Estrogen-Related Receptor Potential Target Genes in Silkworm (Bombyx mori): Insights into Metabolic Regulation
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Sample Collection and Preparation
2.2.1. Extraction of Tissue Protein
2.2.2. Extraction of Nuclear Protein
2.3. Western Blot
2.4. Bioinformatics Analysis
2.5. Electrophoretic Mobility Shift Assay
2.6. Vector Construction
2.7. Cell Transfection and Luciferase Assay
2.8. Statistical Analysis
3. Results
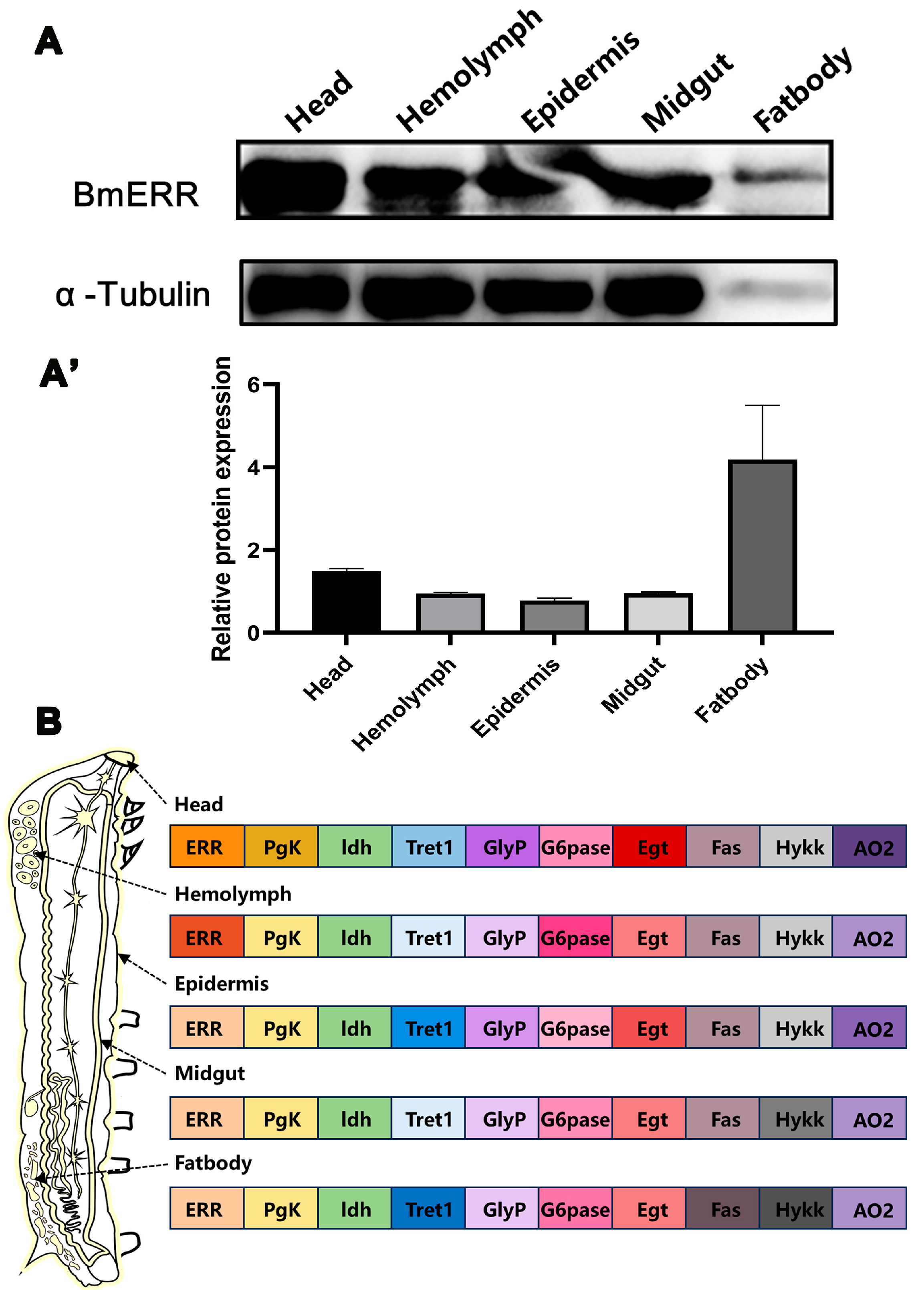
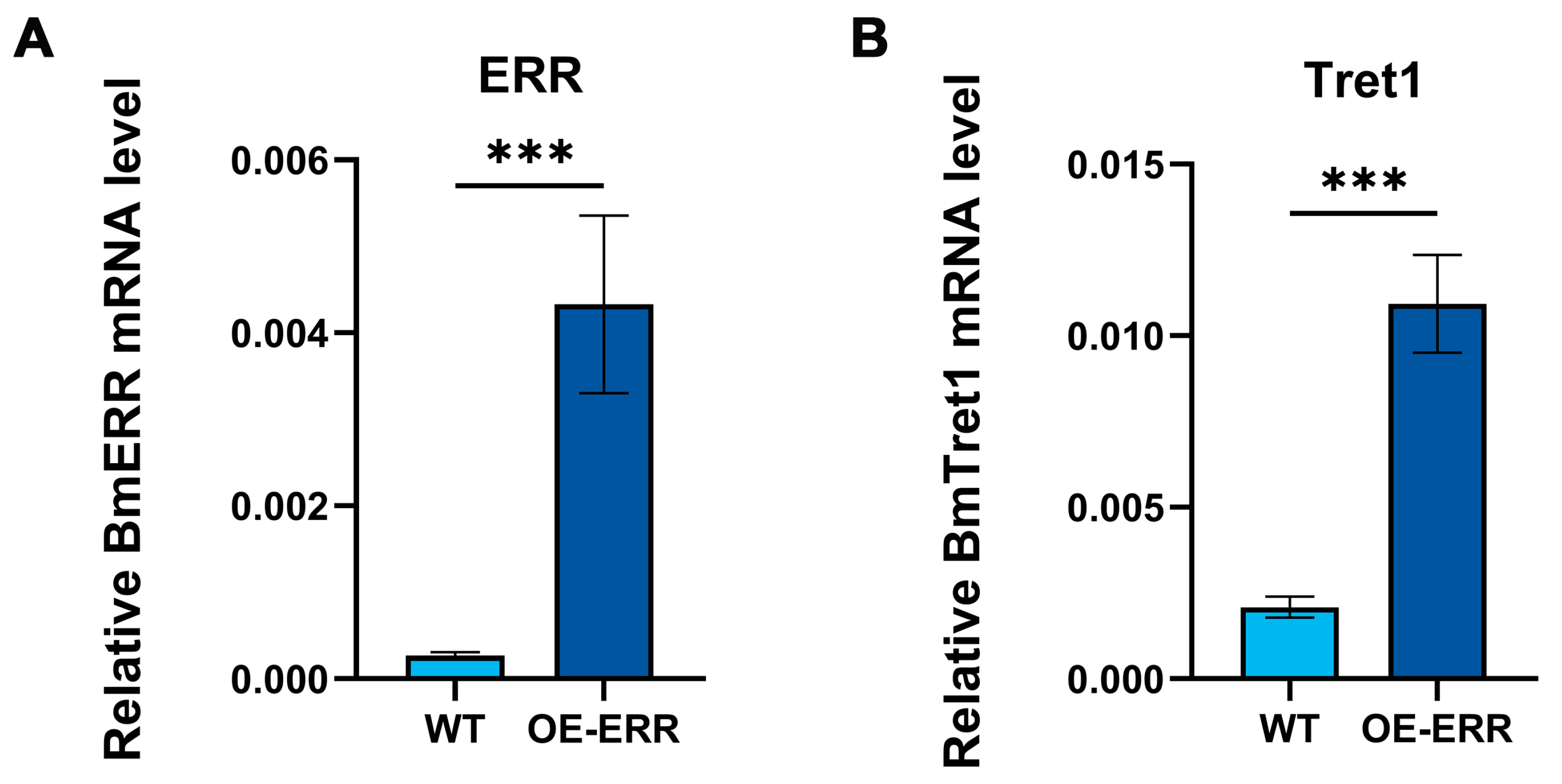
3.1. Detection of ERR Expression and Screening of Target Genes in Silkworm
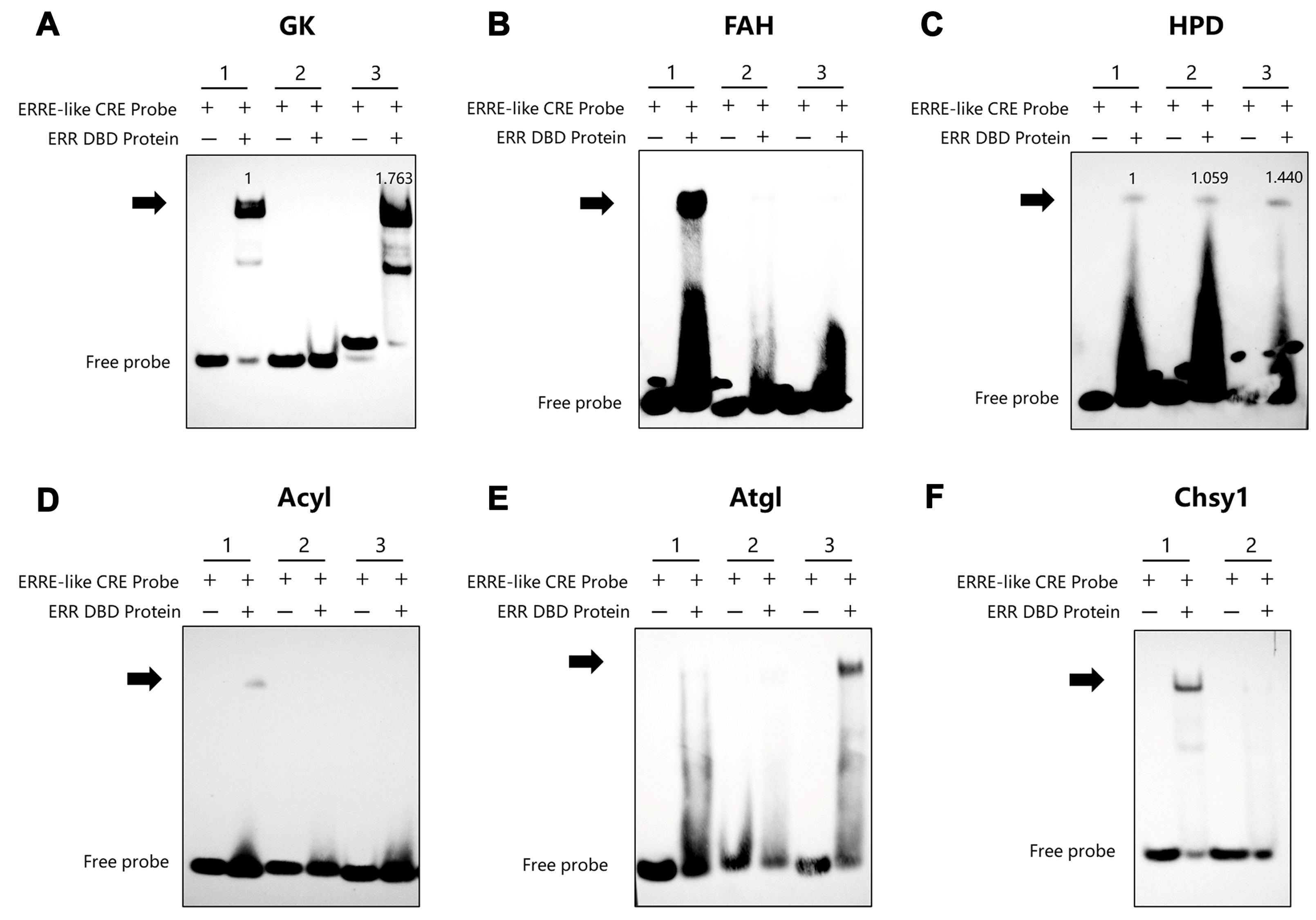
3.2. Identification of BmERR Target Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schreiber, S.N.; Emter, R.; Hock, M.B.; Knutti, D.; Cardenas, J.; Podvinec, M.; Oakeley, E.J.; Kralli, A. The Estrogen-Related Receptor Alpha (ERRalpha) Functions in PPARgamma Coactivator 1Alpha (PGC-1Alpha)-Induced Mitochondrial Biogenesis. Proc. Natl. Acad. Sci. USA 2004, 101, 6472–6477. [Google Scholar] [CrossRef]
- Giguere, V. Transcriptional Control of Energy Homeostasis by the Estrogen-Related Receptors. Endocr. Rev. 2008, 29, 677–696. [Google Scholar] [CrossRef] [PubMed]
- Sladek, R.; Bader, J.A.; Giguère, V. The Orphan Nuclear Receptor Estrogen-Related Receptor Alpha is a Transcriptional Regulator of the Human Medium-Chain Acyl Coenzyme a Dehydrogenase Gene. Mol. Cell. Biol. 1997, 17, 5400–5409. [Google Scholar] [CrossRef] [PubMed]
