absorption spectroscopy for food quality evaluation. In Woodhead Publishing Series in Food Science, Technology and Nutrition, Evaluation Technologies for Food Quality; Jian, Z., Xichang, W., Eds.; Woodhead Publishing: Kuala Lumpur, Malaysia, 2019; pp. 145–173. [Google Scholar] [CrossRef]
- McKinistry, P.J.; Indyka, H.E.; Kim, N.D. The determination of major and minor elements in milk and infant formula by slurry nebulisation and inductively coupled plasma-optical emmission spectrometry (ICP-OES). Food Chem. 1999, 65, 245–252. [Google Scholar] [CrossRef]
- Rahman, N.; Hashem, S.; Akther, S.; Jothi, J.S. Impact of various extraction methods on fatty acid profile, physicochemical properties, and nutritional quality index of pangus fish oil. Food Sci. Nutr. 2023, 11, 4688–4699. [Google Scholar] [CrossRef]
- Truzzi, C.; Giorgini, E.; Annibaldi, A.; Antonucci, M.; Illuminati, S.; Scarponi, G.; Riolo, P.; Isidoro, N.; Conti, C.; Zarantoniello, M.; et al. Fatty acids profile of black soldier fly (Hermetia illucens): Influence of feeding substrate based on coffee-waste silverskin enriched with microalgae. Anim. Feed Sci. Technol. 2020, 259, 114309. [Google Scholar] [CrossRef]
- R version 4.4.2 (Core Team). R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2024; Available online: https://www.r-project.org/ (accessed on 8 March 2025).
- Stabile, C.; Muzzatti, M.J.; Haider, F.; Bertram, S.M.; MacMillan, H.A. Beyond Growth: The Impact of Diet on Body Composition in the Banded Cricket (Gryllodes Sigillatus). A Preprint. 2024. Available online: https://ssrn.com/abstract=5034863 (accessed on 14 March 2025).
- Vaga, M.; Berggren, Å.; Jansson, A. Growth, survival and development of house crickets (Acheta domesticus) fed flowering plants. J. Insects Food Feed 2021, 7, 151–161. [Google Scholar] [CrossRef]
- Hunt, A.S.; Ward, A.M.; Ferguson, G. Effects of a High Calcium Diet on Gut Loading in Varying Ages of Crickets (Acheta domesticus) and Mealworms (Tenebrio molitor). 2001, pp. 94–102. Available online: https://nagonline.net/wp-content/uploads/2014/02/Hunt-HighCaDiet.pdf (accessed on 14 March 2025).
- Peterson, T.N.; Welti, E.A.; Kaspari, M. Dietary sodium levels affect grasshopper growth and performance. Ecosphere 2021, 12, e03392. [Google Scholar] [CrossRef]
- Clissold, F.J.; Tedder, B.J.; Conigrave, A.D.; Simpson, S.J. The Gastrointestinal Tract as a Nutrient-Balancing. Organ. Proc. R. Soc. B 2010, 277, 1751–1759. [Google Scholar] [CrossRef] [PubMed]
- Finke, M.D. Complete Nutrient Content of Four Species of Feeder Insects. Zoo Biol. 2015, 34, 554–564. [Google Scholar] [CrossRef]
- Raubenheimer, D.; Simpson, S.J. Nutrient Balancing in Grasshoppers: Behavioural and Physiological Correlates of Dietary Breadth. J. Exp. Biol. 2003, 206, 1669–1681. [Google Scholar] [CrossRef]
- Roeder, K.A.; Behmer, S.T. Lifetime consequences of food protein-carbohydrate content for an insect herbivore. Funct. Ecol. 2014, 28, 113543. [Google Scholar] [CrossRef]
- Dobermann, D.; Michaelson, L.; Field, L.M. The effect of an initial high-quality feeding regime on the survival of Gryllus bimaculatus (black cricket) on bio-waste. J. Insects Food Feed 2018, 5, 1–8. [Google Scholar] [CrossRef]
- Ajdini, A.; Peñaranda, D.S.; Stawarczyk, M.; Bengtsson, J. Seaweed inclusion reduces fat and alters fatty acid composition in house crickets (Acheta domesticus). Front. Nutr. 2023, 10, 1203215. [Google Scholar]
- Tzompa-Sosa, D.A.; Dewettinck, K.; Provijn, P.; Brouwers, J.F.; Meulenaer, d.B.; Dennis Oonincx, D.G.A.B. Lipidome of cricket species used as food. Food Chem. 2021, 349, 129077. [Google Scholar] [CrossRef]
- Palupi, E.; Nasir, S.Q.; Jayanegara, A.; Susanto, I.; Ismail, A.; Iwansyah, A.C.; Setiawan, B.; Sulaeman, A.; Damanik, M.R.M.; Filianty, F. Meta-analysis on the fatty acid composition of edible insects as a sustainable food and feed. Future Foods 2025, 11, 100529. [Google Scholar] [CrossRef]
- Pastell, H.; Mellberg, S.; Ritvanen, T.; Raatikainen, M.; Mykkänen, S.; Niemi, J.; Latomäki, I.; Wirtanen, G. How does locally produced feed affect the chemical composition of reared house crickets (Acheta domesticus)? ACS Food Sci. Technol. 2021, 1, 625–635. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Zhao, T.; Fitriani, A.; Rahmadhia, S.N.; Alirezalu, K.; Fernando, I. Acheta domesticus (house cricket) as human foods—An approval of the European commission—A systematic review. Food Front. 2024, 5, 435–473. [Google Scholar] [CrossRef]
- NHANES 2013-2016; Agricultural Research Service. Usual Nutrient Intake from Food and Beverages, by Gender and Age, What We Eat in America. USDA: Washington, DC, USA, 2019.
