Investigation of Essential Oil from Cumin (Cuminum cyminum) Seeds and Selected Terpenes as Repellents Against Adult Female Phlebotomus papatasi (Scopoli) (Diptera: Psychodidae) Sand Flies
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sand Fly Maintenance
2.2. Chemical Sources
2.3. Extraction of Cumin Seed EO (Hydrodistillation)
2.4. Gas Chromatography–Mass Spectrometry (GC–MS) Analysis with Electron Ionization (EI)
2.5. Static-Air Repellency Bioassay
2.6. Data Analysis
3. Results
3.1. Chemical Constituents of Cumin Seed EO Revealed by GC-MS
3.2. Bioassay Results
3.2.1. Spatial and Topical Repellency Screening for Cumin Seed EO and Other Test Materials Against Adult Female P. papatasi
Spatial Repellency
Contact Repellency
3.2.2. Determination of EC50 Values for Spatial Repellency of Selected Substances Compared to DEET and Transfluthrin
3.2.3. Knockdown Effects of Cumin Seed EO and Cumin Aldehyde Compared to Transfluthrin
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- González, U.; Pinart, M.; Sinclair, D.; Firooz, A.; Enk, C.; Vélez, I.D.; Esterhuizen, T.M.; Tristan, M.; Alvar, J. Vector and Reservoir Control for Preventing Leishmaniasis. Cochrane Database Syst. Rev. 2015, 8, CD008736. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.L.; Courtenay, O.; Kelly-Hope, L.A.; Scott, T.W.; Takken, W.; Torr, S.J.; Lindsay, S.W. The Importance of Vector Control for the Control and Elimination of Vector-Borne Diseases. PLoS Negl. Trop. Dis. 2020, 14, e0007831. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Leishmaniasis 2023. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis (accessed on 5 August 2024).
- Mikery, O.; Rojas, J.C.; Rebollar-Téllez, E.A.; Valle-Mora, J.; Castillo, A. Assessment of Synthetic Chemicals for the Anthropophilic Sandfly Lutzomyia Cruciata Attraction to Light-Baited Traps. Int. J. Pest Manag. 2022, 70, 1160–1170. [Google Scholar] [CrossRef]
- Moafi, M.; Rezvan, H.; Sherkat, R.; Taleban, R. Leishmania Vaccines Entered in Clinical Trials: A Review of Literature. Int. J. Prev. Med. 2019, 10, 95. [Google Scholar]
- Balaska, S.; Fotakis, E.A.; Chaskopoulou, A.; Vontas, J. Chemical Control and Insecticide Resistance Status of Sand Fly Vectors Worldwide. PLoS Negl. Trop. Dis. 2021, 15, e0009586. [Google Scholar] [CrossRef]
- White, G.B.; Moore, S.J.; Debboun, M. Terminology of Insect Repellents. In Insect Repellents: Principles, Methods and Uses; Debboun, M., Frances, S.P., Strickman, D., Eds.; CRC Press: Boca Raton, FL, USA, 2007; pp. 31–46. [Google Scholar]
- Klun, J.A.; Khrimian, A.; Debboun, M. Repellent and Deterrent Effects of SS220, Picaridin, and Deet Suppress Human Blood Feeding by Aedes aegypti, Anopheles stephensi, and Phlebotomus papatasi. J. Med. Entomol. 2006, 43, 34–39. [Google Scholar] [CrossRef]
- Weeks, E.N.I.; Wasserberg, G.; Logan, J.L.; Agneessens, J.; Stewart, S.A.; Dewhirst, S. Efficacy of the Insect Repellent IR3535 on the Sand Fly Phlebotomus papatasi in Human Volunteers. J. Vector Ecol. 2019, 44, 290–292. [Google Scholar] [CrossRef] [PubMed]
- Naucke, T.J.; Lorentz, S.; Grünewald, H.-W. Laboratory Testing of the Insect Repellents IR3535® and DEET against Phlebotomus mascittii and P. duboscqi (Diptera: Psychodidae). Int. J. Med. Microbiol. 2006, 296, 230–232. [Google Scholar] [CrossRef] [PubMed]
- Temeyer, K.B.; Schlechte, K.G.; Coats, J.R.; Cantrell, C.L.; Rosario-Cruz, R.; Lohmeyer, K.H.; Pérez de León, A.A.; Li, A.Y. In Vitro Evaluation of Essential Oils and Saturated Fatty Acids for Repellency against the Old-World Sand Fly, Phlebotomus papatasi (Scopoli)(Diptera: Psychodidae). Insects 2024, 15, 155. [Google Scholar] [CrossRef]
