Assessing Phenotypic and Genotypic Resistance to Flumethrin in Varroa destructor Populations in Muğla, Türkiye
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Varroa Samples
2.2. Acute LD50 Analysis and Determination of Phenotypic Resistance
2.3. DNA Isolation from Varroa Samples
2.4. Amplification of the VGSC Gene Region by PCR Using Appropriate Primers
2.5. RFLP Analysis of the PCR Products
2.6. DNA Sequencing of Some of the VGSC PCR Products by Sanger and Bioinformatics Analysis
3. Results
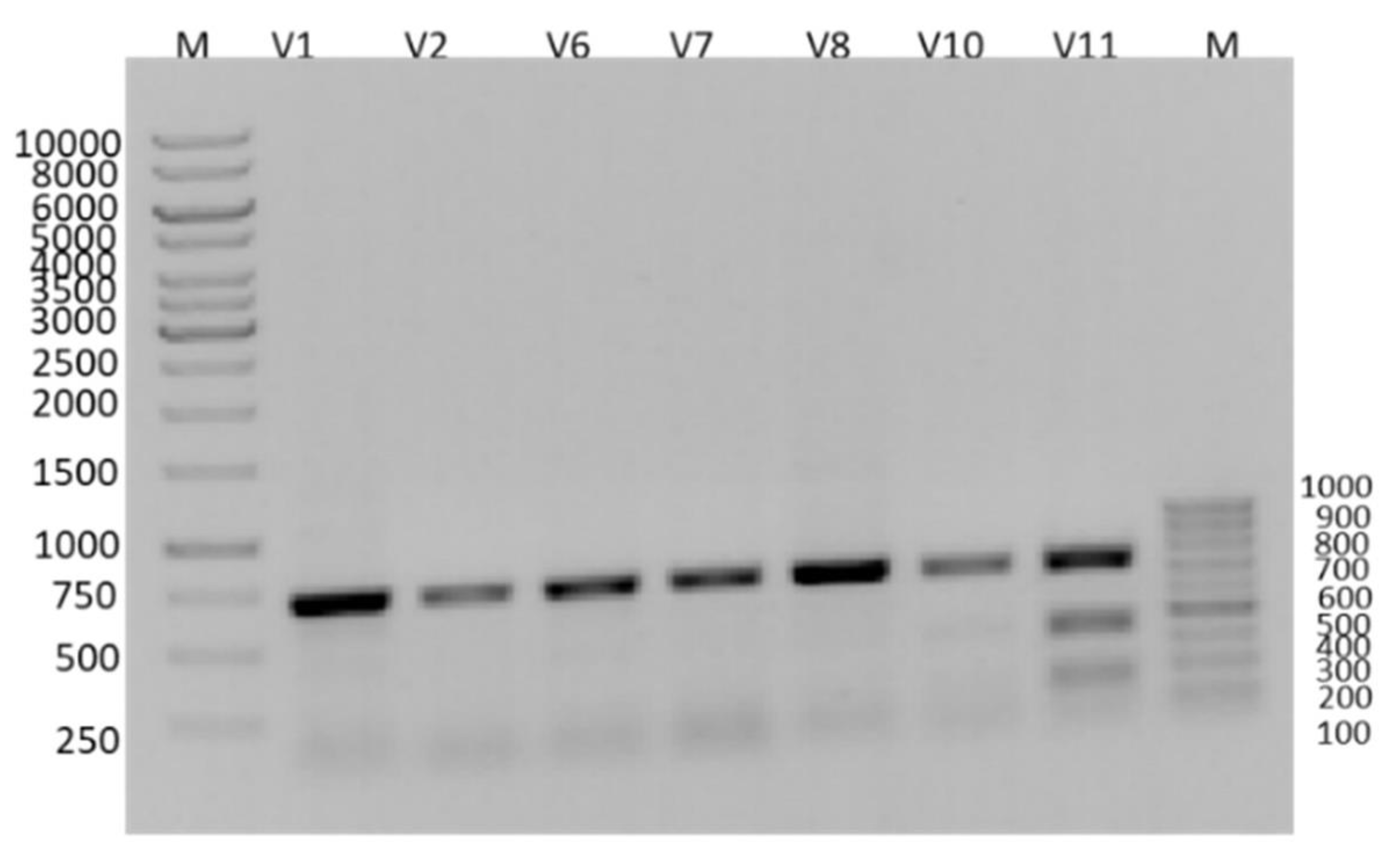
3.1. RFLP Analysis of PCR Products
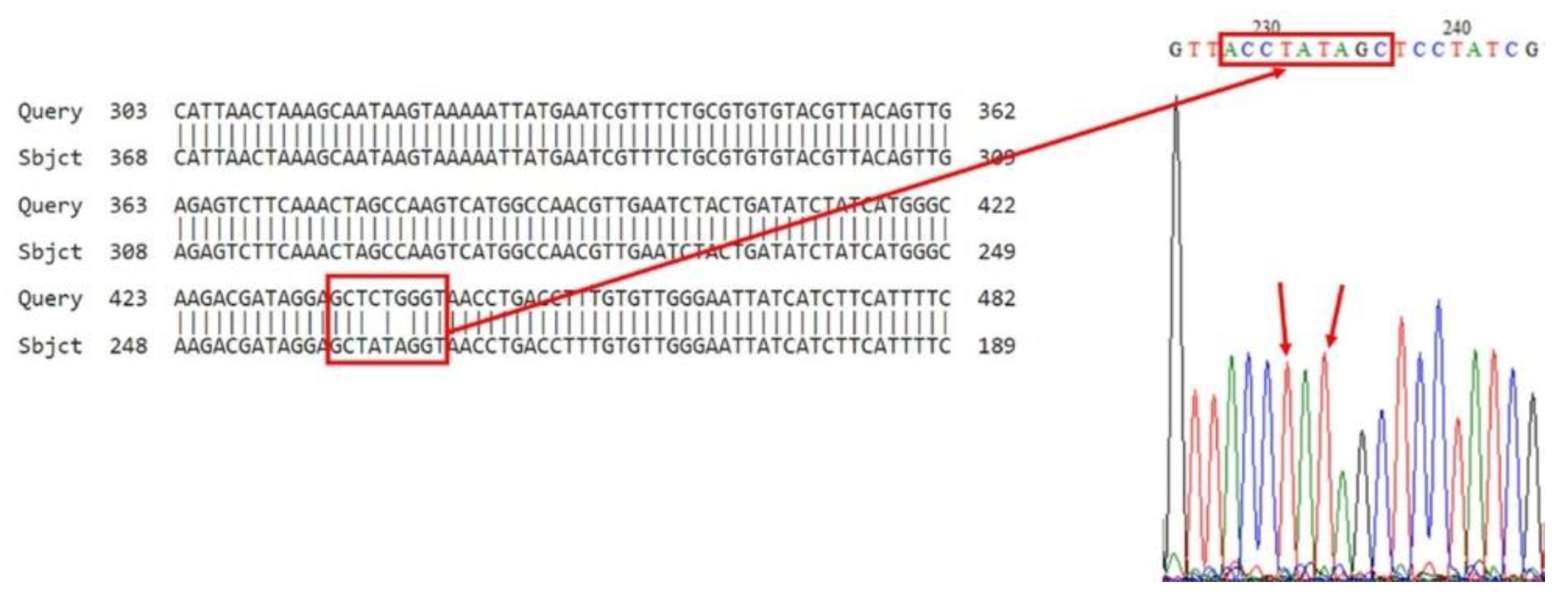
3.2. Bioinformatics Analysis of VGSC PCR Products
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LD50 | Median lethal dose |
| VGSC | Voltage-gated sodium channel |
| RFLP | Restriction Fragment Length Polymorphism |
References
- Benito-Murcia, M.; Martín-Hernández, R.; Meana, A.; Botías, C.; Higes, M. Study of pyrethroid resistance mutations in populations of Varroa destructor across Spain. Res. Vet. Sci. 2022, 152, 34–37. [Google Scholar] [CrossRef] [PubMed]
- Van Der Steen, J.; Vejsnæs, F. Varroa control: A brief overview of available methods. Bee World 2021, 98, 50–56. [Google Scholar] [CrossRef]
- Dietemann, V.; Nazzi, F.; Martin, S.J.; Anderson, D.L.; Locke, B.; Delaplane, K.S.; Wauquiez, Q.; Tannahill, C.; Frey, E.; Ziegelmann, B. Standard methods for varroa research. J. Apic. Res. 2013, 52, 1–54. [Google Scholar] [CrossRef]
- Mitton, G.A.; Quintana, S.; Gimenez Martinez, P.; Mendoza, Y.; Ramallo, G.; Brasesco, C.; Villalba, A.; Eguaras, M.J.; Maggi, M.D.; Ruffinengo, S.R. First record of resistance to flumethrin in a varroa population from Uruguay. J. Apic. Res. 2016, 55, 422–427. [Google Scholar] [CrossRef]
- Gonzalez-Cabrera, J.; Davies, T.E.; Field, L.M.; Kennedy, P.J.; Williamson, M.S. An amino acid substitution (L925V) associated with resistance to pyrethroids in Varroa destructor. PLoS ONE 2013, 8, e82941. [Google Scholar] [CrossRef]
- Vlogiannitis, S.; Jonckheere, W.; Laget, D.; de Graaf, D.C.; Vontas, J.; Van Leeuwen, T. Pyrethroid target-site resistance mutations in populations of the honey bee parasite Varroa destructor (Acari: Varroidae) from Flanders, Belgium. Exp. Appl. Acarol. 2021, 85, 205–221. [Google Scholar] [CrossRef]
