A Comparative Morphological Study of the Ultrastructure of Antennal Sensilla in Sclerodermus guani (Hymenoptera: Bethylidae)
Simple Summary
Abstract
1. Introduction
2. Material and Method
2.1. Insects
2.2. Scanning Electron Microscopy (SEM) Photography
2.3. Sensilla Classification
2.4. Data Analysis
3. Results
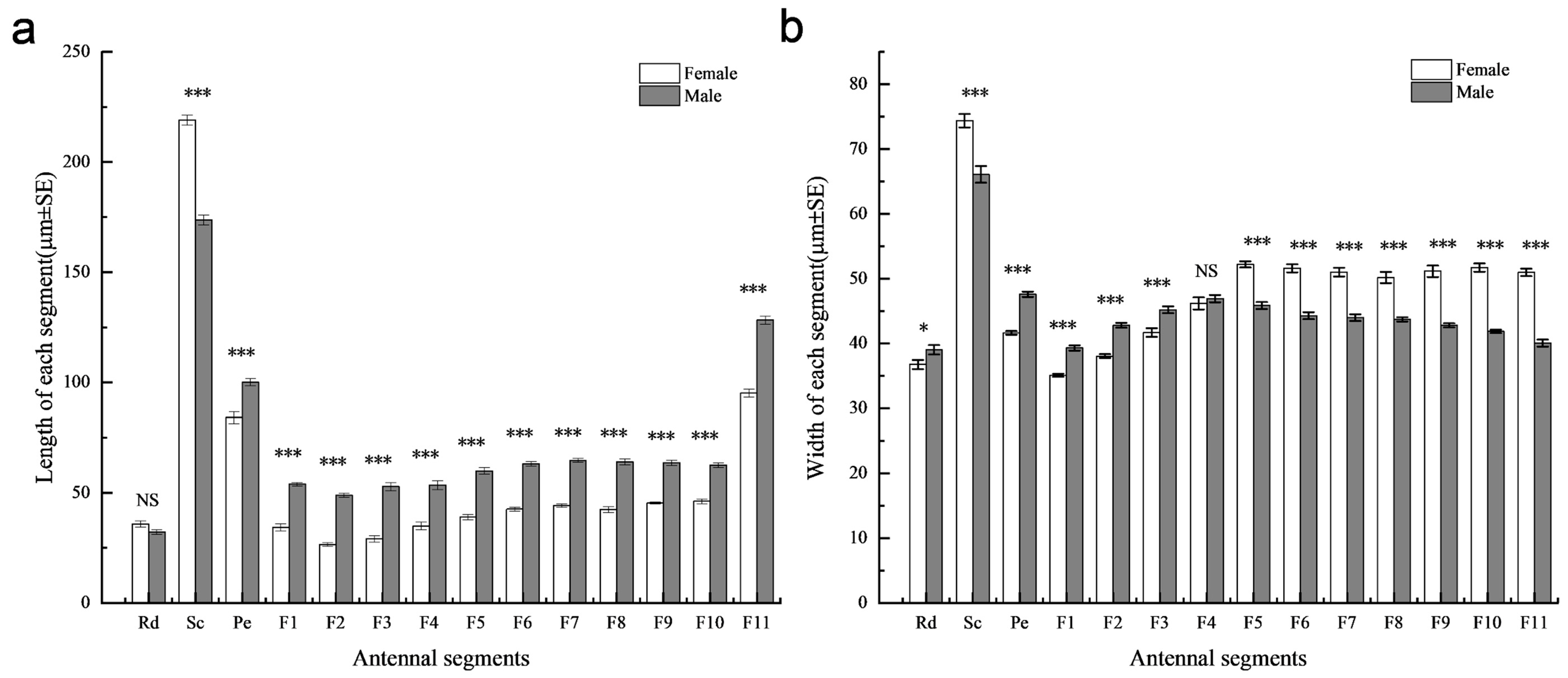
3.1. Comparison of the Antennae Between Female and Male Sclerodermus guani
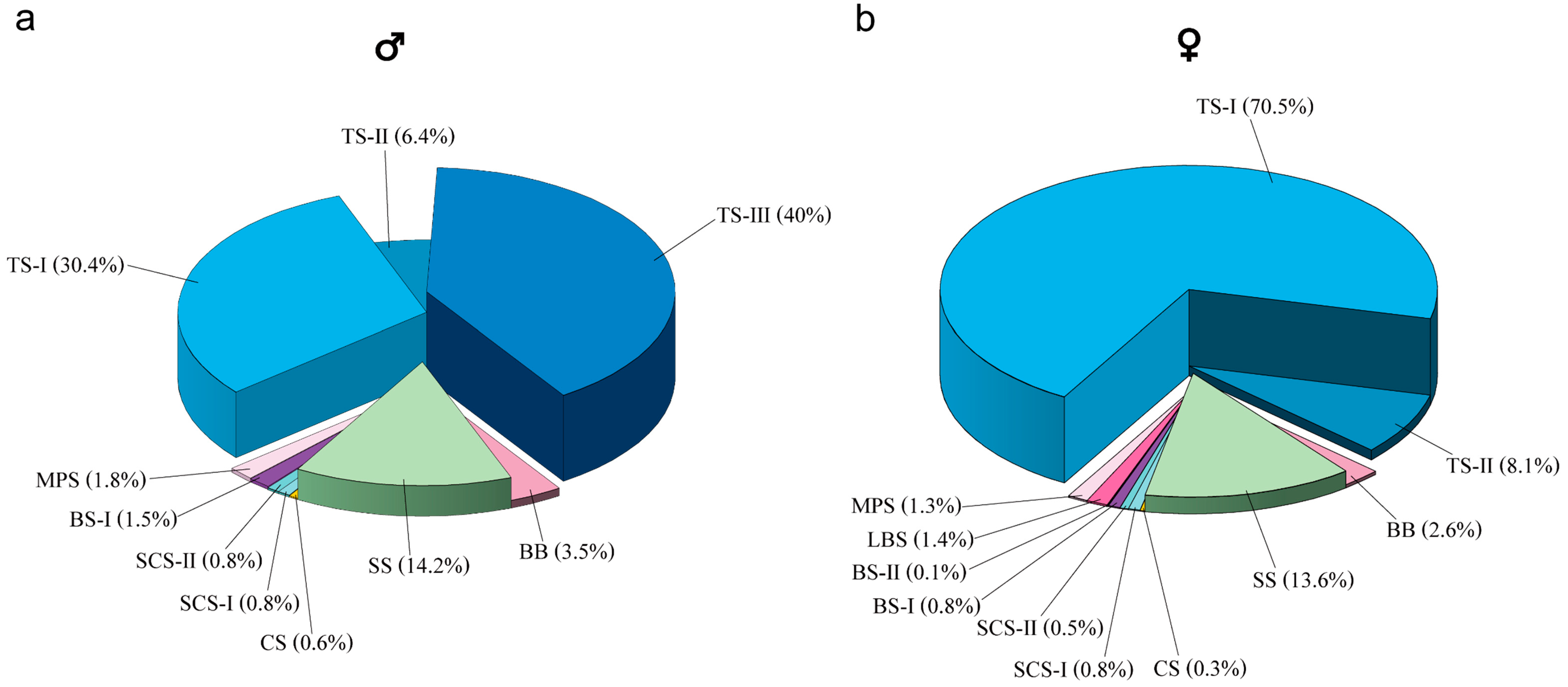
3.2. Comparison of the Number and Types of Sensilla Between Female and Male Sclerodermus guani
4. Discussion
4.1. Böhm Bristles (BB)
4.2. Trichoid Sensilla (TS)
4.3. Squamiform Sensilla (SS)
4.4. Multiporous Plate Sensilla (MPS)
4.5. Long Basiconic Sensilla (LBS)
4.6. Basiconica Sensilla (BS)
4.7. Styloconic Sensilla (SCS)
4.8. The Coeloconica Sensilla (CS)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stillwell, R.C.; Blanckenhorn, W.U.; Teder, T.; Davidowitz, G.; Fox, C.W. Sex differences in phenotypic plasticity affect variation in sexual size dimorphism in insects: From physiology to evolution. Annu. Rev. Entomol. 2010, 55, 227–245. [Google Scholar] [CrossRef]
- Das, P.; Chen, L.; Sharma, K.R.; Fadamiro, H.Y. Abundance of antennal chemosensilla in two parasitoid wasps with different degree of host specificity may explain sexual and species differences in their response to host-related volatiles. Microsc. Res. Tech. 2011, 74, 900–909. [Google Scholar] [CrossRef]
- Dahake, A.; Stöckl, A.L.; Foster, J.J.; Sane, S.P.; Kelber, A. The roles of vision and antennal mechanoreception in hawkmoth flight control. eLife 2018, 7, e37606. [Google Scholar] [CrossRef] [PubMed]
- Nowińska, A.; Brożek, J. Antennal sensory structures in water bugs of Nepoidea (Insecta: Hemiptera: Nepomorpha), their morphology and function. Zoomorphology 2019, 138, 307–319. [Google Scholar] [CrossRef]
- Nakano, M.; Morgan-Richards, M.; Trewick, S.A.; Clavijo-Mccormick, A. Chemical ecology and olfaction in short-horned grasshoppers (Orthoptera: Acrididae). J. Chem. Ecol. 2022, 48, 121–140. [Google Scholar] [CrossRef]
- Kim, J.Y.; Leal, W. Ultrastructure of pheromone-detecting sensillum placodeum of the Japanese beetle, Popillia japonica Newmann (Coleoptera: Scarabaeidae). Arthropod Struct. Dev. 2000, 29, 121–128. [Google Scholar] [CrossRef]
- Wechsler, S.P.; Bhandawat, V. Behavioral algorithms and neural mechanisms underlying odor-modulated locomotion in insects. J. Exp. Biol. 2023, 226, jeb200261. [Google Scholar] [CrossRef] [PubMed]
- Keil, T.A. Morphology and development of the peripheral olfactory organs. In Insect Olfaction; Springer: Berlin/Heidelberg, Germany, 1999; pp. 5–47. [Google Scholar]
- Van Baaren, J.; Boivin, G.; Le Lannic, J.; Nénon, J.P. Comparison of antennal sensilla of Anaphes victus and A. listronoti (Hymenoptera, Mymaridae), egg parasitoids of Curculionidae. Zoomorphology 1999, 119, 1–8. [Google Scholar] [CrossRef]