- Barry, J.B.; Laganiere, J.; Giguere, V. A Single Nucleotide in an Estrogen-Related Receptor Alpha Site Can Dictate Mode of Binding and Peroxisome Proliferator-Activated Receptor Gamma Coactivator 1Alpha Activation of Target Promoters. Mol. Endocrinol. 2006, 20, 302–310. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dufour, C.R.; Wilson, B.J.; Huss, J.M.; Kelly, D.P.; Alaynick, W.A.; Downes, M.; Evans, R.M.; Blanchette, M.; Giguere, V. Genome-Wide Orchestration of Cardiac Functions by the Orphan Nuclear Receptors ERRalpha and Gamma. Cell Metab. 2007, 5, 345–356. [Google Scholar] [CrossRef]
- Shen, G.; Wu, J.; Han, C.; Liu, H.; Xu, Y.; Zhang, H.; Lin, Y.; Xia, Q. Oestrogen-Related Receptor Reduces Vitellogenin Expression by Crosstalk with the Ecdysone Receptor Pathway in Female Silkworm, Bombyx mori. Insect Mol. Biol. 2018, 27, 454–463. [Google Scholar] [CrossRef]
- Jin, W.; Jia, Y.; Tan, E.; Xi, G. Relevance of Estrogen-Related Receptor Gene and Ecdysone Receptor Gene in Adult Testis of the Cricket Teleogryllus emma (Orthoptera: Gryllidae). Sci. Nat. 2017, 104, 97. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, M.; Zhang, G.; Liu, F.; Wang, H.; Guo, X.; Xu, B. Molecular Cloning, Expression, and Stress Response of the Estrogen-Related Receptor Gene (AccERR) from Apis Cerana Cerana. Sci. Nat. 2016, 103, 24. [Google Scholar] [CrossRef]
- Ostberg, T.; Jacobsson, M.; Attersand, A.; Mata, D.U.A.; Jendeberg, L. A Triple Mutant of the Drosophila ERR Confers Ligand-Induced Suppression of Activity. Biochemistry 2003, 42, 6427–6435. [Google Scholar] [CrossRef]
- Thornton, J.W.; Need, E.; Crews, D. Resurrecting the Ancestral Steroid Receptor: Ancient Origin of Estrogen Signaling. Science 2003, 301, 1714–1717. [Google Scholar] [CrossRef]
- Adams, M.D.; Celniker, S.E.; Holt, R.A.; Evans, C.A.; Gocayne, J.D.; Amanatides, P.G.; Scherer, S.E.; Li, P.W.; Hoskins, R.A.; Galle, R.F.; et al. The Genome Sequence of Drosophila melanogaster. Science 2000, 287, 2185–2195. [Google Scholar] [CrossRef] [PubMed]