- Van Peer, M.; Frooninckx, L.; Coudron, C.; Berrens, S.; Álvarez, C.; Deruytter, D.; Verheyen, G.; Van Miert, S.; Savoldelli, S.; Spranghers, T. Insects’ valorisation potential of using organic side streams as feed for Tenebrio molitor, Acheta domesticus, Locust migratoria. Insects 2021, 12, 796. [Google Scholar] [CrossRef] [PubMed]
- Jucker, C.; Belluco, S.; Oddon, S.B.; Ricci, A.; Bonizzi, L.; Lupi, D.; Savoldelli, S.; Biasato, I.; Caimi, C.; Mascaretti, A.; et al. Impact of some local organic by-products on Acheta domesticus growth and meal production. J. Insects Food Feed. 2022, 8, 631–640. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.; Liu, Y.; Lu, Y.; Zhou, M.; Wang, S.; Wang, S. The effect of different dietary sugars on the development and fecundity of Harmonia axyridis. Front. Physiol. 2020, 11, 574851. [Google Scholar] [CrossRef]
- Kulma, M.; Tuůmová, V.; Fialová, A.; Kourimská, L. Insect consumption in the Czech Republic: What the eye does not see, the heart does not grieve over. J. Insects Food Feed. 2020, 6, 525–535. [Google Scholar] [CrossRef]
- Orkusz, A. Nutritional value and health-promoting properties of edible insects. J. Insects Food Feed 2021, 7, 1017–1027. [Google Scholar]
- Rempel, J.; Grover, K.; El-Matary, W. Micronutrient deficiencies and anaemia in children with inflammatory bowel disease. Nutrients 2021, 13, 236. [Google Scholar] [CrossRef]
- Paul, A.; Frederich, M.; Megido, R.C.; Alabi, T.; Malik, P.; Uyttenbroeck, R.; Francis, F.; Blecker, C.; Haubruge, E.; Lognay, G.; et al. Insect fatty acids: A comparison of lipids from three Orthopterans and Tenebrio molitor L. larvae. J. Asia Pac. Entomol. 2017, 20, 334–337. [Google Scholar] [CrossRef]
- Fombong, F.; Kinyuru, J.N.; Ng’ang’a, J.; Ayieko, M.A.; Vanden Broeck, J.; Van Der Borght, M. Affordable processing of edible orthopterans provides a highly nutritive source of food ingredients. Foods 2021, 10, 144. [Google Scholar] [CrossRef]
- Kolobe, S.D.; Manyelo, E.M.; Malematja, E.; Sebola, N.A.; Mabelebele, M. Fats and major fatty acids present in edible insects utilised as food and livestock feed. J. Vet. Sci. 2023, 22, 100312. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.J.; Brainard, J.; Song, F.; Wang, X.; Abdelhamid, A.; Hooper, L.; PUFAH Group. Omega-3, omega-6, and total dietary polyunsaturated fat for prevention and treatment of type 2 Diabetes mellitus: Systematic review and meta-analysis of randomised controlled trials. BMJ 2019, 21, l4697. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Magara, H.J.O.; Hugel, S.; Fisher, L.B. Nutritional composition of four grasshopper species frequently consumed in Madagascar: Insights for nutritional contribution and alternative insect farming. Foods 2025, 14, 1848. [Google Scholar] [CrossRef]




