Effects of Confinement and Wheat Variety on the Performance of Two Aphid Species
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Aphid Populations and Age-Synchronised Cohorts
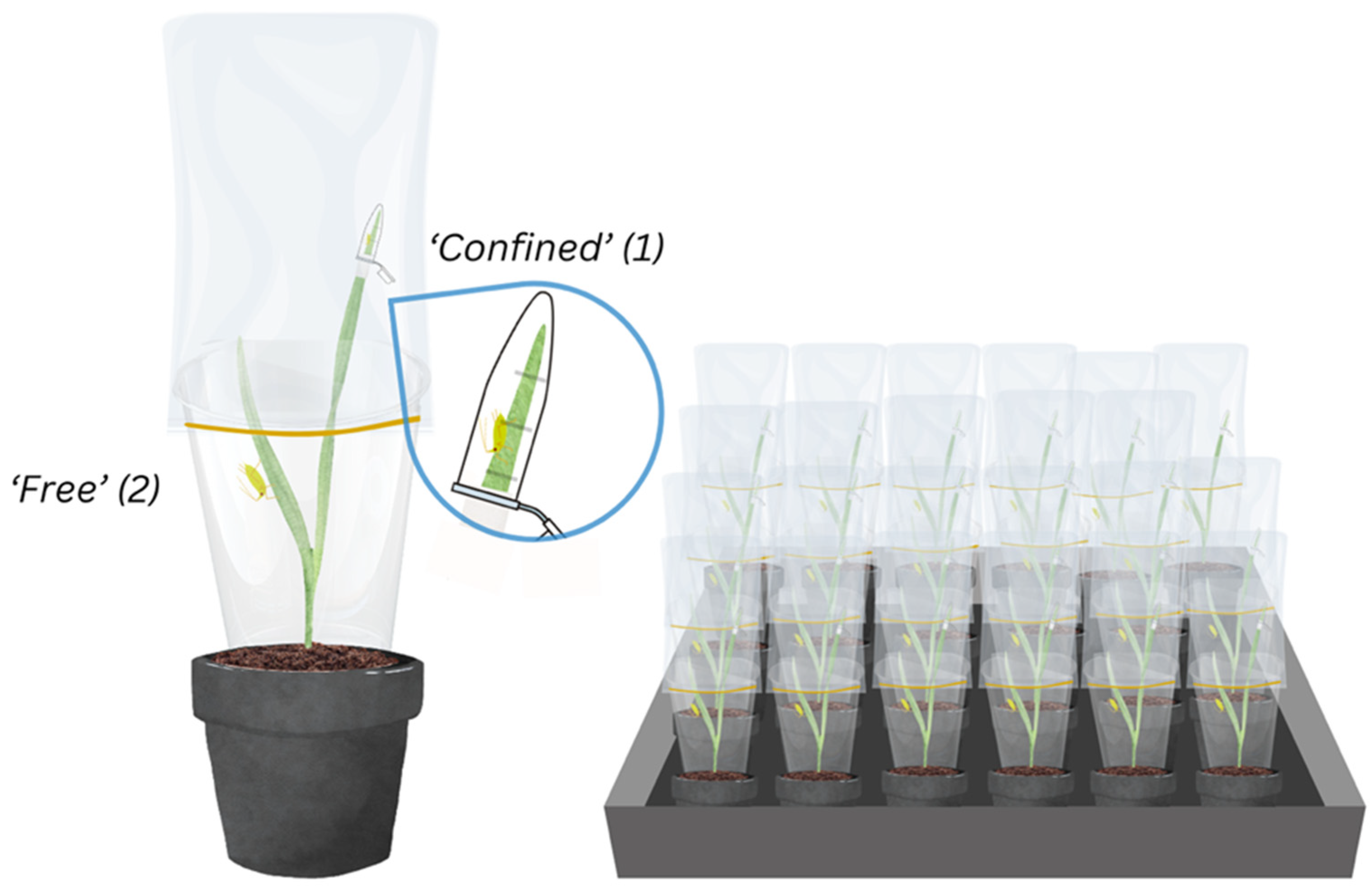
2.3. Experimental Design
2.4. Statistical Analysis
3. Results
3.1. English Grain Aphid (Sitobion avenae)
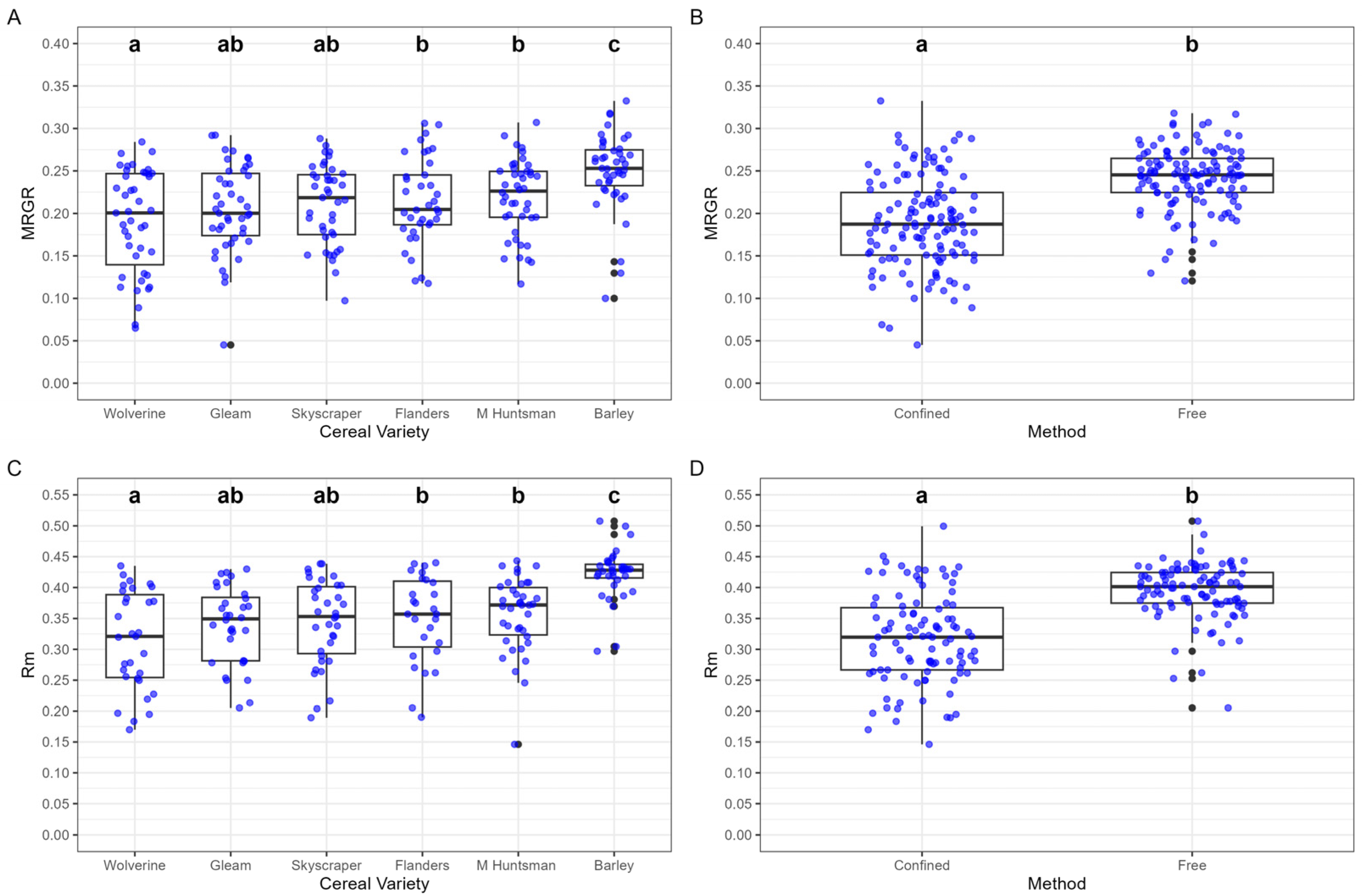
3.1.1. Mean Relative Growth Rate (MRGR)
3.1.2. Intrinsic Rate of Increase (rm)
3.2. Bird Cherry-Oat Aphid (Rhopalosiphum padi)
3.2.1. Mean Relative Growth Rate (MRGR)
3.2.2. Intrinsic Rate of Increase (rm)
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dedryver, C.-A.; Le Ralec, A.; Fabre, F. The Conflicting Relationships Between Aphids and Men: A Review of Aphid Damage and Control Strategies. C. R. Biol. 2010, 333, 539–553. [Google Scholar] [CrossRef] [PubMed]
- Nancarrow, N.; Aftab, M.; Hollaway, G.; Rodoni, B.; Trębicki, P. Yield Losses Caused by Barley Yellow Dwarf Virus-PAV Infection in Wheat and Barley: A Three-Year Field Study in South-Eastern Australia. Microorganisms 2021, 9, 645. [Google Scholar] [CrossRef] [PubMed]
- Gaunce, G.M.; Bockus, W.W. Estimating Yield Losses Due to Barley Yellow Dwarf on Winter Wheat in Kansas Using Disease Phenotypic Data. Plant Health Prog. 2015, 16, 1–6. [Google Scholar] [CrossRef]
- Zhao, C.; Zhou, M. Effect of Barley Yellow Dwarf Virus (BYDV) on Barley: A Precise Assessment of Reductions in Yield Components under Variable Disease Severities. Plant Dis. 2025, 109, 37–42. [Google Scholar] [CrossRef]
- Namara, L.M.; Lacey, S.; Kildea, S.; Schughart, M.; Walsh, L.; Doyle, D.; Gaffney, M.T. Barley Yellow Dwarf Virus in Winter Barley: Control in Light of Resistance Issues and Loss of Neonicotinoid Insecticides. Ann. Appl. Biol. 2024, 186, 132–142. [Google Scholar] [CrossRef]
- Foster, S.P.; Paul, V.L.; Slater, R.; Warren, A.; Denholm, I.; Field, L.M.; Williamson, M.S. A Mutation (L1014F) in the Voltage-Gated Sodium Channel of the Grain Aphid, Sitobion avenae, Is Associated with Resistance to Pyrethroid Insecticides. Pest Manag. Sci. 2014, 70, 1249–1253. [Google Scholar] [CrossRef]