- Mitton, G.A.; Meroi Arcerito, F.; Cooley, H.; Fernandez de Landa, G.; Eguaras, M.J.; Ruffinengo, S.R.; Maggi, M.D. More than sixty years living with Varroa destructor: A review of acaricide resistance. Int. J. Pest Manag. 2022, 1–18. [Google Scholar] [CrossRef]
- Millán-Leiva, A.; Marín, Ó.; Christmon, K.; Vanengelsdorp, D.; González-Cabrera, J. Mutations associated with pyrethroid resistance in Varroa mite, a parasite of honey bees, are widespread across the United States. Pest Manag. Sci. 2021, 77, 3241–3249. [Google Scholar] [CrossRef]
- Morfin, N.; Rawn, D.; Petukhova, T.; Kozak, P.; Eccles, L.; Chaput, J.; Pasma, T.; Guzman-Novoa, E. Surveillance of synthetic acaricide efficacy against Varroa destructor in Ontario, Canada. Can. Entomol. 2022, 154, e17. [Google Scholar] [CrossRef]
- Hansen, G. Guide to Varroa Mite Controls for Commercial Beekeeping Operations. 2021. Available online: https://honeybeehealthcoalition.org/wp-content/uploads/2021/06/Commercial_Beekeeping_060621.pdf (accessed on 13 March 2025).
- McGruddy, R.A.; Bulgarella, M.; Felden, A.; Baty, J.W.; Haywood, J.; Stahlmann-Brown, P.; Lester, P.J. Are increasing honey bee colony losses attributed to Varroa destructor in New Zealand driven by miticide resistance? J. Apic. Res. 2024, 63, 648–659. [Google Scholar] [CrossRef]
- Maggi, M.D.; Ruffinengo, S.R.; Gende, L.B.; Eguaras, M.J.; Sardella, N.H. LC50 baseline levels of amitraz, coumaphos, fluvalinate and flumethrin in populations of Varroa destructor from Buenos Aires Province, Argentina. J. Apic. Res. 2008, 47, 292–295. [Google Scholar] [CrossRef]
- Goodwin, R.; Taylor, M.; McBrydie, H.; Cox, H. Base levels of resistance to common control compounds by a New Zealand population of Varroa destructor. N. Z. J. Crop Hortic. Sci. 2005, 33, 347–352. [Google Scholar] [CrossRef]
- Yarsan, E.; Yilmaz, F.; Sevin, S.; Akdeniz, G.; Celebi, B.; Ozturk, S.H.; Ayikol, S.N.; Karatas, U.; Ese, H.; Fidan, N. Investigation of resistance against to flumethrin using against Varroa destructor in Türkiye. Vet. Res. Commun. 2024, 48, 1683–1696. [Google Scholar] [CrossRef]
- Erdem, E.; Koç-İnak, N.; Rüstemoğlu, M.; İnak, E. Geographical distribution of pyrethroid resistance mutations in Varroa destructor across Türkiye and a European overview. Exp. Appl. Acarol. 2024, 92, 309–321. [Google Scholar] [CrossRef]