| Diets | Diet Ingredients | Quantity g/Kg DM |
|---|---|---|
| Chicken Feed (Control) | Control | Control |
| Cassava–Sugar Diet | Cassava leaves, sugar and baking powder | 980 g + 10 g + 10 g |
| Desmodium–Bran Diet | Silver leaf Desmodium, wheat bran, maize bran, and cowpea bran, SWMC | 200 g + 200 g + 200 g + 400 g |
| Morning Glory–Bean Diet | Tropical African morning glory, navy bean bran, maize bran, maize germ, rice bran | 300 g + 300 g + 200 g + 100 g + 100 g |
| Morning Glory–Cassava Diet | Tropical African morning glory, cassava leaves, maize bran, rice bran, cowpea bran, navy bean bran | 200 g + 200 g + 200 g + 100 g + 100 g + 200 g |
| Morning Glory–Cowpea Diet | Tropical African morning glory, cowpea bran, cassava leaf powder, cowpea bran and navy bean bran | 300 g + 200 g + 400 g + 100 g |
| Mixed Weed–Bran Diet (Optimal) | Tropical African morning glory, gallant soldier, cassava leaves, cowpea bran, navy bean bran, maize bran, wheat bran | 200 g + 200 g + 150 g + 200 g + 100 g + 100 g + 50 g |
| Cassava–Gallant Soldier Diet | Cassava leaves, gallant soldier, cowpea bran, tropical African morning glory, taro leaves | 300 g + 300 g + 200 g + 100 g + 100 g |
| Wheat–Bran Diet | Wheat bran and powder | 990 g + 10 g |
| Maize–Cassava Diet | Maize bran, cassava tuber bran, wheat bran, baking powder | 330 g + 330 g + 330 g + 10 g |
| Diet | DM | g/kg DM | Gross Energy (Kcal/kg) | |||||
|---|---|---|---|---|---|---|---|---|
| Moisture | Protein | Fat | Ash | Fiber | Carbohydrate | |||
| Chicken Feed (Control) | 906.70 ± 1.20 | 93.30 ± 1.20 | 215.00 ± 5.68 | 29.30 ± 0.58 | 65.00 ± 2.14 | 66.20 ± 0.28 | 531.20 ± 7.10 | 3248.70 ± 16.30 |
| Cassava–Sugar Diet | 905.00 ± 0.95 | 95.00 ± 0.95 | 250.00 ± 1.78 | 34.00 ± 0.82 | 53.00 ± 1.40 | 100.00 ± 1.03 | 468.00 ± 4.80 | 3178.00 ± 14.82 |
| Desmodium–Bran Diet | 871.00 ± 0.36 | 129.00 ± 0.36 | 245.00 ± 11.00 | 38.00 ± 0.91 | 84.00 ± 0.74 | 74.00 ± 1.65 | 430.00 ± 10.74 | 3042.00 ± 7.50 |
| Morning Glory–Bean Diet | 928.00 ± 3.96 | 72.00 ± 3.96 | 240.00 ± 0.06 | 71.00 ± 4.70 | 101.00 ± 2.70 | 88.00 ± 1.43 | 428.00 ± 9.58 | 3311.00 ± 12.07 |
| Morning Glory–Cassava diet | 884.00 ± 1.00 | 116.00 ± 1.0 | 235.00 ± 10.26 | 21.00 ± 0.80 | 101.00 ± 0.80 | 106.00 ± 2.97 | 421.00 ± 12.81 | 2813.00 ± 13.80 |
| Morning Glory–Cowpea Diet | 905.00 ± 3.04 | 95.00 ± 3.04 | 225.00 ± 10.51 | 19.00 ± 0.26 | 64.00 ± 0.71 | 62.00 ± 0.44 | 535.00 ± 13.55 | 3211.00 ± 10.73 |