- Huss, J.M.; Garbacz, W.G.; Xie, W. Constitutive Activities of Estrogen-Related Receptors: Transcriptional Regulation of Metabolism by the ERR Pathways in Health and Disease. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2015, 1852, 1912–1927. [Google Scholar] [CrossRef]
- Giguere, V.; Yang, N.; Segui, P.; Evans, R.M. Identification of a New Class of Steroid Hormone Receptors. Nature 1988, 331, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Dufour, C.R.; Giguere, V. ERRalpha as a Bridge Between Transcription and Function: Role in Liver Metabolism and Disease. Front. Endocrinol. 2019, 10, 206. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Lin, T.; Kamarajugadda, S.; Lu, J. Regulation of Glycolysis and the Warburg Effect by Estrogen-Related Receptors. Oncogene 2012, 32, 2079–2086. [Google Scholar] [CrossRef]
- Su, P.; Mao, X.; Ma, J.; Huang, L.; Yu, L.; Tang, S.; Zhuang, M.; Lu, Z.; Osafo, K.S.; Ren, Y.; et al. ERRalpha Promotes Glycolytic Metabolism and Targets the Nlrp3/Caspase-1/Gsdmd Pathway to Regulate Pyroptosis in Endometrial Cancer. J. Exp. Clin. Cancer Res. 2023, 42, 274. [Google Scholar] [CrossRef]
- Gallagher, K.M.; Roderick, J.E.; Tan, S.H.; Tan, T.K.; Murphy, L.; Yu, J.; Li, R.; O’Connor, K.W.; Zhu, J.; Green, M.R.; et al. ESRRB Regulates Glucocorticoid Gene Expression in Mice and Patients with Acute Lymphoblastic Leukemia. Blood Adv. 2020, 4, 3154–3168. [Google Scholar] [CrossRef]
- Yoshihara, E.; Wei, Z.; Lin, C.S.; Fang, S.; Ahmadian, M.; Kida, Y.; Tseng, T.; Dai, Y.; Yu, R.T.; Liddle, C.; et al. ERRgamma is Required for the Metabolic Maturation of Therapeutically Functional Glucose-Responsive Beta Cells. Cell Metab. 2016, 23, 622–634. [Google Scholar] [CrossRef]