- Siegel, E.L.; Olivera, M.; Roig, E.M.; Perry, M.; Li, A.Y.; D’hers, S.; Elman, N.M.; Rich, S.M. Spatial Repellents Transfluthrin and Metofluthrin Affect the Behavior of Dermacentor variabilis, Amblyomma americanum, and Ixodes scapularis in an in Vitro Vertical Climb Assay. PLoS ONE 2022, 17, e0269150. [Google Scholar] [CrossRef]
- Fongnikin, A.; Ahoga, J.; Ndombidje, B.; Hueha, C.; de Souza, E.; Oti-Tossou, R.; Govoetchan, R.; Ngufor, C. Mosquito ShieldTM, a Transfluthrin Passive Emanator, Protects against Pyrethroid-Resistant Anopheles gambiae Sensu Lato in Central Benin. Malar. J. 2024, 23, 225. [Google Scholar] [CrossRef] [PubMed]
- Powell, R.T.; Miaoulis, M.; Tsafrakidou, P.; Giantsis, I.A.; Linthicum, K.J.; Kline, D.L.; Chaskopoulou, A.; Gibson, S. Efficacy of Transfluthrin Varies by Species and Placement in a Warm Temperate Mediterranean Environment. J. Am. Mosq. Control Assoc. 2024, 40, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Zollner, G.; Orshan, L. Evaluation of a Metofluthrin Fan Vaporizer Device against Phlebotomine Sand Flies (Diptera: Psychodidae) in a Cutaneous Leishmaniasis Focus in the Judean Desert, Israel. J. Vector Ecol. 2011, 36, S157–S165. [Google Scholar] [CrossRef] [PubMed]
- Wagman, J.M.; Achee, N.L.; Grieco, J.P. Insensitivity to the Spatial Repellent Action of Transfluthrin in Aedes aegypti: A Heritable Trait Associated with Decreased Insecticide Susceptibility. PLoS Negl. Trop. Dis. 2015, 9, e0003726. [Google Scholar] [CrossRef]
- Agramonte, N.M.; Bloomquist, J.R.; Bernier, U.R. Pyrethroid Resistance Alters the Blood-Feeding Behavior in Puerto Rican Aedes aegypti mosquitoes Exposed to Treated Fabric. PLoS Negl. Trop. Dis. 2017, 11, e0005954. [Google Scholar] [CrossRef]
- Deletre, E.; Martin, T.; Duménil, C.; Chandre, F. Insecticide Resistance Modifies Mosquito Response to DEET and Natural Repellents. Parasites Vectors 2019, 12, 1–10. [Google Scholar] [CrossRef]
- Isman, M.B. Botanical Insecticides in the Twenty-First Century—Fulfilling Their Promise? Annu. Rev. Entomol. 2020, 65, 233–249. [Google Scholar] [CrossRef]
- Brosset, A.; Blande, J.D. Volatile-Mediated Plant–Plant Interactions: Volatile Organic Compounds as Modulators of Receiver Plant Defence, Growth, and Reproduction. J. Exp. Bot. 2022, 73, 511–528. [Google Scholar] [CrossRef]
- Rojas, E.; Scorza, J.V. The Use of Lemon Essential Oil as a Sandfly Repellent. Trans. R. Soc. Trop. Med. Hyg. 1991, 85, 803. [Google Scholar] [CrossRef]
- Yaghoobi-Ershadi, M.R.; Akhavan, A.A.; Jahanifard, E.; Vatandoost, H.; Amin, G.H.; Moosavi, L.; Zahraei Ramazani, A.R.; Abdoli, H.; Arandian, M.H. Repellency effect of myrtle essential oil and DEET against Phlebotomus papatasi, under laboratory conditions. Iran. J. Public Health 2006, 35, 7–13. [Google Scholar]
- Kimutai, A.; Ngeiywa, M.; Mulaa, M.; Njagi, P.G.N.; Ingonga, J.; Nyamwamu, L.B.; Ombati, C.; Ngumbi, P. Repellent Effects of the Essential Oils of Cymbopogon citratus and Tagetes minuta on the Sandfly, Phlebotomus duboscqi. BMC Res. Notes 2017, 10, 98. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, G.; Fattahi, M.; Alirezalu, A.; Ghosta, Y. Antioxidant and Antifungal Activities of a New Chemovar of Cumin (Cuminum cyminum L.). Food Sci. Biotechnol. 2018, 28, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G.; Pavela, R.; Petrelli, R.; Cappellacci, L.; Canale, A.; Senthil-Nathan, S.; Maggi, F. Not Just Popular Spices! Essential Oils from Cuminum cyminum and Pimpinella anisum Are Toxic to Insect Pests and Vectors without Affecting Non-Target Invertebrates. Ind. Crops Prod. 2018, 124, 236–243. [Google Scholar] [CrossRef]