- Walsh, L.; Ferrari, E.; Foster, S.; Gaffney, M.T. Evidence of Pyrethroid Tolerance in the Bird Cherry-Oat Aphid Rhopalosiphum padi in Ireland. Outlooks Pest Manag. 2020, 31, 5–9. [Google Scholar] [CrossRef]
- Shah, F.; Coulter, J.A.; Ye, C.; Wu, W. Yield Penalty Due to Delayed Sowing of Winter Wheat and the Mitigatory Role of Increased Seeding Rate. Eur. J. Agron. 2020, 119, 126120. [Google Scholar] [CrossRef]
- Slafer, G.A.; Savin, R.; Sadras, V.O. Wheat Yield Is Not Causally Related to the Duration of the Growing Season. Eur. J. Agron. 2023, 148, 126885. [Google Scholar] [CrossRef]
- Plaza-Bonilla, D.; Álvaro-Fuentes, J.; Bareche, J.; Masgoret, A.; Cantero-Martínez, C. Delayed Sowing Improved Barley Yield in a No-till Rainfed Mediterranean Agroecosystem. Agron. J. 2017, 109, 1249–1260. [Google Scholar] [CrossRef]
- Greenslade, A.F.C.; Ward, J.L.; Martin, J.L.; Corol, D.I.; Clark, S.J.; Smart, L.E.; Aradottir, G.I. Triticum monococcum Lines with Distinct Metabolic Phenotypes and Phloem-Based Partial Resistance to the Bird Cherry-Oat Aphid Rhopalosiphum padi. Ann. Appl. Biol. 2016, 168, 435–449. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, M.P.; Borlaug, N.E. Impacts of Breeding on International Collaborative Wheat Improvement. J. Agric. Sci. 2006, 144, 3–17. [Google Scholar] [CrossRef]
- Awmack, C.S.; Leather, S.R. Host Plant Quality and Fecundity in Herbivorous Insects. Annu. Rev. Entomol. 2002, 47, 817–844. [Google Scholar] [CrossRef] [PubMed]
- Borg, A.N.; Vuts, J.; Caulfield, J.C.; Birkett, M.A. Metabolite-Based Resistance in Wheat Varieties to Aphid Virus Vectors: Progress and Future Opportunities. Pest Manag. Sci. 2025. [Google Scholar] [CrossRef]
- Borg, A.N.; Vuts, J.; Caulfield, J.C.; Withall, D.M.; Foulkes, M.J.; Birkett, M.A. Characterisation of Aphid Antixenosis in Aphid-Resistant Ancestor Wheat, Triticum monococcum. Pest Manag. Sci. 2024. [Google Scholar] [CrossRef]
- Wojciechowicz-Zytko, E.; Emden, H.F. Are Aphid Mean Relative Growth Rate and Intrinsic Rate of Increase Likely to Show a Correlation in Plant Resistance Studies? J. Appl. Entomol. 1995, 119, 405–409. [Google Scholar] [CrossRef]
- Khan, M.; Port, G. Performance of Clones and Morphs of Two Cereal Aphids on Wheat Plants with High and Low Nitrogen Content. Entomol. Sci. 2008, 11, 159–165. [Google Scholar] [CrossRef]