- Zhu, Q.; Wu, N.; Brożek, J.; Dai, W. Antennal morphology and sexual dimorphism of antennal sensilla in Callitettix versicolor (Fabricius)(Hemiptera: Cercopidae). Insects 2019, 10, 56. [Google Scholar] [CrossRef]
- Elgar, M.A.; Zhang, D.; Wang, Q.; Wittwer, B.; Pham, H.T.; Johnson, T.L.; Freelance, C.B.; Coquilleau, M. Focus: Ecology and evolution: Insect antennal morphology: The evolution of diverse solutions to odorant perception. Yale J. Biol. Med. 2018, 91, 457. [Google Scholar]
- Howard, R.W.; Charlton, M.; Charlton, R.E. Host-finding, host-recognition, and host-acceptance behavior of Cephalonomia tarsalis (Hymenoptera: Bethylidae). Ann. Entomol. Soc. Am. 1998, 91, 879–889. [Google Scholar] [CrossRef]
- Wajnberg, E.; Colazza, S. Chemical Ecology of Insect Parasitoids; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Meiners, T.; Peri, E. Chemical ecology of insect parasitoids: Essential elements for developing effective biological control programmes. In Chemical Ecology of Insect Parasitoids; Wiley: Hoboken, NJ, USA, 2013; pp. 191–224. [Google Scholar]
- Fürstenau, B.; Adler, C.; Schulz, H.; Hilker, M. Host habitat volatiles enhance the olfactory response of the larval parasitoid Holepyris sylvanidis to specifically host-associated cues. Chem. Senses 2016, 41, 611–621. [Google Scholar]
- Chiu-Alvarado, P.; Barrera, J.F.; Rojas, J.C. Attraction of Prorops nasuta (Hymenoptera: Bethylidae), a parasitoid of the coffee berry borer (Coleoptera: Curculionidae), to host-associated olfactory cues. Ann. Entomol. Soc. Am. 2009, 102, 166–171. [Google Scholar] [CrossRef]
- Vinson, S.B. The general host selection behavior of parasitoid Hymenoptera and a comparison of initial strategies utilized by larvaphagous and oophagous species. Biol. Control 1998, 11, 79–96. [Google Scholar] [CrossRef]
- Steiner, S.; Mumm, R.; Ruther, J. Courtship pheromones in parasitic wasps: Comparison of bioactive and inactive hydrocarbon profiles by multivariate statistical methods. J. Chem. Ecol. 2007, 33, 825–838. [Google Scholar] [CrossRef]
- Hallem, E.A.; Dahanukar, A.; Carlson, J.R. Insect odor and taste receptors. Annu. Rev. Entomol. 2006, 51, 113–135. [Google Scholar] [CrossRef] [PubMed]
- Gokhman, V.; Krutov, V. On the external structure of the male antennae in the subfamily Ichneumoninae (Hymenoptera, Ichneumonidae) and related groups. Zool. Zhurnal 1996, 75, 1182–1194. [Google Scholar]
- Wang, Y.; Brożek, J.; Dai, W. Functional morphology and sexual dimorphism of antennae of the pear lace bug Stephanitis nashi (Hemiptera: Tingidae). Zool. Anz. 2020, 286, 11–19. [Google Scholar] [CrossRef]
- Song, L.M.; Wang, X.M.; Huang, J.P.; Zhu, F.; Jiang, X.; Zhang, S.G.; Ban, L.P. Ultrastructure and morphology of antennal sensilla of the adult diving beetle Cybister japonicus Sharp. PLoS ONE 2017, 12, e0174643. [Google Scholar] [CrossRef]