- Deletre, E.; Martin, T.; Campagne, P.; Bourguet, D.; Cadin, A.; Menut, C.; Bonafos, R.; Chandre, F. Repellent, Irritant and Toxic Effects of 20 Plant Extracts on Adults of the Malaria Vector Anopheles gambiae Mosquito. PLoS ONE 2013, 8, e82103. [Google Scholar] [CrossRef]
- Mahran, H.A. Using Nanoemulsions of the Essential Oils of a Selection of Medicinal Plants from Jazan, Saudi Arabia, as a Green Larvicidal against Culex pipiens. PLoS ONE 2022, 17, e0267150. [Google Scholar] [CrossRef]
- Mohamadi, N.; Sharifi, I.; Afgar, A.; Sharififar, F.; Sharifi, F. Antileishmanial Effects of Bunium Persicum Crude Extract, Essential Oil, and Cuminaldehyde on Leishmania Major: In Silico and in Vitro Properties. Acta Parasitol. 2023, 68, 103–113. [Google Scholar] [CrossRef]
- Kemme, J.A.; Van Essen, P.H.; Ritchie, S.A.; Kay, B.H. Response of Mosquitoes to Carbon Dioxide and 1-Octen-3-Ol in Southeast Queensland, Australia. J. Am. Mosq. Control Assoc. 1993, 9, 431–435. [Google Scholar]
- Xu, P.; Zhu, F.; Buss, G.K.; Leal, W.S. 1-Octen-3-Ol–the Attractant That Repels. F1000Research 2015, 4, 156. [Google Scholar] [CrossRef]
- Magalhães-Junior, J.T.; Barrouin-Melo, S.M.; Corrêa, A.G.; da Rocha Silva, F.B.; Machado, V.E.; Govone, J.S.; Pinto, M.C. A Laboratory Evaluation of Alcohols as Attractants for the Sandfly Lutzomyia longipalpis (Diptera: Psychodidae). Parasites Vectors 2014, 7, 1–5. [Google Scholar] [CrossRef]
- von Oppen, S.; Masuh, H.; Licastro, S.; Zerba, E.; Gonzalez-Audino, P. A Floral-Derived Attractant for Edes aegypti Mosquitoes. Entomol. Exp. Appl. 2015, 155, 184–192. [Google Scholar] [CrossRef]
- Machado, V.E.; Corrêa, A.G.; Goulart, T.M.; Silva, F.B.d.R.; Ortiz, D.G.S.; Pinto, M.C. Attraction of the Sand Fly Nyssomyia Neivai (Diptera: Psychodidae) to Chemical Compounds in a Wind Tunnel. Parasites Vectors 2015, 8, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Lawyer, P.; Rowland, T.; Meneses, C.; Rowton, E. Care and Maintenance of Phlebotomine Sand Flies; BEI Resources; Walter Reed Army Institute of Research: Bethesda, MD, USA, 2016; Available online: https://www.beiresources.org/Portals/2/VectorResources/Methods%20in%20Sand%20fly%20Research.pdf (accessed on 2 June 2025).
- Council of Europe. European Pharmacopoeia Vol. 1, 5th ed.; Council of Europe: Strasbourg, France, 2005; p. 217. [Google Scholar]
- van Den Dool, H.; Dec. Kratz, P. A Generalization of the Retention Index System Including Linear Temperature Programmed Gas—Liquid Partition Chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Paluch, G.; Grodnitzky, J.; Bartholomay, L.; Coats, J. Quantitative Structure−Activity Relationship of Botanical Sesquiterpenes: Spatial and Contact Repellency to the Yellow Fever Mosquito, Aedes aegypti. J. Agric. Food Chem. 2009, 57, 7618–7625. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Yang, L.; Bloomquist, J.R. High-throughput Screening Method for Evaluating Spatial Repellency and Vapour Toxicity to Mosquitoes. Med. Vet. Entomol. 2019, 33, 388–396. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.r-project.org/ (accessed on 4 June 2025).
- RStudio Team. RStudio: Integrated Development for R; RStudio PBC: Boston, MA, USA, 2024; Available online: http://www.rstudio.com/ (accessed on 4 June 2025).
- AAT Bioquest, Inc. Quest Graph™ EC50 Calculator. AAT Bioquest. Available online: https://www.aatbio.com/tools/ec50-calculator (accessed on 4 June 2025).