- Dahlin, I.; Ninkovic, V. Aphid Performance and Population Development on Their Host Plants Is Affected by Weed–Crop Interactions. J. Appl. Ecol. 2013, 50, 1281–1288. [Google Scholar] [CrossRef]
- Moreno-Delafuente, A.; Viñuela, E.; Fereres, A.; Medina, P.; Trębicki, P. Simultaneous Increase in CO2 and Temperature Alters Wheat Growth and Aphid Performance Differently Depending on Virus Infection. Insects 2020, 11, 459. [Google Scholar] [CrossRef]
- Birch, L.C. The Intrinsic Rate of Natural Increase of an Insect Population. J. Anim. Ecol. 1948, 17, 15–26. [Google Scholar] [CrossRef]
- Unger, L.M.; Quisenberry, S.S. Categorization of Six Wheat Plant Introduction Lines for Resistance to the Russian Wheat Aphid (Homoptera: Aphididae). J. Econ. Entomol. 1997, 90, 1408–1413. [Google Scholar] [CrossRef]
- Fiebig, M.; Poehling, H.-M.; Borgemeister, C. Barley Yellow Dwarf Virus, Wheat, and Sitobion avenae: A Case of Trilateral Interactions. Entomol. Exp. Appl. 2004, 110, 11–21. [Google Scholar] [CrossRef]
- Leather, S.R.; Dixon, A.F.G. Aphid Growth and Reproductive Rates. Entomol. Exp. Appl. 1984, 35, 137–140. [Google Scholar] [CrossRef]
- Klueken, A.M.; Poehling, H.-M.; Hau, B. Attractiveness and Host Suitability of Winter Wheat Cultivars for Cereal Aphids (Homoptera: Aphididae). J. Plant Dis. Prot. 2008, 115, 114–121. [Google Scholar] [CrossRef]
- Sotherton, N.W.; van Emden, H.F. Laboratory Assessments of Resistance to the Aphids Sitobion avenae and Metopolophium dirhodum in Three Triticum Species and Two Modern Wheat Cultivars. Ann. Appl. Biol. 1982, 101, 99–107. [Google Scholar] [CrossRef]
- Martinez-Chavez, L.M.; Roberts, J.M.; Karley, A.J.; Shaw, B.; Pope, T.W. The Clip Cage Conundrum: Assessing the Interplay of Confinement Method and Aphid Genotype in Fitness Studies. Insect Sci. 2024, 31, 1591–1602. [Google Scholar] [CrossRef]
- Nalam, V.J.; Han, J.; Pitt, W.J.; Acharya, S.R.; Nachappa, P. Location, Location, Location: Feeding Site Affects Aphid Performance by Altering Access and Quality of Nutrients. PLoS ONE 2021, 16, e0245380. [Google Scholar] [CrossRef]