- Fan, Y.; Na, S.Y.; Jung, Y.S.; Radhakrishnan, K.; Choi, H.S. Estrogen-Related Receptor Gamma (ERRgamma) is a Key Regulator of Lysyl Oxidase Gene Expression in Mouse Hepatocytes. Steroids 2023, 194, 109226. [Google Scholar] [CrossRef]
- Eichner, L.J.; Giguere, V. Estrogen Related Receptors (ERRs): A New Dawn in Transcriptional Control of Mitochondrial Gene Networks. Mitochondrion 2011, 11, 544–552. [Google Scholar] [CrossRef]
- Tennessen, J.M.; Baker, K.D.; Lam, G.; Evans, J.; Thummel, C.S. The Drosophila Estrogen-Related Receptor Directs a Metabolic Switch that Supports Developmental Growth. Cell Metab. 2011, 13, 139–148. [Google Scholar] [CrossRef]
- Beebe, K.; Robins, M.M.; Hernandez, E.J.; Lam, G.; Horner, M.A.; Thummel, C.S. Drosophila Estrogen-Related Receptor Directs a Transcriptional Switch that Supports Adult Glycolysis and Lipogenesis. Genes Dev. 2020, 34, 701–714. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Padmanabha, D.; Gentile, L.B.; Dumur, C.I.; Beckstead, R.B.; Baker, K.D. HIF- and Non-HIF-Regulated Hypoxic Responses Require the Estrogen-Related Receptor in Drosophila melanogaster. PLoS Genet. 2013, 9, e1003230. [Google Scholar] [CrossRef]
- Long, W.; Wu, J.; Shen, G.; Zhang, H.; Liu, H.; Xu, Y.; Gu, J.; Jia, L.; Lin, Y.; Xia, Q. Estrogen-Related Receptor Participates in Regulating Glycolysis and Influences Embryonic Development in Silkworm Bombyx mori. Insect Mol. Biol. 2019, 29, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Liu, D.; Xu, H.; Wu, J.; Hou, L.; Yang, C.; Xia, Q.; Lin, P. A Study on the Effect of Energy on the Development of Silkworm Embryos Using an Estrogen-Related Receptor. Int. J. Mol. Sci. 2023, 24, 14485. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.; Pandey, A.K.; Gupta, S.; Kumar, A.; Khanna, P.; Shankar, J.; Ravi, R.K. Estrogen Related Receptor is Required for the Testicular Development and for the Normal Sperm Axoneme/Mitochondrial Derivatives in Drosophila Males. Sci. Rep. 2017, 7, 40372. [Google Scholar] [CrossRef]
- Park, W.R.; Lim, D.J.; Sang, H.; Kim, E.; Moon, J.H.; Choi, H.S.; Kim, I.S.; Kim, D.K. Aphid Estrogen-Related Receptor Controls Glycolytic Gene Expression and Fecundity. Insect Biochem. Mol. Biol. 2021, 130, 103529. [Google Scholar] [CrossRef]
- Bozzolan, F.; Durand, N.; Demondion, E.; Bourgeois, T.; Gassias, E.; Debernard, S. Evidence for a Role of Oestrogen Receptor-Related Receptor in the Regulation of Male Sexual Behaviour in the Moth Agrotis ipsilon. Insect Mol. Biol. 2017, 26, 403–413. [Google Scholar] [CrossRef]
- Jiang, X.; Zheng, S.; Bamu, A.; Dai, H.; Lin, X. Nilaparvata lugens ERR2 Regulates Moulting and Ovary Development is Related to Hormone Signalling. Insect Mol. Biol. 2023, 32, 376–386. [Google Scholar] [CrossRef]
- Shen, G.; Wu, J.; Lin, Y.; Hua, X.; Xia, Q.; Zhao, P. Estrogen-Related Receptor Influences the Hemolymph Glucose Content by Regulating Midgut Trehalase Gene Expression in the Last Instar Larvae of Bombyx mori. Int. J. Mol. Sci. 2021, 22, 4343. [Google Scholar] [CrossRef]
- Wu, J.; Shen, G.; Liu, D.; Xu, H.; Jiao, M.; Zhang, Y.; Lin, Y.; Zhao, P. The Response of the Estrogen-Related Receptor to 20-Hydroxyecdysone in Bombyx mori: Insight into the Function of Estrogen-Related Receptor in Insect 20-Hydroxyecdysone Signaling Pathway. Front. Physiol. 2021, 12, 785637. [Google Scholar] [CrossRef]