- Zauli, A.; Maurizi, E.; Carpaneto, G.M.; Chiari, S.; Merivee, E.; Svensson, G.; Di Giulio, A. Scanning electron microscopy analysis of the antennal sensilla in the rare saproxylic beetle Elater ferrugineus (Coleoptera: Elateridae). Ital. J. Zoöl. 2016, 83, 338–350. [Google Scholar] [CrossRef][Green Version]
- Meng, J.; Huang, D.; Xiao, J.; Bu, W. Antennal sensilla of fig wasps (Hymenoptera: Agaonidae): Function-driven elaboration in females and degeneration in males. Ann. Entomol. Soc. Am. 2016, 109, 99–105. [Google Scholar] [CrossRef]
- Boivin, G. Phenotypic plasticity and fitness in egg parasitoids. Neotropical Entomol. 2010, 39, 457–463. [Google Scholar] [CrossRef]
- Quicke, D.L. Biology, Systematics, Evolution and Ecology of Braconid and Ichneumonid Parasitoid Wasps; Wiley Blackwell: Hoboken, NJ, USA, 2015. [Google Scholar]
- Chen, J.; Cheng, H.Z. Advances in applied research on Scleroderma spp. Chin. J. Biol. Control 2000, 16, 166. [Google Scholar]
- Li, X.; Lu, D.; Liu, X.; Zhang, Q.; Zhou, X. Ultrastructural characterization of olfactory sensilla and immunolocalization of odorant binding and chemosensory proteins from an ectoparasitoid Scleroderma guani (Hymenoptera: Bethylidae). Int. J. Biol. Sci. 2011, 7, 848. [Google Scholar] [CrossRef]
- Lai, Y.; Wang, Y.; Wang, X.; Yang, Z.; Tang, Y.; Qin, R.; Zhang, Y.; Zhang, Y. A field test of controlling the pine wilt disease with the technique of isolating Monochamus alternatus on forest spot and releasing parasitoid Sclerodermus guani. Chin. J. Biol. Control 2012, 28, 460. [Google Scholar]
- Wang, L.; Li, C.; Luo, Y.; Wang, G.; Dou, Z.; Haq, I.U.; Shang, S.; Cui, M. Current and future control of the wood-boring pest Anoplophora glabripennis. Insect Sci. 2023, 30, 1534–1551. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xu, B.; Li, L.; Sun, J. Host-size mediated trade-off in a parasitoid Sclerodermus harmandi. PLoS ONE 2011, 6, e23260. [Google Scholar] [CrossRef]
- Liu, Z.; Deng, L.; Wang, S.; Zheng, X.; Holyoak, M.; Wickham, J.D.; Tao, Y.; Sun, J. Mortality risk promotes cooperation of wasps when paralysing hosts. Anim. Behav. 2021, 172, 135–144. [Google Scholar] [CrossRef]
- Xu, K.Q.; Xu, F.Y.; Wang, M.M.; Zhao, J.L.; Jiang, Q.G.; Zhang, P.; Xu, D.; He, R.Q.; Jiang, X.G. The techniques of Scleroderma guani Xiao et Wu to control pine sawyer beetles. J. Nanjing For. Univ. 2002, 26, 48–52. [Google Scholar]
- Cheng, S.; Yu, J.; Zhu, X.; Wu, Y.; Huang, H.; Ding, Z. Parasitism of Scleroderma guani on the larva of Monochamus alternatus in the xylem of Pinus massoniana in lab and in the field. Forest Pest Dis. 2007, 26, 9–11. [Google Scholar]
- Yao, W.; Yang, Z. Studies on biological control of Anoplophora glabripennis (Coleoptera: Cerambycidae) with a parasitoid, Sclerodermus guani (Hymenoptera: Bethylidae). J. Environ. Entomol. 2008, 30, 127–134. [Google Scholar]