| Mixed Weed–Bran Diet (Optimal) | 921.00 ± 1.99 | 79.00 ± 1.99 | 215.00 ± 4.97 | 63.00 ± 2.70 | 118.00 ± 0.79 | 125.00 ± 1.40 | 400.00 ± 4.43 | 3028.00 ± 20.77 |
| Cassava–Gallant Soldier Diet | 912.00 ± 0.40 | 88.00 ± 0.40 | 200.00 ± 10.06 | 53.00 ± 0.36 | 113.00 ± 1.05 | 128.00 ± 4.00 | 418.00 ± 11.99 | 2949.00 ± 14.60 |
| Wheat–Bran Diet | 901.00 ± 2.44 | 99.00 ± 2.14 | 145.0 ± 9.19 | 34.000 ± 0.55 | 43.00 ± 0.75 | 99.80 ± 3.69 | 569.00 ± 53.32 | 3162.00 ± 22.68 |
| Maize–Cassava Diet | 880.00 ± 4.04 | 120.00 ± 4.04 | 135.0 ± 2.53 | 13.0 ± 0.33 | 52.00 ± 0.47 | 114.00 ± 4.71 | 566.00 ± 9.48 | 2021.00 ± 35.03 |
| Diet | mg/kg DM | g/kg DM | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Fe | Cu | Zn | Mn | Na | Mg | Ca | P | K | |
| Chicken Feed | 228.10 ± 10.74 | 16.20 ± 0.47 | 67.00 ± 1.18 | 63.90 ± 5.81 | 1065.40 ± 38.75 | 2.00 ± 0.01 | 5.00 ± 0.01 | 2.00 ± 0.01 | 13.00 ± 0.01 |
| Cassava–Sugar Diet | 61.10 ± 2.64 | 5.30 ± 0.01 | 49.50 ± 0.66 | 73.90 ± 0.48 | 100.00 ± 2.46 | 0.10 ± 0.01 | 0.10 ± 0.01 | 0.20 ± 0.01 | 13.00 ± 0.02 |
| Desmodium–Bran Diet | 171.10 ± 0.60 | 6.80 ± 0.63 | 37.10 ± 2.76 | 35.90 ± 4.94 | 1939.30 ± 24.36 | 2.00 ± 0.01 | 9.00 ± 0.02 | 2.00 ± 0.01 | 12.00 ± 0.01 |
| Morning Glory–Bean Diet | 327.20 ± 4.35 | 18.00 ± 0.74 | 62.50 ± 1.20 | 52.80 ± 3.67 | 1935.00 ± 15.95 | 3.00 ± 0.01 | 9.00 ± 0.01 | 2.00 ± 0.01 | 17.00 ± 0.02 |
| Morning Glory–Cassava Diet | 288.20 ± 64.85 | 7.30 ± 0.09 | 45.00 ± 1.53 | 57.60 ± 0.79 | 2033.40 ± 34.85 | 2.00 ± 0.01 | 7.00 ± 0.01 | 3.00 ± 0.01 | 13.00 ± 0.01 |
| Morning Glory–Cowpea Diet | 212.50 ± 1.96 | 19.70 ± 0.05 | 28.80 ± 0.78 | 10.20 ± 0.17 | 1040.80 ± 1.31 | 2.00 ± 0.01 | 10.0 ± 0.02 | 2.00 ± 0.01 | 12.00 ± 0.02 |
| Mixed Weed–Bran Diet | 881.40 ± 19.10 | 9.70 ± 1.37 | 64.20 ± 1.03 | 79.30 ± 0.59 | 2399.90 ± 58.98 | 3.00 ± 0.01 | 11.00 ± 0.01 | 2.00 ± 0.01 | 16.00 ± 0.02 |
| Cassava–Gallant Soldier Diet | 172.60 ± 12.26 | 8.60 ± 0.65 | 35.80 ± 0.61 | 52.80 ± 2.59 | 1935.00 ± 123.85 | 2.00 ± 0.01 | 6.00 ± 0.01 | 2.00 ± 0.01 | 10.00 ± 0.01 |
| Wheat–Bran Diet | 46.20 ± 4.35 | 5.37 ± 0.02 | 57.90 ± 1.96 | 83.60 ± 0.54 | 21.30 ± 0.15 | 3.00 ± 0.01 | 1.00 ± 0.01 | 3.00 ± 0.01 | 12.00 ± 0.01 |
| Maize–Cassava Diet | 38.40 ± 19.15 | 3.50 ± 0.09 | 18.90 ± 0.98 | 85.20 ± 0.35 | 19.30 ± 0.15 | 3.00 ± 0.01 | 1.00 ± 0.01 | 0.20 ± 0.01 | 15.00 ± 0.02 |
| G. sigillatus Reared On | DM | Fat | Protein | Crude Fiber | Ash | Carbohydrates | Gross Energy (kcal/kg) |
|---|---|---|---|---|---|---|---|
| Chicken Feed (Control) | 96.29 ± 0.02 a | 12.83 ± 0.092 d | 61.08 ± 0.35 bc | 6.18 ± 0.083 cd | 6.63 ± 0.025 c | 9.57 ± 0.417 bc | 3981.03 ± 6.11 b |