- Crafts-Brandner, S.J.; Chu, C.-C. Insect Clip Cages Rapidly Alter Photosynthetic Traits of Leaves. Crop Sci. 1999, 39, 1896–1899. [Google Scholar] [CrossRef]
- Haas, J.; Lozano, E.R.; Poppy, G.M. A Simple, Light Clip-Cage for Experiments with Aphids. Agric. For. Entomol. 2018, 20, 589–592. [Google Scholar] [CrossRef]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A Decimal Code for the Growth Stages of Cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Hu, X.-S.; Zhang, Z.-F.; Zhu, T.-Y.; Song, Y.; Wu, L.-J.; Liu, X.-F.; Zhao, H.-Y.; Liu, T.-X. Maternal Effects of the English Grain Aphids Feeding on the Wheat Varieties with Different Resistance Traits. Sci. Rep. 2018, 8, 7344. [Google Scholar] [CrossRef]
- Jiménez-Martínez, E.S.; Bosque-Pérez, N.A.; Berger, P.H.; Zemetra, R.S. Life History of the Bird Cherry-Oat Aphid, Rhopalosiphum padi (Homoptera: Aphididae), on Transgenic and Untransformed Wheat Challenged with Barley Yellow Dwarf Virus. J. Econ. Entomol. 2004, 97, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, I.J.; White, P.F. Simple Estimation of Intrinsic Increase Rates for Aphids and Tetranychid Mites. J. Appl. Ecol. 1977, 14, 757. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. arXiv 2014. [Google Scholar] [CrossRef]
- Estimated Marginal Means, Aka Least-Squares Means. Available online: https://rvlenth.github.io/emmeans/ (accessed on 11 April 2025).
- Leather, S.R.; Dixon, A.F.G. The Effect of Cereal Growth Stage and Feeding Site on the Reproductive Activity of the Bird-cherry Aphid, Rhopalosiphum padi. Ann. Appl. Biol. 1981, 97, 135–141. [Google Scholar] [CrossRef]
- Yin, W.; Xue, Q.; Su, L.; Feng, X.; Feng, X.; Zheng, Y.; Hoffmann, A.A. Microhabitat Separation Between the Pest Aphids Rhopalosiphum padi and Sitobion avenae: Food Resource or Microclimate Selection? J. Pest Sci. 2021, 94, 795–804. [Google Scholar] [CrossRef]
- Ma, G.; Bai, C.-M.; Wang, X.-J.; Majeed, M.Z.; Ma, C.-S. Behavioural Thermoregulation Alters Microhabitat Utilization and Demographic Rates in Ectothermic Invertebrates. Anim. Behav. 2018, 142, 49–57. [Google Scholar] [CrossRef]