- Kai, H.; Zhiqiang, X.; Pingli, D. The parasitizing behavior of Scleroderma guani Xiao et Wu (Hymenoptera: Bethylidae) wasps on Tenebrio molitor pupae. Kun Chong Xue Bao Acta Entomol. Sinica 2006, 49, 454–460. [Google Scholar]
- Zuji, Z.; Chuihui, Z.; Wei, Y.; Demin, Y.; Weijun, Y. A preliminary study on the bionomics of Scleroderma sichuanensis (Hymenoptera, Bethylidae). Sci. Silvae Sin. 1997, 33, 475–480. [Google Scholar]
- Zhongqi, Y.; Xiaoyi, W.; Zhaoyao, D.; Yanlong, Z.; Yi’nan, Z.; Liangming, C.; Ke, W. Sclerodermus alternatusi (Hymenoptera: Bethylidae), a new species from China, parasitizing Monochamus alternatus (Coleoptera: Cerambycidae). Zool. Syst. 2024, 49, 258–266. [Google Scholar]
- Yang, Z.Q.; Wang, X.Y.; Yao, Y.X.; Gould, J.R.; Cao, L.M. A new species of Sclerodermus (Hymenoptera: Bethylidae) parasitizing Agrilus planipennis (Coleoptera: Buprestidae) from China, with a key to Chinese species in the genus. Ann. Entomol. Soc. Am. 2012, 105, 619–627. [Google Scholar] [CrossRef]
- Kieffer, J.J. Description de nouveaux Dryininae et Bethylinae. Du Musée Civique de Gênes. Ann. Mus. Civico Stor. Nat. Genova 1904, 1, 351–412. [Google Scholar]
- Tian, S.P.; Xu, Z.Q. Scanning electron microscopic observation of sensilla on the antenna of Scleroderma guani. Entomol. Knowl. 2003, 1, 59–62. [Google Scholar]
- Yang, P.; Li, Z.B.; Yang, D.R.; Peng, Y.Q.; Kjellberg, F. Comparison of the antennal sensilla of females of four fig-wasps associated with Ficus auriculata. Acta Oecol. 2018, 90, 99–108. [Google Scholar] [CrossRef]
- Schneider, D. Insect antennae. Annu. Rev. Entomol. 1964, 9, 103–122. [Google Scholar] [CrossRef]
- Van Baaren, J.; Boivin, G.; Bourdais, D.; Roux, O. Antennal sensilla of hymenopteran parasitic wasps: Variations linked to host exploitation behavior. Modern Res. Educ. Top. Microsc. 2007, 1, 345–352. [Google Scholar]
- Ando, T.; Sekine, S.; Inagaki, S.; Misaki, K.; Badel, L.; Moriya, H.; Sami, M.M.; Itakura, Y.; Chihara, T.; Kazama, H. Nanopore formation in the cuticle of an insect olfactory sensillum. Curr. Biol. 2019, 29, 1512–1520.e1516. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Hu, X.; Zhou, Z.J.; Jiang, X.J.; Zhan, H.J. The ultrastructure observation of the antenna of Scleroderma sichuanensis. J. Liaoning For. Sci. Technol. 2006, 02, 4–7. [Google Scholar]
- Hazarika, H.N.; Khanikor, B. Integration of morphological and molecular taxonomic characters for identification of Odontoponera denticulata (Hymenoptera: Formicidae: Ponerinae) with the description of the antennal sensilla. Zool. Anz. 2021, 293, 89–100. [Google Scholar] [CrossRef]
- Wei, K.; Wang, X.Y. Ultrastructure of the Antennal Sensilla of Parasitic Wasps, Sclerodermus pupariae. For. Res. 2023, 36, 1–11. [Google Scholar]
- Zhou, C.X.; Sun, X.; Mi, F.; Chen, J.; Wang, M.Q. Antennal sensilla in the parasitoid Sclerodermus sp.(Hymenoptera: Bethylidae). J. Insect Sci. 2015, 15, 36. [Google Scholar] [CrossRef] [PubMed]