- Koç, N.; İnak, E.; Jonckheere, W.; Van Leeuwen, T. Genetic analysis and screening of pyrethroid resistance mutations in Varroa destructor populations from Turkey. Exp. Appl. Acarol. 2021, 84, 433–444. [Google Scholar] [CrossRef]
- Millán-Leiva, A.; Hernández-Rodríguez, C.S.; González-Cabrera, J. New PCR–RFLP diagnostics methodology for detecting Varroa destructor resistant to synthetic pyrethroids. J. Pest. Sci. 2018, 91, 937–941. [Google Scholar] [CrossRef]
- Stara, J.; Pekar, S.; Nesvorna, M.; Erban, T.; Vinsova, H.; Kopecky, J.; Doskocil, I.; Kamler, M.; Hubert, J. Detection of tau-fluvalinate resistance in the mite Varroa destructor based on the comparison of vial test and PCR–RFLP of kdr mutation in sodium channel gene. Exp. Appl. Acarol. 2019, 77, 161–171. [Google Scholar] [CrossRef]
- Mitton, G.A.; Quintana, S.; Mendoza, Y.; Eguaras, M.; Maggi, M.D.; Ruffinengo, S.R. L925V mutation in voltage-gated sodium channel of Varroa destructor populations from Argentina and Uruguay, with different degree of susceptibility to pyrethroids. Int. J. Acarol. 2021, 47, 374–380. [Google Scholar] [CrossRef]
- Wu, X.; Li, Z.; Yang, H.; He, X.; Yan, W.; Zeng, Z. The adverse impact on lifespan, immunity, and forage behavior of worker bees (Apis mellifera Linnaeus 1758) after exposure to flumethrin. Sci. Total Environ. 2023, 858, 160146. [Google Scholar] [CrossRef]
- Lodesani, M.; Colombo, M.; Spreafico, M. Ineffectiveness of Apistan® treatment against the mite Varroa jacobsoni Oud in several districts of Lombardy (Italy). Apidologie 1995, 26, 67–72. [Google Scholar] [CrossRef]
- Millán-Leiva, A.; Marín, Ó.; De la Rúa, P.; Muñoz, I.; Tsagkarakou, A.; Eversol, H.; Christmon, K.; VanEngelsdorp, D.; González-Cabrera, J. Mutations associated with pyrethroid resistance in the honey bee parasite Varroa destructor evolved as a series of parallel and sequential events. J. Pest Sci. 2021, 94, 1505–1517. [Google Scholar] [CrossRef]
- González-Cabrera, J.; Bumann, H.; Rodríguez-Vargas, S.; Kennedy, P.J.; Krieger, K.; Altreuther, G.; Hertel, A.; Hertlein, G.; Nauen, R.; Williamson, M.S. A single mutation is driving resistance to pyrethroids in European populations of the parasitic mite, Varroa destructor. J. Pest. Sci. 2018, 91, 1137–1144. [Google Scholar] [CrossRef]