- Gross, A.D.; Coats, J.R. Can Green Chemistry Provide Effective Repellents. In Insect Repellents Handbook; Debboun, M., Frances, S.P., Strickman, D., Eds.; CRC Press: Boca Raton, FL, USA, 2015; pp. 75–90. [Google Scholar]
- Merah, O.; Sayed-Ahmad, B.; Talou, T.; Saad, Z.; Cerny, M.; Grivot, S.; Evon, P.; Hijazi, A. Biochemical Composition of Cumin Seeds, and Biorefining Study. Biomolecules 2020, 10, 1054. [Google Scholar] [CrossRef]
- Kan, Y.; Kartal, M.; Ozek, T.; Aslan, S.; Baser, K.H.C. Composition of Essential Oil of Cuminum cyminum L. According to Harvesting Times. Turkish J. Pharm. Sci. 2007, 4, 25–29. [Google Scholar]
- Wei, J.; Zhang, X.; Bi, Y.; Miao, R.; Zhang, Z.; Su, H. Anti-inflammatory Effects of Cumin Essential Oil by Blocking JNK, ERK, and NF-κB Signaling Pathways in LPS-stimulated RAW 264.7 Cells. Evid.-B. Complement. Alternat. Med. 2015, 2015, 474509. [Google Scholar] [CrossRef] [PubMed]
- Moawad, S.A.; El-Ghorab, A.H.; Hassan, M.; Nour-Eldin, H.; El-Gharabli, M.M. Chemical and Microbiological Characterization of Egyptian Cultivars for Some Spices and Herbs Commonly Exported Abroad. Food Nutr. Sci. 2015, 6, 643. [Google Scholar] [CrossRef]
- Martinez-Velazquez, M.; Castillo-Herrera, G.A.; Rosario-Cruz, R.; Flores-Fernandez, J.M.; Lopez-Ramirez, J.; Hernandez-Gutierrez, R.; del Carmen Lugo-Cervantes, E. Acaricidal Effect and Chemical Composition of Essential Oils Extracted from Cuminum cyminum, Pimenta dioica and Ocimum basilicum against the Cattle Tick Rhipicephalus (Boophilus) Microplus (Acari: Ixodidae). Parasitol. Res. 2011, 108, 481–487. [Google Scholar] [CrossRef]
- Zheljazkov, V.D.; Jeliazkova, E.A.; Astatkie, T. Allelopathic Effects of Essential Oils on Seed Germination of Barley and Wheat. Plants 2021, 10, 2728. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Y.; Ma, G.; Yang, F.; Zhan, Z.; Guan, L.; Kuang, W.; Wang, J.; Li, J.; Han, F. Piperonyl Butoxide Synergizes the Larvicidal Activity of Origanum Vulgare Essential Oil and Its Major Constituents against the Larvae of Aedes albopictus and Culex pipiens quinquefasciatus. J. Asia Pac. Entomol. 2023, 26, 102025. [Google Scholar] [CrossRef]
- Deletre, E.; Chandre, F.; Williams, L.; Duménil, C.; Menut, C.; Martin, T. Electrophysiological and Behavioral Characterization of Bioactive Compounds of the Thymus vulgaris, Cymbopogon winterianus, Cuminum cyminum and Cinnamomum zeylanicum Essential Oils against Anopheles gambiae and Prospects for Their Use as Bednet Treatments. Parasites Vectors 2015, 8, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Mota, T.F.; Silva, C.M.d.A.; Conceição, M.d.S.; Fraga, D.B.M.; Brodskyn, C.I.; Neto, M.F.d.A.; Santana, I.B.; Mesquita, P.R.R.; Leite, F.H.A.; Magalhães-Júnior, J.T. Screening Organic Repellent Compounds against Lutzomyia longipalpis (Diptera: Psychodidae) Present in Plant Essential Oils: Bioassay plus an in Silico Approach. Acta Trop. 2022, 229, 106367. [Google Scholar] [CrossRef] [PubMed]