- Kou, X.; Bai, S.; Luo, Y.; Yu, J.; Guo, H.; Wang, C.; Zhang, H.; Chen, C.; Liu, X.; Ji, W. Construction of a Modified Clip Cage and Its Effects on the Life-History Parameters of Sitobion avenae (Fabricius) and Defense Responses of Triticum aestivum. Insects 2022, 13, 777. [Google Scholar] [CrossRef]
- Aradottir, G.I.; Martin, J.L.; Clark, S.J.; Pickett, J.A.; Smart, L.E. Searching for Wheat Resistance to Aphids and Wheat Bulb Fly in the Historical Watkins and Gediflux Wheat Collections. Ann. Appl. Biol. 2017, 170, 179–188. [Google Scholar] [CrossRef]
- Stout, M.J. Reevaluating the Conceptual Framework for Applied Research on Host-Plant Resistance. Insect Sci. 2013, 20, 263–272. [Google Scholar] [CrossRef]
- Tous-Fandos, A.; Gallinger, J.; Enting, A.; Chamorro-Lorenzo, L.; Sans, F.X.; Ninkovic, V. Effect of Plant Identity in Wheat Mixtures on English Grain Aphid (Sitobion avenae) Control. J. Appl. Entomol. 2024, 149, 132–140. [Google Scholar] [CrossRef]
- Bourke, P.M.; Evers, J.B.; Bijma, P.; van Apeldoorn, D.F.; Smulders, M.J.M.; Kuyper, T.W.; Mommer, L.; Bonnema, G. Breeding beyond Monoculture: Putting the ‘Intercrop’ into Crops. Front. Plant Sci. 2021, 12, 734167. [Google Scholar] [CrossRef] [PubMed]
- Tanguy, S.; Dedryver, C.-A. Reduced BYDV–PAV Transmission by the Grain Aphid in a Triticum monococcum Line. Eur. J. Plant Pathol. 2009, 123, 281–289. [Google Scholar] [CrossRef]
- Lamb, R.J.; Migui, S.M.; Lamb, R.J. Patterns of Resistance to Three Cereal Aphids Among Wheats in the Genus Triticum (Poaceae). Bull. Entomol. Res. 2003, 93, 323–333. [Google Scholar] [CrossRef]
- Eleftherianos, I.; Vamvatsikos, P.; Ward, D.; Gravanis, F. Changes in the Levels of Plant Total Phenols and Free Amino Acids Induced by Two Cereal Aphids and Effects on Aphid Fecundity. J. Appl. Entomol. 2006, 130, 15–19. [Google Scholar] [CrossRef]
- Mousseau, T.A.; Dingle, H. Maternal Effects in Insect Life Histories. Annu. Rev. Entomol. 1991, 36, 511–534. [Google Scholar] [CrossRef]
- Hales, F.D.; Wilson, A.C.C.; Sloane, M.A.; Simon, J.-C.; le Gallic, J.-F.; Sunnucks, P. Lack of Detectable Genetic Recombination on the X Chromosome during the Parthenogenetic Production of Female and Male Aphids. Genet. Res. 2002, 79, 203–209. [Google Scholar] [CrossRef]