- Masini, P.; Piersanti, S.; Lupi, D.; Salerno, G.; Rebora, M. Antennal chemoreceptors in the European ectoparasitoid Sclerodermus cereicollis (Hymenoptera: Bethylidae). Microsc. Res. Tech. 2024, 87, 2275–2291. [Google Scholar] [CrossRef]
- Ochieng, S.; Park, K.; Zhu, J.; Baker, T. Functional morphology of antennal chemoreceptors of the parasitoid Microplitis croceipes (Hymenoptera: Braconidae). Arthropod Struct. Dev. 2000, 29, 231–240. [Google Scholar] [CrossRef]
- Li, Z.; Yang, P.; Peng, Y.; Yang, D. Ultrastructure of antennal sensilla of female Ceratosolen solmsi marchali (Hymenoptera: Chalcidoidea: Agaonidae: Agaoninae). Can. Entomol. 2009, 141, 463–477. [Google Scholar] [CrossRef]
- Chen, Q.; Li, L.; Kang, G.; Zuo, T.; Zhang, K.; Song, L.; Zhu, X.; Ke, H.; Huang, M.; Zhao, J. Morphology and ultrastructure of antennal sensilla of the parasitic wasp Baryscapus dioryctriae (Hymenoptera: Eulophidae). Microsc. Res. Tech. 2023, 86, 12–27. [Google Scholar] [CrossRef]
- Liao, A.Q.; Song, C.C.; Sun, Z.; Wang, M.Q. SEM-based analysis of antennal sensilla of the parasitoid, Encarsia formosa Gahan (Hymenoptera: Aphelinidae). Orient. Insects 2019, 53, 251–266. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Chen, D.Y.; Chao, X.T.; Dong, Z.S.; Huang, Z.Y.; Zheng, X.L.; Lu, W. Ultrastructure of antennal sensilla of Copidosomopsis nacoleiae (Eady)(Hymenoptera: Chalcidoidea: Encyrtidae), a parasitoid of Diaphania angustalis (Snellen) (Lepidoptera: Crambidae). Microsc. Res. Tech. 2021, 84, 2149–2165. [Google Scholar] [CrossRef] [PubMed]
- Merivee, E.; Ploomi, A.; Rahi, M.; Bresciani, J.; Ravn, H.P.; Luik, A.; Sammelselg, V. Antennal sensilla of the ground beetle Bembidion properans Steph.(Coleoptera, Carabidae). Micron 2002, 33, 429–440. [Google Scholar] [CrossRef]
- Silva, I.M.D.; Pereira, K.D.S.; Spranghers, T.; Zanuncio, J.C.; Serrão, J.E. Antennal sensilla and sexual dimorphism of the parasitoid Trichospilus pupivorus (Hymenoptera: Eulophidae). Microsc. Microanal. 2016, 22, 913–921. [Google Scholar] [CrossRef]
- Shields, V.D.; Hildebrand, J.G. Recent advances in insect olfaction, specifically regarding the morphology and sensory physiology of antennal sensilla of the female sphinx moth Manduca sexta. Microsc. Res. Tech. 2001, 55, 307–329. [Google Scholar] [CrossRef]
- Zhang, X.M.; Wang, S.; Li, S.; Luo, C.; Li, Y.X.; Zhang, F. Comparison of the antennal sensilla ultrastructure of two cryptic species in Bemisia tabaci. PLoS ONE 2015, 10, e0121820. [Google Scholar] [CrossRef]
- Yang, H.Y.; Zheng, L.X.; Zhang, Z.F.; Zhang, Y.; Wu, W.J. The structure and morphologic changes of antennae of Cyrtorhinus lividipennis (Hemiptera: Miridae: Orthotylinae) in different instars. PLoS ONE 2018, 13, e0207551. [Google Scholar] [CrossRef] [PubMed]
