Hydrogen Stable Isotopes Indicate Reverse Migration of Fall Armyworm in North America
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Stable Hydrogen Isotopes Analysis
2.3. Inferences on the Probability of Origin
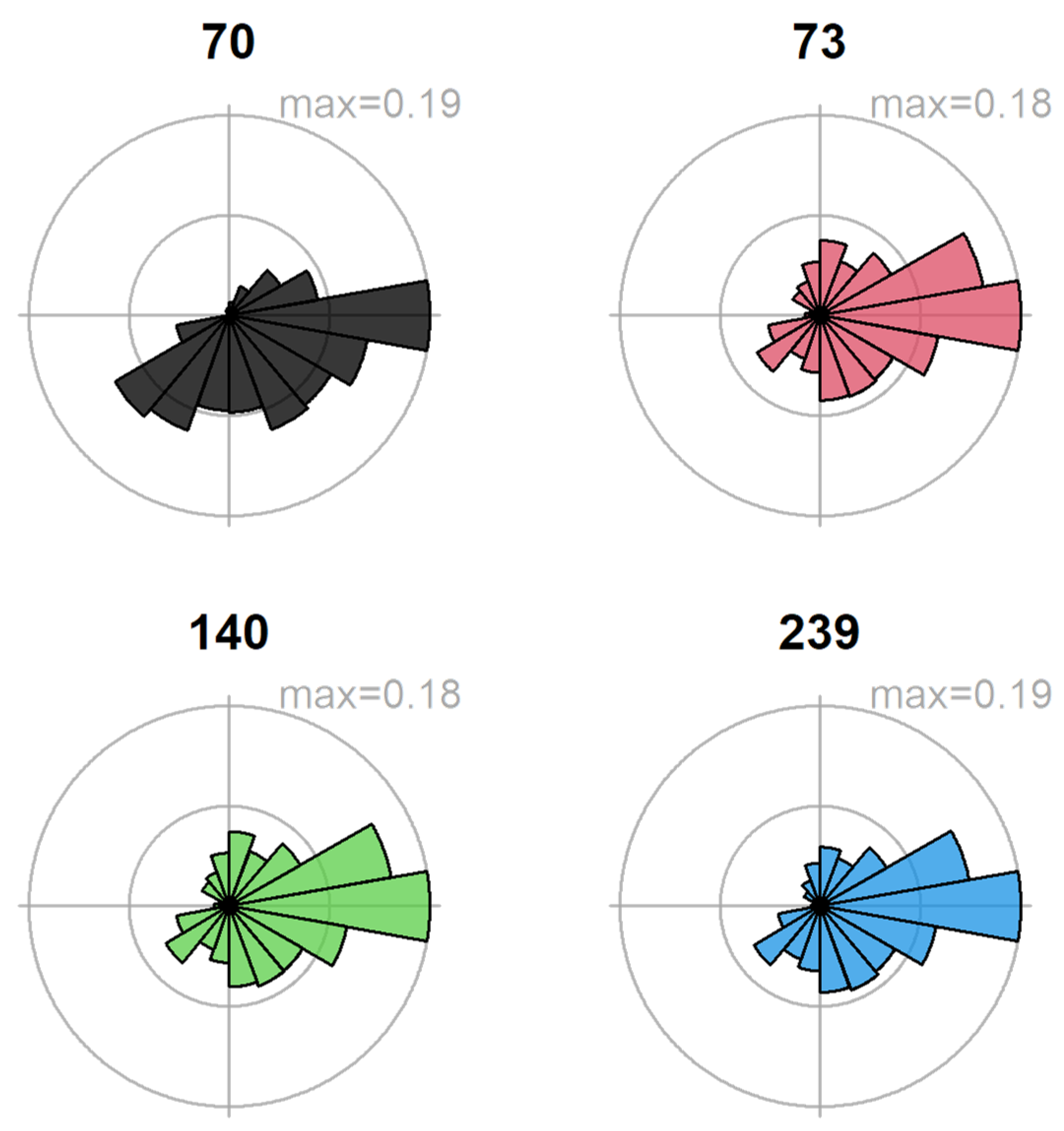
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FAW | Fall armyworm |
| IPM | Integrated pest management |
| IRM | Insect resistance management |
References
- Montezano, D.G.; Sosa-Gómez, D.; Specht, A.; Roque-Specht, V.F.; Sousa-Silva, J.C.; de Paula-Moraes, S.; Peterson, J.A.; Hunt, T. Host plants of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas. Afr. Entomol. 2018, 26, 286–300. [Google Scholar] [CrossRef]
- Goergen, G.; Kumar, P.L.; Sankung, S.B.; Togola, A.; Tamò, M. First Report of Outbreaks of the Fall Armyworm Spodoptera frugiperda (J E Smith) (Lepidoptera, Noctuidae), a New Alien Invasive Pest in West and Central Africa. PLoS ONE 2016, 11, e0165632. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.J. Migration and the Life History Strategy of the Fall Armyworm, Spodoptera frugiperda in the Western Hemisphere. Int. J. Trop. Insect Sci. 1987, 8, 543–549. [Google Scholar] [CrossRef]
- Rose, A.H.; Silversides, R.H.; Lindquist, O.H. Migration flight by an aphid, Rhopalosiphum maidis (Hemiptera: Aphididae), and a noctuid, Spodoptera frugiperda (Lepidoptera: Noctuidae). Can. Entomol. 1975, 107, 567–576. [Google Scholar] [CrossRef]
- Nagoshi, R.N.; Meagher, R.L. Review of Fall Armyworm (Lepidoptera: Noctuidae) Genetic Complexity and Migration. Fla. Entomol. 2008, 91, 546–554. [Google Scholar] [CrossRef]
- Dingle, H. Function of migration in the seasonal synchronization of insects. Entomol. Exp. Appl. 1982, 31, 36–48. [Google Scholar] [CrossRef]
- Luginbill, P. The Fall Army Worm; US Department of Agriculture: Washington, DC, USA, 1928. [Google Scholar]
- Tessnow, A.E.; Nagoshi, R.N.; Meagher, R.L.; Fleischer, S.J. Revisiting fall armyworm population movement in the United States and Canada. Front. Insect Sci. 2023, 3, 1104793. [Google Scholar] [CrossRef]
- Nagoshi, R.N.; Meagher, R.L.; Hay-Roe, M. Inferring the annual migration patterns of fall armyworm (Lepidoptera: Noctuidae) in the United States from mitochondrial haplotypes. Ecol. Evol. 2012, 2, 1458–1467. [Google Scholar] [CrossRef]
- Pair, S.; Raulston, J.; Rummel, D.; Westbrook, J.; Wolf, W.; Sparks, A.; Schuster, M. Development and production of corn earworm and fall armyworm in the Texas high plains: Evidence for reverse fall migration. Southwest. Entomol. 1987, 12, 89–99. [Google Scholar]
- Mitchell, E.R.; McNeil, J.N.; Westbrook, J.K.; Silvain, J.F.; Lalanne-Cassou, B.; Chalfant, R.B.; Pair, S.D.; Waddill, V.H.; Sotomayor-Rios, A.; Proshold, F.I. Seasonal Periodicity of Fall Armyworm, (Lepidoptera: Noctuidae) in the Caribbean Basin and Northward to Canada. J. Entomol. Sci. 1991, 26, 39–50. [Google Scholar] [CrossRef]