- Farjamfar, M.; Saboori, A.; González-Cabrera, J.; Hernandez Rodriguez, C.S. Genetic variability and pyrethroid susceptibility of the parasitic honey bee mite Varroa destructor (Acari: Varroidae) in Iran. Exp. Appl. Acarol. 2018, 76, 139–148. [Google Scholar] [CrossRef]
- Almecija, G.; Schimmerling, M.; Del Cont, A.; Poirot, B.; Duquesne, V. Varroa destructor resistance to tau-fluvalinate: Relationship between in vitro phenotypic test and VGSC L925V mutation. Pest Manag. Sci. 2022, 78, 5097–5105. [Google Scholar] [CrossRef]
- Panini, M.; Reguzzi, M.C.; Chiesa, O.; Cominelli, F.; Lupi, D.; Moores, G.; Mazzoni, E. Pyrethroid resistance in Italian populations of the mite Varroa destructor: A focus on the Lombardy region. Bull. Insectol. 2019, 72, 227–232. [Google Scholar]
- Alissandrakis, E.; Ilias, A.; Tsagkarakou, A. Pyrethroid target site resistance in Greek populations of the honey bee parasite Varroa destructor (Acari: Varroidae). J. Apic. Res. 2017, 56, 625–630. [Google Scholar] [CrossRef]
- Bahreini, R.; González-Cabrera, J.; Hernández-Rodríguez, C.S.; Moreno-Martí, S.; Muirhead, S.; Labuschagne, R.B.; Rueppell, O. Arising amitraz and pyrethroids resistance mutations in the ectoparasitic Varroa destructor mite in Canada. Sci. Rep. 2025, 15, 1587. [Google Scholar] [CrossRef]



| Apiaries | Location | LD50 (µg) | DOSES µg/petri (Diameter = 8.5 cm) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 10 | 20 | 40 | 50 | 60 | 80 | 100 | |||
| Average Mortality a | |||||||||
| V1 | Menteşe | 42 | 0 | 2 | 4 | 7 | 8 | 10 | 10 |
| V2 | Ula | 39.2 | 2.1 | 2 | 5.3 | 6 | 8.5 | 10 | 10 |
| V3 | Milas | 61.5 | 0 | 0 | 2 | 3 | 5 | 5 | 10 |
| V4 | Milas | 46 | 0 | 1 | 4 | 6 | 7 | 10 | 10 |
| V5 | Milas | 51.5 | 0 | 1 | 3 | 5 | 6 | 8.2 | 10 |
| V6 | Milas | 51.4 | 0 | 1 | 1 | 6 | 6.4 | 8 | 10 |
| V7 | Bodrum | 44.6 | 0 | 2 | 4 | 6 | 8 | 8.2 | 10 |
| V8 | Milas | 36.5 | 0 | 3 | 6 | 8 | 8 | 10 | 10 |
| V9 | Menteşe | 48 | 0 | 1 | 3 | 4 | 8 | 10 | 10 |
| V10 | Menteşe | 47.5 | 0 | 1 | 2 | 6 | 9 | 10 | 10 |
| V11 | Köyceğiz | 53.6 | 0 | 0 | 1.8 | 2.5 | 8 | 10 | 10 |
| V12 | Milas | 38.4 | 0.7 | 3.1 | 4.7 | 6.5 | 8.3 | 10 | 10 |
| V13 | Milas | 45 | 0 | 1 | 3 | 7 | 8 | 10 | 10 |
| V14 | Milas | 54.5 | 0 | 0 | 2.5 | 4.2 | 5 | 10 | 10 |