- Verhulst, N.O.; Cavegn, J.C.; Mathis, A. Spatial Repellency and Vapour Toxicity of Transfluthrin against the Biting Midges Culicoides Nubeculosus and C. sonorensis (Ceratopogonidae). Curr. Res. Insect Sci. 2021, 1, 100002. [Google Scholar] [CrossRef] [PubMed]
- Cuba, I.H.; Richoux, G.R.; Norris, E.J.; Bernier, U.R.; Linthicum, K.J.; Bloomquist, J.R. Vapor Phase Repellency and Insecticidal Activity of Pyridinyl Amides against Anopheline mosquitoes. Curr. Res. Parasitol. Vector-Borne Dis. 2021, 1, 100062. [Google Scholar] [CrossRef]
- Bibbs, C.S.; Kaufman, P.E. Volatile Pyrethroids as a Potential Mosquito Abatement Tool: A Review of Pyrethroid-Containing Spatial Repellents. J. Integr. Pest. Manag. 2017, 8, 21. [Google Scholar] [CrossRef]
- Rapley, L.P.; Russell, R.C.; Montgomery, B.L.; Ritchie, S.A. The Effects of Sustained Release Metofluthrin on the Biting, Movement, and Mortality of Aedes aegypti in a Domestic Setting. Am. J. Trop. Med. Hyg. 2009, 81, 94–99. [Google Scholar] [CrossRef]
- Yousefi, S.; Reza Zahraei-Ramazani, A.; Rassi, Y.; Vatandoost, H.; Reza Yaghoobi-Ershadi, M.; Reza Aflatoonian, M.; Ahmad Akhavan, A.; Aghaei-Afshar, A.; Amin, M.; Paksa, A. Evaluation of Different Attractive Traps for Capturing Sand Flies (Diptera: Psychodidae) in an Endemic Area of Leishmaniasis, Southeast of Iran. J. Arthropod. Borne Dis. 2020, 14, 202–213. [Google Scholar] [CrossRef]
- Kempraj, V.; Park, S.J.; Cameron, D.N.S.; Taylor, P.W. 1-Octanol Emitted by Oecophylla Smaragdina Weaver Ants Repels and Deters Oviposition in Queensland Fruit Fly. Sci. Rep. 2022, 12, 15768. [Google Scholar] [CrossRef]
- Rebollar-Tellez, E.A.; Hamilton, J.G.C.; Ward, R.D. Response of Female Lutzomyia longipalpis to Host Odour Kairomones from Human Skin. Physiol. Entomol. 1999, 24, 220–226. [Google Scholar] [CrossRef]
- Andrade, A.J.; Andrade, M.R.; Dias, E.S.; Pinto, M.C.; Eiras, Á.E. Are Light Traps Baited with Kairomones Effective in the Capture of Lutzomyia longipalpis and Lutzomyia intermedia? An Evaluation of Synthetic Human Odor as an Attractant for Phlebotomine Sand Flies (Diptera: Psychodidae: Phlebotominae). Mem. Inst. Oswaldo Cruz 2008, 103, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Tchouassi, D.P.; Jacob, J.W.; Cheseto, X.; Chepkemoi, L.S.; Hassaballa, I.B.; Torto, B. Enzyme-Catalyzed Kinetic Resolution of Racemic 1-Octen-3-Ol and Field Evaluation of Its Enantiomeric Isomers as Attractants of Sandflies. Front. Trop. Dis. 2024, 4, 1327349. [Google Scholar] [CrossRef]