| Cassava–Sugar Diet | 92.41 ± 0.16 e | 7.44 ± 0.56 e | 62.79 ± 0.56 ab | 10.34 ± 0.14 a | 6.92 ± 0.084 b | 4.92 ± 0.453 de | 3388.00 ± 22.61 d |
| Desmodium–Bran Diet | 95.97 ± 0.29 ab | 16.65 ± 0.85 bc | 59.38 ± 0.35 cd | 5.53 ± 0.28 d | 6.79 ± 0.100 bc | 7.63 ± 0.902 bcd | 4164.93 ± 63.89 a |
| Morning Glory–Bean Diet | 95.61 ± 0.27 b | 15.32 ± 1.01 bc | 60.89 ± 1.61 bc | 5.72 ± 1.74 d | 5.20 ± 0.034 e | 8.49 ± 2.767 bc | 4154.13 ± 94.07 a |
| Morning Glory–Cowpea Diet | 94.51 ± 0.58 cd | 13.14 ± 0.41 d | 64.39 ± 2.10 a | 8.07 ± 0.41 b | 6.04 ± 0.032 d | 2.86 ± 1.952 ef | 3872.70 ± 41.96 c |
| Morning Glory–Cowpea Diet | 95.02 ± 0.17 c | 16.17 ± 2.29 bc | 52.90 ± 0.59 f | 6.37 ± 0.19 cd | 4.67 ± 0.205 f | 14.91 ± 1.833 a | 4167.56 ± 119.97 a |
| Mixed Weed–Bran Diet (Optimal) | 96.41 ± 0.12 a | 12.52 ± 0.79 d | 64.44 ± 1.61 a | 3.36 ± 0.25 e | 5.14 ± 0.098 e | 10.94 ± 0.792 b | 4241.97 ± 41.61 a |
| Cassava–Gallant Soldier Diet | 96.02 ± 0.16 ab | 16.66 ± 0.48 bc | 58.30 ± 0.48 d | 7.72 ± 0.06 bc | 6.24 ± 0.078 d | 7.09 ± 0.189 cd | 4115.43 ± 27.47 a |
| Wheat–Bran Diet | 94.76 ± 0.35 cd | 17.71 ± 1.17 ab | 56.17 ± 0.14 e | 6.94 ± 0.80 bcd | 4.43 ± 0.115 g | 9.50 ± 1.882 bc | 4220.67 ± 54.08 a |
| Maize–Cassava Diet | 94.25 ± 0.12 d | 19.06 ± 0.43 a | 51.52 ± 0.12 f | 6.59 ± 0.24 cd | 15.41 ± 0.251 a | 1.59 ± 0.261 f | 3839.93 ± 32.44 c |
| F9,20 | 62.38 | 32.48 | 51.24 | 24.43 | 1956.00 | 22.77 | 56.61 |
| p | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magara, H.J.O.; Hugel, S.; Fisher, B.L. Growth Performance and Nutritional Content of Tropical House Cricket (Gryllodes sigillatus (Walker, 1969)) Reared on Diets Formulated from Weeds and Agro By-Products. Insects 2025, 16, 600. https://doi.org/10.3390/insects16060600
Magara HJO, Hugel S, Fisher BL. Growth Performance and Nutritional Content of Tropical House Cricket (Gryllodes sigillatus (Walker, 1969)) Reared on Diets Formulated from Weeds and Agro By-Products. Insects. 2025; 16(6):600. https://doi.org/10.3390/insects16060600
Chicago/Turabian StyleMagara, Henlay J. O., Sylvain Hugel, and Brian L. Fisher. 2025. "Growth Performance and Nutritional Content of Tropical House Cricket (Gryllodes sigillatus (Walker, 1969)) Reared on Diets Formulated from Weeds and Agro By-Products" Insects 16, no. 6: 600. https://doi.org/10.3390/insects16060600
APA StyleMagara, H. J. O., Hugel, S., & Fisher, B. L. (2025). Growth Performance and Nutritional Content of Tropical House Cricket (Gryllodes sigillatus (Walker, 1969)) Reared on Diets Formulated from Weeds and Agro By-Products. Insects, 16(6), 600. https://doi.org/10.3390/insects16060600