| V15 | Milas | 61.8 | 0 | 0 | 1.3 | 2 | 5 | 8.6 | 10 |
| V16 | Milas | 44.4 | 1 | 1.7 | 2.5 | 5 | 8 | 10 | 10 |
| V17 | Köyceğiz | 54.2 | 1 | 0.9 | 1.3 | 5 | 7 | 10 | 10 |
| V18 | Köyceğiz | 57.5 | 0 | 0 | 1.5 | 2.7 | 5.5 | 10 | 10 |
| V19 | Milas | 45.5 | 0.9 | 0.7 | 4.7 | 6.3 | 6 | 9.3 | 10 |
| V20 | Ula | 60.2 | 0 | 0 | 3 | 3 | 4 | 8 | 10 |
| V21 | Ula | 54.3 | 0 | 1.4 | 2.9 | 3.8 | 7.3 | 6.9 | 10 |
| V22 | Dalaman | 31 | 1 | 2 | 7 | 10 | 10 | 10 | 10 |
| V23 | Dalaman | 50 | 0 | 1.7 | 3.3 | 4 | 6 | 10 | 10 |
| V24 | Dalaman | 47 | 0 | 2 | 4 | 5 | 6 | 10 | 10 |
| V25 | Dalaman | 46.5 | 0 | 2 | 3 | 4 | 8 | 10 | 10 |
| V26 | Dalaman | 61 | 0 | 1 | 2 | 2 | 3 | 8 | 10 |
| V27 | Dalaman | 53.5 | 0 | 0 | 3 | 5 | 6 | 8 | 10 |
| 49.13 | 0.24 | 1.17 | 3.18 | 4.99 | 6.85 | 9.19 | 10 | ||
| VGSC Alleles | Varroa Samples | Genotype Probabilities |
|---|---|---|
| L/L homozygous susceptible alleles | - | SS (Leu/Leu) |
| V/V homozygous resistant alleles | V1, V2, V3, V4, V5 V6, V7, V8, V9, V12, V13, V14, V15, V16, V19, V21, V22 | RR (Ile/Ile, Ile/Met, Ile/Val, Val/Val, Val/Met, Met/Met) |
| L/V heterozygous alleles (some of 5 samples) | V10x, V11x, V17x, V18x, V20x | SR (Leu/X resistant allele) |
| No a, b | VGSC Alleles | No a,b | VGSC Alleles | No a,b | VGSC Alleles | No a,b | VGSC Alleles |
|---|---|---|---|---|---|---|---|
| V1a V1b V1c V1d V1e | 5 samples Homozygote RR (Ile/Ile) (CTG→ATA) (5 RR) | V7a V7b V7c V7d V7e | 5 samples Homozygote RR (Ile/Ile) (CTG→ATA) (5 RR) | V13a V13b V13c V13d V13e | 5 samples Homozygote RR allele (5 RR) | V19a V19b V19c V19d V19e | 5 samples Homozygote RR allele (5 RR) |
| V2a V2b V2c V2d V2e | 5 samples Homozygote RR (Ile/Ile) (CTG→ATA) (5 RR) | V8a V8b V8c V8d V8e | Of the 10 alleles, 70% were RR (Ile/Ile), and 30% were GTG (Val) alleles (RR). (5 RR) | V14a V14b V14c V14d V14e | 5 samples Homozygote RR allele (5 RR) | V20a V20b V20c V20d V20e | Of the 10 alleles, 70% were RR (Ile/Ile), 20% were ATG RR (Met) allele (RR), and 10% were CTG (Leu) S allele. (4 RR and 1SR) |
| V3a V3b V3c V3d V3e | 5 samples Homozygote RR Allel (5 RR) | V9a V9b V9c V9d V9e | Of the 10 alleles, 70% were RR (Ile/Ile), and 30% were ATG (Met) alleles (RR). (5 RR) | V15a V15b V15c V15d V15e | 5 samples Homozygote RR allele (5 RR) | V21a V21b V21c V21d V21e | 5 samples Homozygote RR allele (5 RR) |