- Mann, R.S.; Kaufman, P.E.; Butler, J.F. Lutzomyia Spp.(Diptera: Psychodidae) Response to Olfactory Attractant-and Light Emitting Diode-Modified Mosquito Magnet X (MM-X) Traps. J. Med. Entomol. 2009, 46, 1052–1061. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.-S.; Park, B.-S.; Ku, S.-K.; Lee, S.-E. Repellent Activities of Essential Oils and Monoterpenes against Culex pipiens Pallens. J. Am. Mosq. Control Assoc. 2002, 18, 348–351. [Google Scholar]
- Tian, Y.; Hogsette, J.A.; Norris, E.J.; Hu, X.P. Topical Toxicity and Repellency Profiles of 17 Essential Oil Components Against Insecticide-Resistant and Susceptible Strains of Adult Musca domestica (Diptera: Muscidae). Insects 2024, 15, 384. [Google Scholar] [CrossRef]

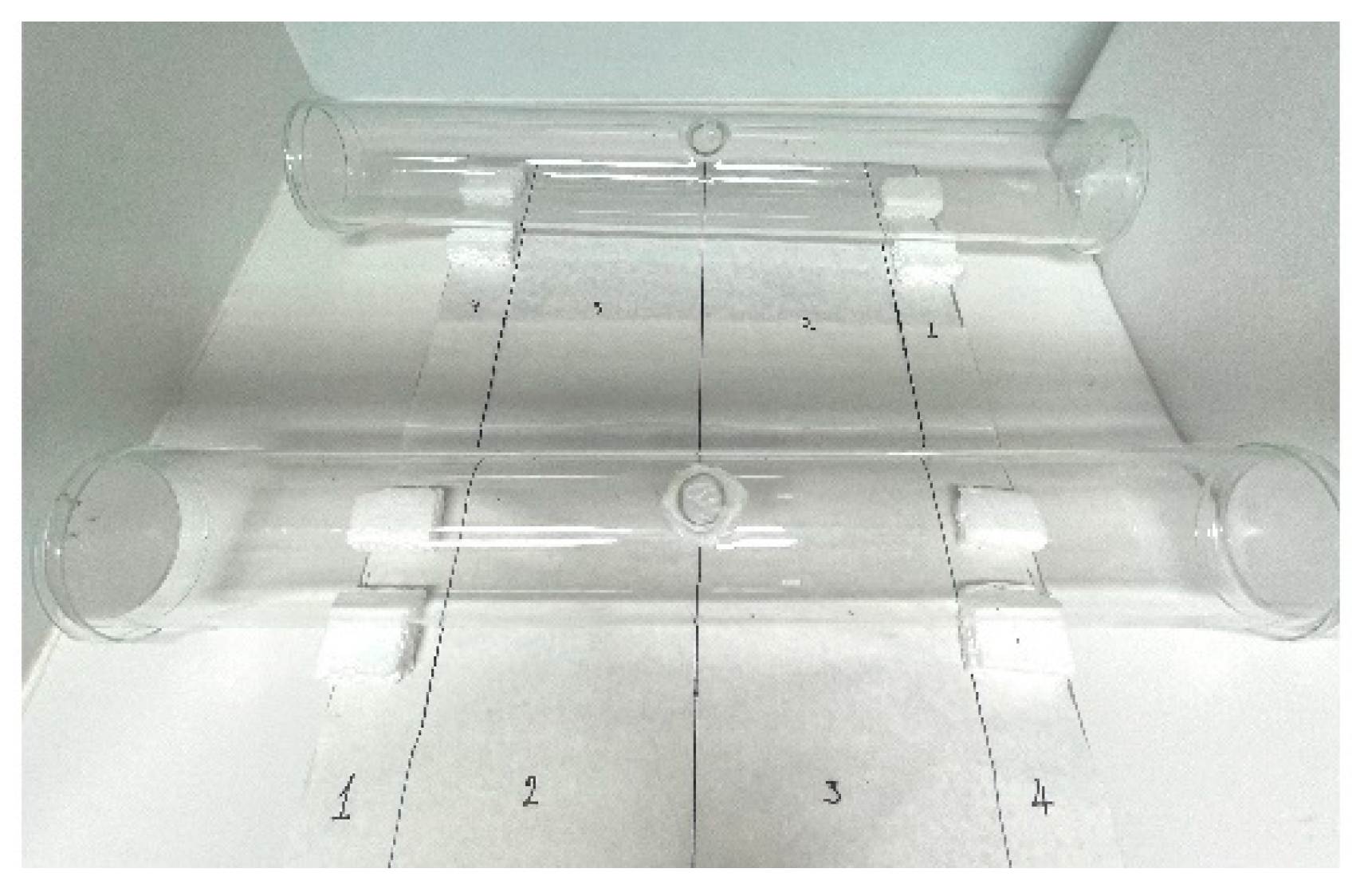

| # | Compound | RT | RI | Peak Relative Area, % | Identification |
|---|---|---|---|---|---|
| 1 | α-pinene | 17.17 | 929 | 0.46 | RRI, MS, S |
| 2 | β-pinene | 20.041 | 972 | 11.45 | RRI, MS, S |
| 3 | β-myrcene | 21.44 | 991 | 0.93 | RRI, MS, S |
| 4 | p-cymene | 23.37 | 1022 | 5.14 | RRI, MS, S |
| 5 | D-limonene | 23.55 | 1025 | 0.82 | RRI, MS |
| 6 | 1,8-cineol | 23.68 | 1027 | 0.38 | RRI, MS |
| 7 | γ-terpinene | 25.46 | 1057 | 10.79 | RRI, MS, S |
| 8 | trans-sabinene hydrate | 27.76 | 1095 | 0.15 | RRI, MS |
| 9 | linalool | 28.02 | 1099 | 0.14 | RRI, MS |
| 10 | cis-p-menth-2-en-1-ol | 29.04 | 1118 | 0.06 | RRI, MS |
| 11 | terpinen-4-ol | 32.09 | 1172 | 0.38 | RRI, MS |
| 12 | α-terpineol | 32.89 | 1187 | 0.10 | RRI, MS |
| 13 | p-menth-3-en-7-al | 33.08 | 1190 | 4.08 | RRI, MS |
| 14 | cumin aldehyde | 36.12 | 1230 | 26.98 | RRI, MS, S |
| 15 | phellandral | 38.85 | 1264 | 0.44 | RRI, MS |
| 16 | p-mentha-1,3-dien-7-al | 39.77 | 1275 | 15.83 | RRI, MS |
| 17 | p-mentha-1,4-dien-7-al | 40.52 | 1284 | 20.27 | RRI, MS |
| 18 | p-mentha-1,4-dien-7-ol | 44.16 | 1333 | 0.63 | RRI, MS |
| 19 | RT:44.734 | 44.71 | 1340 | 0.15 | RRI, MS |
| 20 | RT:46.185 | 46.19 | 1361 | 0.07 | RRI, MS |
| 21 | 1-tetradecene | 48.579 | 1394 | 0.45 | RRI, MS, S |
| 22 | trans-β-farnesene | 51.93 | 1460 | 0.04 | RRI, MS |
| 23 | β-acoradiene | 52.59 | 1474 | 0.19 | RRI, MS |
| 24 | carotol | 57.57 | 1596 | 0.04 | RRI, MS |
| 25 | phytone | 65.276 | 1844 | 0.02 | RRI, MS |