- Robinson, J. Conditioning Host Plant Affects Antixenosis and Antibiosis to Russian Wheat Aphid (Homoptera: Aphididae). J. Econ. Entomol. 1993, 86, 602–606. [Google Scholar] [CrossRef]
- Martín, B.; Fereres, A. Evaluation of a Choice-Test Method to Assess Resistance of Melon to Aphis gossypii Glover (Homoptera: Aphididae) by Comparison with Conventional Antibiosis and Antixenosis Trials. Appl. Entomol. Zool. 2003, 38, 405–411. [Google Scholar] [CrossRef]

| Breeder | Wheat Variety | End Use Group 1 | Parents |
|---|---|---|---|
| Desprez | Flanders | Old (1976–1983) | Champlein × FD 2816-348 |
| Syngenta | Gleam | Hard group 4 | Kielder × Hereford |
| Plant Breeding Institute | Maris Huntsman | Old (1972–1983) | [(CI 12633 × Cappellle Desprez × 5) × Hybrid 46] × Professeur Marchal |
| Limagrain | Skyscraper | Soft group 4 | (Cassius × NAWW29) × KWS Santiago |
| RAGT | Wolverine | Hard group 4 | (09TC2654 × Panorama) × Coronation |
| Biological Parameter | Measurement |
|---|---|
| Development Time (DT) | Duration from birth to adult emergence + 0.5 d [31] |
| Weight Gain (Wg) | Wa − Wn 1 [31] |
| Mean Relative Growth Rate (MRGR) | (lnWa − lnWn)/DT [23,31] |
| Intrinsic Rate of Natural Increase (rm) | 0.738 ln(fecundity)/DT [23,33] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leandro, M.E.D.A.; Roberts, J.M.; Dickin, E.T.; Pope, T.W. Effects of Confinement and Wheat Variety on the Performance of Two Aphid Species. Insects 2025, 16, 477. https://doi.org/10.3390/insects16050477
Leandro MEDA, Roberts JM, Dickin ET, Pope TW. Effects of Confinement and Wheat Variety on the Performance of Two Aphid Species. Insects. 2025; 16(5):477. https://doi.org/10.3390/insects16050477
Chicago/Turabian StyleLeandro, Maria Elisa D. A., Joe M. Roberts, Ed T. Dickin, and Tom W. Pope. 2025. "Effects of Confinement and Wheat Variety on the Performance of Two Aphid Species" Insects 16, no. 5: 477. https://doi.org/10.3390/insects16050477
APA StyleLeandro, M. E. D. A., Roberts, J. M., Dickin, E. T., & Pope, T. W. (2025). Effects of Confinement and Wheat Variety on the Performance of Two Aphid Species. Insects, 16(5), 477. https://doi.org/10.3390/insects16050477