- Taszakowski, A.; Masłowski, A.; Daane, K.M.; Brożek, J. Closer view of antennal sensory organs of two Leptoglossus species (Insecta, Hemiptera, Coreidae). Sci. Rep. 2023, 13, 617. [Google Scholar] [CrossRef]
- Wang, X.Y.; Yang, Z.Q.; Gould, J.R. Sensilla on the antennae, legs and ovipositor of Spathius agrili Yang (Hymenoptera: Braconidae), a parasitoid of the emerald ash borer Agrilus planipennis Fairmaire (Coleoptera: Buprestidae). Microsc. Res. Tech. 2010, 73, 560–571. [Google Scholar] [CrossRef]
- Barlin, M.R.; Vinson, S.B. The multiporous plate sensillum and its potential use in braconid systematics (Hymenoptera: Braconidae). Can. Entomol. 1981, 113, 931–938. [Google Scholar] [CrossRef]
- Ware, A.B.; Compton, S.G. Repeated evolution of elongate multiporous plate sensilla in female fig wasps (Hymenoptera: Agaonidae: Agaoninae). Proc. K. Ned. Akad. Wet. 1992, 95, 275–292. [Google Scholar]
- Gao, Y.; Luo, L.Z.; Hammond, A. Antennal morphology, structure and sensilla distribution in Microplitis pallidipes (Hymenoptera: Braconidae). Micron 2007, 38, 684–693. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.N.; Peng, Y.; Lu, Z.-Y.; Dhiloo, K.H.; Zheng, Y.; Shan, S.; Li, R.-J.; Zhang, Y.-J.; Guo, Y.Y. Cloning and expression profile of ionotropic receptors in the parasitoid wasp Microplitis mediator (Hymenoptera: Braconidae). J. Insect Physiol. 2016, 90, 27–35. [Google Scholar] [CrossRef]
- Yang, L.L.; Wang, B.; Shen, J.; Wang, G.R. Comparative morphology and plant volatile responses of antennal sensilla in Cinara cedri (Hemiptera: Lachninae), Eriosoma lanigerum (Hemiptera: Eriosomatinae), and Therioaphis trifolii (Hemiptera: Calaphidinae). Front. Cell. Neurosci. 2023, 17, 1162349. [Google Scholar] [CrossRef] [PubMed]
- Altner, H.; Loftus, R. Ultrastructure and function of insect thermo-and hygroreceptors. Ann. Rev. Entomol. 1985, 30, 273–295. [Google Scholar] [CrossRef]
- Yao, C.A.; Ignell, R.; Carlson, J.R. Chemosensory coding by neurons in the coeloconic sensilla of the Drosophila antenna. J. Neurosci. 2005, 25, 8359–8367. [Google Scholar] [CrossRef]
- Benton, R.; Vannice, K.S.; Gomez-Diaz, C.; Vosshall, L.B. Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell 2009, 136, 149–162. [Google Scholar] [CrossRef]
- Teder, T.; Kaasik, A.; Taits, K.; Tammaru, T. Why do males emerge before females? Sexual size dimorphism drives sexual bimaturism in insects. Biol. Rev. 2021, 96, 2461–2475. [Google Scholar] [CrossRef]

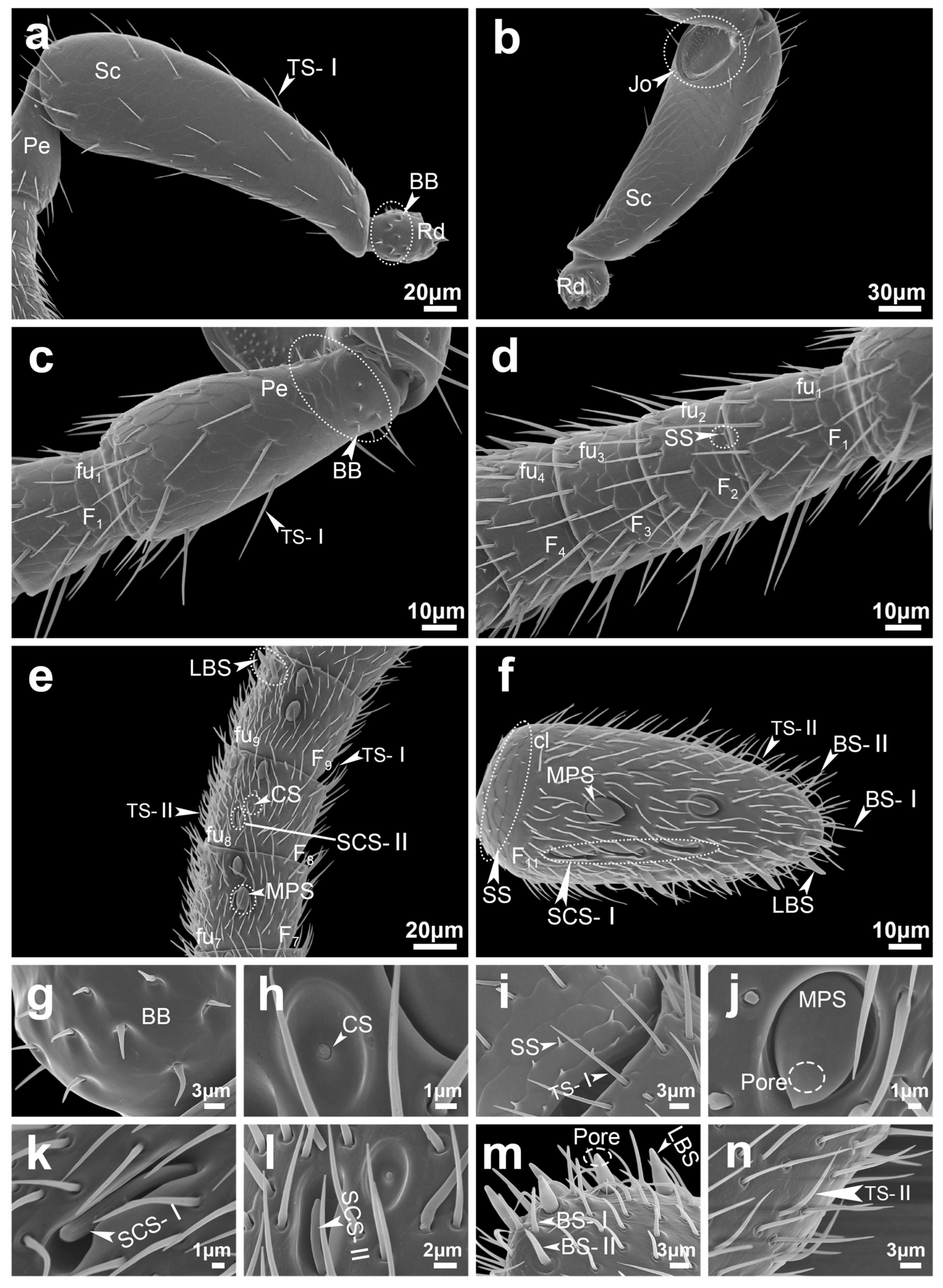
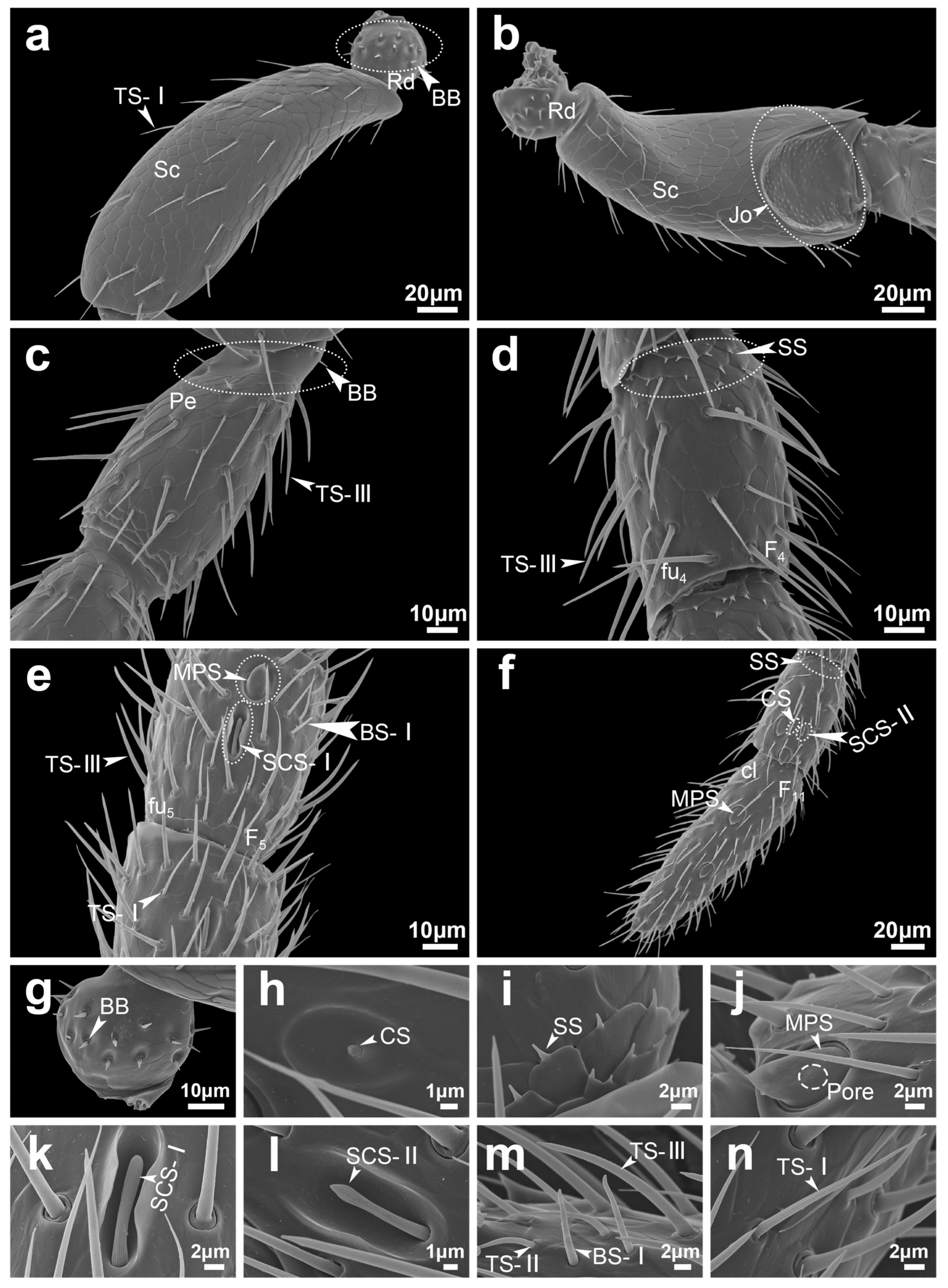

| Abbreviation | Name |
|---|---|
| Rd | Radicula |
| Sc | Scape |
| Pe | Pedicel |
| Fu | Funicle |
| Cl | Clava |
| Fl | Flagellum |
| Jo | Wide membranous joint between the scape and the pedicel |
| fu1-fu10 | 1–10 Funicle |
| F1-F11 | 1–11 Flagellomeres |
| BB | Böhm bristles |
| TS-I | Trichodea sensilla type I |
| TS-II | Trichodea sensilla type II |
| TS-III | Trichodea sensilla type III |
| MPS | Multiporous plate sensilla |
| CS | Coeloconica sensilla |