- Kovalenko, E.V.; Mazina, M.Y.; Krasnov, A.N.; Vorobyeva, N.E. The Drosophila Nuclear Receptors EcR and ERR Jointly Regulate the Expression of Genes Involved in Carbohydrate Metabolism. Insect Biochem. Mol. Biol. 2019, 112, 103184. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Li, Y.; Zhao, X.; Zhang, M.; Gu, W. Ethylparaben Affects Lifespan, Fecundity, and the Expression Levels of ERR, EcR and YPR in Drosophila melanogaster. J. Insect Physiol. 2014, 71, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Sachan, A.; Ravi, R.K. Estrogen-Related Receptor is Critical for Testicular Mitochondrial Homeostasis and Sperm Motility: A Drosophila-Based Study. FS Sci. 2022, 3, 217–227. [Google Scholar] [CrossRef]
- Geng, D.Q.; Wang, X.L.; Lyu, X.Y.; Raikhel, A.S.; Zou, Z. Ecdysone-Controlled Nuclear Receptor ERR Regulates Metabolic Homeostasis in the Disease Vector Mosquito Aedes aegypti. PLoS Genet. 2024, 20, e1011196. [Google Scholar] [CrossRef]
- Park, K.; Kwak, I.S. Molecular Effects of Endocrine-Disrupting Chemicals on the Chironomus riparius Estrogen-Related Receptor Gene. Chemosphere 2010, 79, 934–941. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Paz, P.; Morales, M.; Urien, J.; Morcillo, G.; Martinez-Guitarte, J.L. Endocrine-Related Genes are Altered by Antibacterial Agent Triclosan in Chironomus riparius Aquatic Larvae. Ecotoxicol. Environ. Saf. 2017, 140, 185–190. [Google Scholar] [CrossRef]
- Zhang, J.J.; Xi, G.S. Morphological Changes in Different Caste Adult Ant Specificities of Polyrhachis vicina roger (Hymenoptera, Formicidae) Caused in Estrogen-Related Receptor. Gen. Comp. Endocrinol. 2018, 266, 29–37. [Google Scholar] [CrossRef]