| V4a V4b V4c V4d V4e | 5 samples Homozygote RR (Ile/Ile) (CTG→ATA) (5 RR) | V10a V10b V10c V10d V10e | Of the 10 alleles, 60% were RR (Ile/Ile), 20% were ATG RR (Met) allele (RR), and 20% were CTG (Leu) S allele. 3 RR, 2SR or 4RR, 1SS | V16a V16b V16c V16d V16e | 5 samples Homozygote RR allele (5 RR) | V22a V22b V22c V22d V22e | 5 samples Homozygote RR (Ile/Ile) (CTG→ATA) (5 RR) |
| V5a V5b V5c V5d V5e | 5 samples Homozygote RR (Ile/Ile) (CTG→ATA) (5 RR) | V11a V11b V11c V11d V11e | Of the 10 alleles, 60% were RR and 40% were CTG (Leu) S alleles 2SS+3RR or 4SR+1RR or 1SS+2SR+2RR | V17a V17b V17c V17d V17e | Of the 10 alleles, 80% were R, and 20% were CTG (Leu) S alleles. 3 RR, 2SR or 4RR, 1SS | The total number of mites being pooled was 22 × 5 = 110. There were 220 alleles for 110 diploid mites. When allele frequencies were evaluated, 95% of the alleles detected were resistant, while 5% of the alleles detected were sensitive. The populations were highly resistant to the drug. | |
| V6a V6b V6c V6d V6e | 5 samples Homozygote RR (Ile/Ile) (CTG→ATA) (5 RR) | V12a V12b V12c V12d V12e | 5 samples Homozygote RR allele (5 RR) | V18a V18b V18c V18d V18e | 10 allelden %80’i R alleli, %20’si CTG (Leu) S alleli. 3 RR, 2SR veya 4RR, 1SS. | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sorucu, A.; Çöl, B.; Dibek, E.; Babayeva, A. Assessing Phenotypic and Genotypic Resistance to Flumethrin in Varroa destructor Populations in Muğla, Türkiye. Insects 2025, 16, 548. https://doi.org/10.3390/insects16060548
Sorucu A, Çöl B, Dibek E, Babayeva A. Assessing Phenotypic and Genotypic Resistance to Flumethrin in Varroa destructor Populations in Muğla, Türkiye. Insects. 2025; 16(6):548. https://doi.org/10.3390/insects16060548
Chicago/Turabian StyleSorucu, Ali, Bekir Çöl, Esra Dibek, and Anara Babayeva. 2025. "Assessing Phenotypic and Genotypic Resistance to Flumethrin in Varroa destructor Populations in Muğla, Türkiye" Insects 16, no. 6: 548. https://doi.org/10.3390/insects16060548
APA StyleSorucu, A., Çöl, B., Dibek, E., & Babayeva, A. (2025). Assessing Phenotypic and Genotypic Resistance to Flumethrin in Varroa destructor Populations in Muğla, Türkiye. Insects, 16(6), 548. https://doi.org/10.3390/insects16060548