| SS | Squamiform sensilla |
| BS-I | Basiconica sensilla type I |
| BS-II | Basiconica sensilla type II |
| LBS | Long basiconica sensilla |
| SCS-I | Styloconic sensilla type I |
| SCS-II | Styloconic sensilla type II |
| Sensilla | Subtype | Genders | Rd | Sc | Pe | Flagellomeres | Total | p | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 | F10 | F11 | ||||||||
| BB | - | ♂ | 22 ± 0.42 | 11 ± 0 | - | - | - | - | - | - | - | - | - | - | - | - | 33 ± 0.42 | 0.699 |
| ♀ | 22 ± 0.31 | 11 ± 0 | - | - | - | - | - | - | - | - | - | - | - | - | 33 ± 0.31 | |||
| TS | I | ♂ | - | 46 ± 0.56 | 14 ± 0.23 | 11 ± 0.56 | 12 ± 0.48 | 11 ± 0.48 | 14 ± 0.56 | 14 ± 0.7 | 17 ± 0.49 | 23 ± 0.58 | 25 ± 0.31 | 25 ± 0.67 | 33 ± 0.92 | 45 ± 1.07 | 290 ± 2.23 | <0.01 |
| ♀ | 54 ± 1.12 | 40 ± 1.45 | 30 ± 0.6 | 40 ± 1.17 | 49 ± 1.1 | 68 ± 1.41 | 75 ± 1.89 | 71 ± 1.37 | 82 ± 1.2 | 83 ± 1.51 | 85 ± 1.08 | 84 ± 2 | 141 ± 1.94 | 901 ± 8.5 | ||||
| II | ♂ | - | - | - | - | - | - | 4 ± 0.21 | 7 ± 0.33 | 8 ± 0.21 | 7 ± 0.34 | 7 ± 0.26 | 7 ± 0.42 | 8 ± 0.22 | 14 ± 0.42 | 61 ± 1.05 | <0.01 | |
| ♀ | 8 ± 0.31 | 11 ± 0.33 | 12 ± 0.54 | 12 ± 0.42 | 12 ± 0.48 | 12 ± 0.48 | 14 ± 0.26 | 22 ± 0.62 | 103 ± 1.33 | |||||||||
| III | ♂ | - | - | 31 ± 0.72 | 24 ± 0.8 | 26 ± 1.2 | 29 ± 1.18 | 32 ± 0.61 | 30 ± 0.9 | 33 ± 1.15 | 31 ± 0.76 | 33 ± 0.77 | 31 ± 0.68 | 32 ± 0.89 | 51 ± 1.15 | 382 ± 6.04 | - | |
| ♀ | - | - | - | - | - | - | - | - | - | - | - | - | - | |||||
| MPS | - | ♂ | - | - | - | - | - | 1 ± 0 | 2 ± 0 | 2 ± 0 | 2 ± 0 | 2 ± 0 | 2 ± 0 | 2 ± 0 | 2 ± 0 | 2 ± 0 | 17 ± 0 | 1 |
| ♀ | - | 1 ± 0 | 1 ± 0 | 2 ± 0 | 2 ± 0 | 2 ± 0 | 2 ± 0 | 3 ± 0 | 4 ± 0 | 17 ± 0 | ||||||||
| CS | - | ♂ | - | - | - | - | - | - | - | 1 ± 0 | 1 ± 0 | 1 ± 0 | 1 ± 0 | 1 ± 0 | 1 ± 0 | - | 6 ± 0 | <0.01 |
| ♀ | - | - | 1 ± 0 | 1 ± 0 | 1 ± 0 | 1 ± 0 | - | 4 ± 0 | ||||||||||
| SCS | I | ♂ | - | - | - | - | - | 1 ± 0 | 1 ± 0 | 1 ± 0 | 1 ± 0 | 1 ± 0 | 1 ± 0 | 1 ± 0 | 1 ± 0 | - | 8 ± 0 | <0.01 |
| ♀ | - | 1 ± 0 | 1 ± 0 | 1 ± 0 | 1 ± 0 | 1 ± 0 | 1 ± 0 | 1 ± 0 | 3 ± 0 | 10 ± 0 | ||||||||
| II | ♂ | - | - | - | - | - | 1 ± 0 | 1 ± 0 | 1 ± 0 | 1 ± 0 | 1 ± 0 | 1 ± 0 | 1 ± 0 | 1 ± 0 | - | 8 ± 0 | <0.01 | |
| ♀ | - | 1 ± 0 | 1 ± 0 | 1 ± 0 | 1 ± 0 | 1 ± 0 | 1 ± 0 | 1 ± 0 | - | 7 ± 0 | ||||||||
| BS | I | ♂ | - | - | - | - | - | - | 2 ± 0 | 2 ± 0 | 2 ± 0 | 2 ± 0 | 2 ± 0 | 2 ± 0 | 2 ± 0 | - | 14 ± 0 | <0.01 |
| ♀ | - | - | - | - | - | - | - | 10 ± 0.42 | 10 ± 0.42 | |||||||||
| II | ♂ | - | - | - | - | - | - | - | - | - | - | |||||||
| ♀ | - | - | - | - | - | - | - | 1 | 1 ± 0 | |||||||||
| LBS | - | ♂ | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| ♀ | 2 ± 0 | 2 ± 0 | 2 ± 0 | 2 ± 0 | 2 ± 0 | 2 ± 0 | 6 ± 0 | 18 ± 0 | ||||||||||
| SS | - | ♂ | - | - | - | - | 4 ± 0.4 | 5 ± 0.37 | 8 ± 0.45 | 12 ± 0.56 | 12 ± 0.89 | 17 ± 0.6 | 17 ± 0.56 | 16 ± 0.09 | 16 ± 0.58 | 27 ± 0.65 | 135 ± 2.65 | <0.01 |