| 26 | hexadecanoic acid | 68.54 | 1956 | 0.01 | RRI, MS |
| Total identified | 99.78 | ||||
| Monoterpene hydrocarbons | 29.59 | ||||
| Oxygenated monoterpenes | 69.44 | ||||
| Sesquiterpene hydrocarbons | 0.23 | ||||
| Oxygenated sesquiterpenes | 0.06 | ||||
| Others | 0.46 | ||||
| Mean Spatial Repellency (%) ± SEM | Mean Contact Repellency | |||||||
|---|---|---|---|---|---|---|---|---|
| 15 min | 30 min | 60 min | 90 min | 120 min | 180 min | Avoidance Ratios (p-Values) | Avoidance Frequency (Scores) | |
| Treatment | Concentration: 78.6 μg/cm2 | |||||||
| γ-terpinene | 22.0 ± 7.6 a | 26.0 ± 2.5 a | 37.9 ± 9.1 **a | ** | 0 | |||
| α-pinene | 32.6 ± 4.6 *a | 29.9 ± 7.0 *a | 34.3 ± 7.6 **a | 22.1 ± 8.8 a | 29.0 ± 9.5 *a | 14.9 ± 10.0 a | *** | 0.2 |
| β-pinene | 39.3 ± 9.9 **a | 37.2 ± 5.4 **a | 10.3 ± 7.8 a | 13.7 ± 17.6 a | 50.9 ± 17.5 **a | 25.6 ± 6.3 a | * | 0.3 |
| β-myrcene | 13.8 ± 16.5 a | 18.2 ± 21.0 ab | 51.1 ± 13.2 ***ab | 59.3 ± 0.70 **ab | 75.0 ± 5.83 ***b | 75.0 ± 5.83 ***b | * | 0.1 |
| p-cymene | 54.7 ± 16.2 ***a | 66.8 ± 12.1 ***ab | 68.0 ± 17.9 ***ab | 81.5 ± 9.8 ***ab | 93.8 ± 3.3 ***b | 97.5 ± 2.5 ***b | *** | 1 |
| octanol | 100 ± 0 ***a | 100 ± 0 ***a | 100 ± 0 ***a | 100 ± 0 ***a | 100 ± 0 ***a | 100 ± 0 ***a | *** | 1 |
| 1-octen-3-ol | 43.4 ± 7.6 **a | 88.5 ± 5.8 ***b | 97.2 ± 2.8 ***b | 97.4 ± 2.6 ***b | 100 ± 0 ***b | 97.4 ± 2.6 ***b | *** | 1 |
| DEET | 25.4 ± 10.3 a | 24.2 ± 19.4 *a | 45.4 ± 12.4 ***ab | 66.5 ± 12.0 ***b | 80.6 ± 11.5 ***b | 76.6 ± 13.7 ***b | *** | 1 |
| Concentration: 19.6 μg/cm2 | ||||||||
| cumin seed EO | 100 ± 0 ***a | 97.8 ± 2.2 ***a | 100 ± 0 ***a | 100 ± 0 ***a | 100 ± 0 ***a | 97.6 ± 2.4 ***a | *** | 1 |
| cumin aldehyde | 97.2 ± 2.8 ***a | 100 ± 0 ***a | 94.7 ± 5.2 ***a | 75.0 ± 14.0 ***a | 57.2 ± 11.7 ***ab | 9.4 ± 26.5 b | *** | 0.97 |
| Concentration: 0.1572 μg/cm2 | ||||||||
| transfluthrin | 32.8 ± 19.9 *a | 24.4 ± 10.9 a | 22.7 ± 7.4 a | 20.6 ± 14.5 a | 22.3 ± 2.9 a | 53.4 ± 10.4 **a | *** | 0.7 |
| Control | ||||||||
| −7.2 ± 8.7 a | −13.7 ± 10.2 a | −5.0 ± 9.3 a | −1.8 ± 13.2 a | −4.8 ± 4.8 a | −11.0 ± 3.9 a | 0 | ||
| EC50 (95%CI) μg/cm2, Slope, R2 | |||
|---|---|---|---|
| Treatment | 15 min | 30 min | 60 min |
| cumin seed EO | 0.97 (0.78–1.16), 3.92, 0.92 | 0.88 (0.68–1.08), 3.68, 0.90 | 0.34 (0.01–0.67), 1.90, 0.89 |
| cumin aldehyde | 0.15 (0.04–0.25), 0.96, 0.95 | 0.13 (0.07–0.18), 1.10, 0.96 | 0.07 (0.04–0.10), 2.44, 0.88 |
| octanol | 1.40 (0.67–2.12), 4.61, 0.97 | 0.85 (0.44–1.26), 3.14, 0.94 | 0.60 (0.30–0.91), 1.74, 0.91 |