- Gould, F.; Blair, N.; Reid, M.; Rennie, T.; Lopez, J.; Micinski, S. Bacillus thuringiensis—Toxin resistance management: Stable isotope assessment of alternate host use by Helicoverpa zea. Proc. Natl. Acad. Sci. USA 2002, 99, 16581–16586. [Google Scholar] [CrossRef] [PubMed]
- Westbrook, J.K. Noctuid migration in Texas within the nocturnal aeroecological boundary layer. Integr. Comp. Biol. 2008, 48, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Paula-Moraes, S.V.; Calixto, E.S.; Santos, A.A.; Reay-Jones, F.P.F.; Reisig, D.D.; Farhan, Y.; Smith, J.L.; Hutchison, W.D. Continental-scale migration patterns and origin of Helicoverpa zea (Lepidoptera: Noctuidae) based on a biogeochemical marker. Environ. Entomol. 2024, 53, 487–497. [Google Scholar] [CrossRef]
- Walker, T.J. Migrating Lepidoptera: Are butterflies better than moths? Fla. Entomol. 1980, 63, 79–98. [Google Scholar] [CrossRef]
- Stinner, R.; Barfield, C.; Stimac, J.; Dohse, L. Dispersal and movement of insect pests. Annu. Rev. Entomol. 1983, 28, 319–335. [Google Scholar] [CrossRef]
- Hobson, K.A.; Wassenaar, L.I.; Taylor, O.R. Stable isotopes (δD and δ13C) are geographic indicators of natal origins of monarch butterflies in eastern North America. Oecologia 1999, 120, 397–404. [Google Scholar] [CrossRef]
- Wassenaar, L.; Hobson, K. Comparative equilibration and online technique for determination of non-exchangeable hydrogen of keratins for use in animal migration studies. Isot. Environ. Health Stud. 2003, 39, 211–217. [Google Scholar] [CrossRef]
- Ma, C.; Vander Zanden, H.B.; Wunder, M.B.; Bowen, G.J. assignR: An R package for isotope-based geographic assignment. Methods Ecol. Evol. 2020, 11, 996–1001. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2024. [Google Scholar]
- Hobson, K.A.; Kardynal, K.J.; Koehler, G. Expanding the Isotopic Toolbox to Track Monarch Butterfly (Danaus plexippus) Origins and Migration: On the Utility of Stable Oxygen Isotope (δ18O) Measurements. Front. Ecol. Evol. 2019, 7, 224. [Google Scholar] [CrossRef]
- Westbrook, J.; Fleischer, S.; Jairam, S.; Meagher, R.; Nagoshi, R. Multigenerational migration of fall armyworm, a pest insect. Ecosphere 2019, 10, e02919. [Google Scholar] [CrossRef]
- Lopez, J.R.; Winter, J.M.; Elliott, J.; Ruane, A.C.; Porter, C.; Hoogenboom, G.; Anderson, M.; Hain, C. Sustainable Use of Groundwater May Dramatically Reduce Irrigated Production of Maize, Soybean, and Wheat. Earth’s Future 2022, 10, e2021EF002018. [Google Scholar] [CrossRef]
- Kovač, Z.; Krevh, V.; Filipović, L.; Defterdarović, J.; Balaž, B.-I.; Filipović, V. Estimation of Precipitation Fraction in the Soil Water of the Hillslope Vineyard Using Stable Isotopes of Water. Water 2023, 15, 988. [Google Scholar] [CrossRef]
- Wolf, W.; Westbrook, J.; Raulston, J.; Pair, S.; Hobbs, S. Recent Airborne Radar Observations of Migrant Pests in the United States. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1990, 328, 619–630. [Google Scholar] [CrossRef]