| ♀ | 5 ± 0.29 | 6 ± 0.65 | 8 ± 0.65 | 13 ± 0.58 | 15 ± 0.65 | 18 ± 0.58 | 18 ± 0.65 | 20 ± 0.65 | 24 ± 0.65 | 49 ± 1.11 | 174 ± 3.65 | |||||||
| Sensilla Type | Sensilla Length/μm | Basal Width (Pit Diameter)/μm | Shape | Tip | Surface | Socket | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| ♂ | ♀ | p | ♂ | ♀ | p | |||||
| BB | 5.45 ± 0.48 | 4.5 ± 0.28 | 0.101 | 0.97 ± 0.04 | 0.94 ± 0.06 | 0.635 | peg (straight) | sharp | smooth | concave |
| TS-I (Sc, Pe) | 22.35 ± 0.68 | 23.49 ± 0.71 | 0.255 | 1.18 ± 0.04 | 1.22 ± 0.05 | 0.593 | hair (straight) | sharp | grooved | concave |
| TS-I (Fl) | 16.51 ± 0.24 | 13.22 ± 0.35 | <0.01 | 0.97 ± 0.03 | 0.82 ± 0.03 | <0.01 | ||||
| TS-II | 16.07 ± 0.45 | 11.88 ± 0.19 | <0.01 | 0.87 ± 0.01 | 0.86 ± 0.02 | 0.631 | hair (curved) | sharp | smooth | convex |
| TS-III | 24.34 ± 0.47 | - | - | 2.06 ± 0.05 | - | - | hair (straight) | sharp | smooth | convex |
| MPS | 11.25 ± 0.24 | 10.19 ± 0.27 | <0.01 | 6.29 ± 0.14 | 5.89 ± 0.21 | 0.113 | round | sharp/blunt | smooth | concave |
| CS | 7.27 ± 0.19 | 6.64 ± 0.19 | <0.05 | 4.41 ± 0.22 | 3.97 ± 0.16 | 0.139 | round | blunt | grooved | concave |
| SS | 1.69 ± 0.25 | 0.91 ± 0.16 | <0.01 | 0.75 ± 0.08 | 0.54 ± 0.06 | <0.01 | peg (straight) | sharp | smooth | convex |
| BS-I | 9.93 ± 0.13 | 8.38 ± 0.39 | <0.01 | 1.03 ± 0.04 | 1.05 ± 0.05 | 0.743 | peg (straight) | blunt | grooved | convex |
| BS-II | - | 11.08 ± 0.23 | - | - | 1.70 ± 0.02 | - | peg (straight) | sharp | smooth | convex |
| LBS | - | 8.15 ± 0.27 | - | - | 2.45 ± 0.05 | - | peg (straight) | blunt | grooved | convex |
| SCS-I | 12.32 ± 0.18 | 12.77 ± 0.16 | 0.083 | 1.74 ± 0.02 | 1.29 ± 0.11 | <0.01 | rod (slightly curved) | blunt | grooved | convex |
| SCS-II | 6.96 ± 0.19 | 7.05 ± 0.13 | 0.697 | 0.93 ± 0.02 | 0.92 ± 0.02 | 0.452 | rod (slightly curved) | Sharp | grooved | convex |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Wang, C.; Yu, X.; Wang, B.; Liu, Z. A Comparative Morphological Study of the Ultrastructure of Antennal Sensilla in Sclerodermus guani (Hymenoptera: Bethylidae). Insects 2025, 16, 547. https://doi.org/10.3390/insects16050547
Chen Y, Wang C, Yu X, Wang B, Liu Z. A Comparative Morphological Study of the Ultrastructure of Antennal Sensilla in Sclerodermus guani (Hymenoptera: Bethylidae). Insects. 2025; 16(5):547. https://doi.org/10.3390/insects16050547
Chicago/Turabian StyleChen, Youcheng, Chunxia Wang, Xiuju Yu, Bo Wang, and Zhudong Liu. 2025. "A Comparative Morphological Study of the Ultrastructure of Antennal Sensilla in Sclerodermus guani (Hymenoptera: Bethylidae)" Insects 16, no. 5: 547. https://doi.org/10.3390/insects16050547
APA StyleChen, Y., Wang, C., Yu, X., Wang, B., & Liu, Z. (2025). A Comparative Morphological Study of the Ultrastructure of Antennal Sensilla in Sclerodermus guani (Hymenoptera: Bethylidae). Insects, 16(5), 547. https://doi.org/10.3390/insects16050547