| DEET | 90.72 (67.82–113.62), 6.32, 0.83 | 83.81 (31.79–135.83), 26.41, 0.94 | 78.95 (63.41–94.50), 13.48, 0.71 |
| transfluthrin | 0.06 (0.02–0.10), 1.83, 0.83 | 0.03 (0.02–0.04), 3.14, 0.86 | 0.04 (0.02–0.05), 4.37, 0.73 |
| Treatment | Concentration, μg/cm2 | KND ± SEM, % | |||||
|---|---|---|---|---|---|---|---|
| 15 min | 30 min | 60 min | 90 min | 120 min | 180 min | ||
| Cumin seed EO | 157.2 | 0 ± 0 Aa | 2.05 ± 1.2 Aa | 8.3 ± 3.6 Aa | 70.2 ± 12.7 Ab | 100 ± 0 Ac | 100 ± 0 Ac |
| 78.6 | 0 ± 0 Aa | 0 ± 0 Aa | 25.0 ± 16.0 Aa | 30.8 ± 23.3 Aab | 30.8 ± 23.3 Bab | 96.2 ± 3.8 Ab | |
| cumin aldehyde | 78.6 | 0 ± 0 Aa | 0 ± 0 Aa | 25.0 ± 25.0 Aab | 75.0 ± 25.0 Aab | 63.8 ± 22.8 Aab | 97.4 ± 2.6 Ab |
| 39.2 | 2.2 ± 2.2 Aa | 0.00 Aa | 2.3 ± 1.3 Aa | 17.0 ± 9.0 ABa | 63.8 ± 19.2 Ab | 98.8 ± 1.2 Ab | |
| 19.6 | 0 ± 0 Aa | 0 ± 0 Aa | 0 ± 0 Aa | 0 ± 0 Aa | 0 ± 0 Ba | 57.3 ± 25.3 Ab | |
| transfluthrin | 7.86 | 43.1 ± 5.9 Aa | 53.8 ± 2.7 Aab | 59.8 ± 6.7 Aab | 61.7 ± 2.6 Aab | 59.7 ± 4.9 Aab | 73.7 ± 2.4 Ab |
| 0.786 | 18.5 ± 8.5 Aa | 16.8 ± 5.0 Ba | 14.4 ± 5.6 Ba | 10.4 ± 6.9 Ba | 14.3 ± 10.7 Ba | 9.7 ± 5.2 Ba | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsikolia, M.; Tsafrakidou, P.; Miaoulis, M.; Li, A.Y.; Gundersen-Rindal, D.; Chaskopoulou, A. Investigation of Essential Oil from Cumin (Cuminum cyminum) Seeds and Selected Terpenes as Repellents Against Adult Female Phlebotomus papatasi (Scopoli) (Diptera: Psychodidae) Sand Flies. Insects 2025, 16, 599. https://doi.org/10.3390/insects16060599
Tsikolia M, Tsafrakidou P, Miaoulis M, Li AY, Gundersen-Rindal D, Chaskopoulou A. Investigation of Essential Oil from Cumin (Cuminum cyminum) Seeds and Selected Terpenes as Repellents Against Adult Female Phlebotomus papatasi (Scopoli) (Diptera: Psychodidae) Sand Flies. Insects. 2025; 16(6):599. https://doi.org/10.3390/insects16060599
Chicago/Turabian StyleTsikolia, Maia, Panagiota Tsafrakidou, Michael Miaoulis, Andrew Y. Li, Dawn Gundersen-Rindal, and Alexandra Chaskopoulou. 2025. "Investigation of Essential Oil from Cumin (Cuminum cyminum) Seeds and Selected Terpenes as Repellents Against Adult Female Phlebotomus papatasi (Scopoli) (Diptera: Psychodidae) Sand Flies" Insects 16, no. 6: 599. https://doi.org/10.3390/insects16060599
APA StyleTsikolia, M., Tsafrakidou, P., Miaoulis, M., Li, A. Y., Gundersen-Rindal, D., & Chaskopoulou, A. (2025). Investigation of Essential Oil from Cumin (Cuminum cyminum) Seeds and Selected Terpenes as Repellents Against Adult Female Phlebotomus papatasi (Scopoli) (Diptera: Psychodidae) Sand Flies. Insects, 16(6), 599. https://doi.org/10.3390/insects16060599