- Beerwinkle, K.R.; Lopez, J.D.; Witz, J.A.; Schleider, P.G.; Eyster, R.S.; Lingren, P.D. Seasonal Radar and Meteorological Observations Associated with Nocturnal Insect Flight at Altitudes to 900 Meters. Environ. Entomol. 1994, 23, 676–683. [Google Scholar] [CrossRef]
- Chapman, J.W.; Drake, V.A.; Reynolds, D.R. Recent Insights from Radar Studies of Insect Flight. Annu. Rev. Entomol. 2011, 56, 337–356. [Google Scholar] [CrossRef]
- Storer, N.P.; Babcock, J.M.; Schlenz, M.; Meade, T.; Thompson, G.D.; Bing, J.W.; Huckaba, R.M. Discovery and Characterization of Field Resistance to Bt Maize: Spodoptera frugiperda (Lepidoptera: Noctuidae) in Puerto Rico. J. Econ. Entomol. 2010, 103, 1031–1038. [Google Scholar] [CrossRef]
- Huang, F.; Qureshi, J.A.; Meagher, R.L.; Reisig, D.D.; Head, G.P.; Andow, D.A.; Ni, X.; Kerns, D.; Buntin, G.D.; Niu, Y.; et al. Cry1F Resistance in Fall Armyworm Spodoptera frugiperda: Single Gene versus Pyramided Bt Maize. PLoS ONE 2014, 9, e112958. [Google Scholar] [CrossRef]
- Yang, F.; Wang, Z.; Kerns, D.L. Resistance of Spodoptera frugiperda to Cry1, Cry2, and Vip3Aa Proteins in Bt Corn and Cotton in the Americas: Implications for the Rest of the World. J. Econ. Entomol. 2022, 115, 1752–1760. [Google Scholar] [CrossRef]
- Gutiérrez-Moreno, R.; Mota-Sanchez, D.; Blanco, C.A.; Whalon, M.E.; Terán-Santofimio, H.; Rodriguez-Maciel, J.C.; DiFonzo, C. Field-Evolved Resistance of the Fall Armyworm (Lepidoptera: Noctuidae) to Synthetic Insecticides in Puerto Rico and Mexico. J. Econ. Entomol. 2019, 112, 792–802. [Google Scholar] [CrossRef]
- Mota-Sanchez, D.; Wise, J. Arthropod Pesticide Resistance Database. Available online: https://www.pesticideresistance.org/ (accessed on 18 November 2024).
- Dobush, G.R.; Ankney, C.D.; Krementz, D.G. The Effect of Apparatus, Extraction Time, and Solvent Type on Lipid Extractions of Snow Geese. Can. J. Zool. 1985, 63, 1917–1920. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calixto, E.S.; Paula-Moraes, S.V. Hydrogen Stable Isotopes Indicate Reverse Migration of Fall Armyworm in North America. Insects 2025, 16, 471. https://doi.org/10.3390/insects16050471
Calixto ES, Paula-Moraes SV. Hydrogen Stable Isotopes Indicate Reverse Migration of Fall Armyworm in North America. Insects. 2025; 16(5):471. https://doi.org/10.3390/insects16050471
Chicago/Turabian StyleCalixto, Eduardo S., and Silvana V. Paula-Moraes. 2025. "Hydrogen Stable Isotopes Indicate Reverse Migration of Fall Armyworm in North America" Insects 16, no. 5: 471. https://doi.org/10.3390/insects16050471
APA StyleCalixto, E. S., & Paula-Moraes, S. V. (2025). Hydrogen Stable Isotopes Indicate Reverse Migration of Fall Armyworm in North America. Insects, 16(5), 471. https://doi.org/10.3390/insects16050471