- Zhang, J.J.; Xi, G.S.; Zhao, J. Vitellogenin Regulates Estrogen-Related Receptor Expression by Crosstalk with the JH and IIS-TOR Signaling Pathway in Polyrhachis vicina roger (Hymenoptera, Formicidae). Gen. Comp. Endocrinol. 2021, 310, 113836. [Google Scholar] [CrossRef]
- Park, W.R.; Choi, H.S.; Moon, J.H.; Kim, I.S.; Kim, D.K. 3-Methylcatechol Mediates Anti-Fecundity Effect by Inhibiting Estrogen-Related Receptor-Induced Glycolytic Gene Expression in Myzus persicae. Pestic. Biochem. Physiol. 2024, 200, 105802. [Google Scholar] [CrossRef]
- Towbin, H.; Staehelin, T.; Gordon, J. Electrophoretic Transfer of Proteins from Polyacrylamide Gels to Nitrocellulose Sheets: Procedure and some Applications. Proc. Natl. Acad. Sci. USA 1979, 76, 4350–4354. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, J.; Qin, Q.; Wu, P.; Zhang, H.; Meng, Q. Metabolic Changes During Larval-Pupal Metamorphosis of Helicoverpa armigera. Insect Sci. 2023, 30, 1663–1676. [Google Scholar] [CrossRef]
- Sterkel, M.; Oliveira, P.L. Developmental Roles of Tyrosine Metabolism Enzymes in the Blood-Sucking Insect Rhodnius prolixus. Proc. Biol. Sci. 2017, 284, 20162607. [Google Scholar] [CrossRef] [PubMed]
- Vatanparast, M.; Park, Y. Cold Tolerance Strategies of the Fall Armyworm, Spodoptera Frugiperda (Smith) (Lepidoptera: Noctuidae). Sci. Rep. 2022, 12, 4129. [Google Scholar] [CrossRef]
- Chen, G.; Gao, X.; Zhang, Y.; Ma, C.; Ma, W.; Zhou, Z. The Carboxypeptidase B and Carbonic Anhydrase Genes Play a Reproductive Regulatory Role During Multiple Matings in Ophraella communa. Front. Mol. Biosci. 2023, 10, 1095645. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, D.; Yao, Q.; Zhang, J.; Dong, X.; Tian, H.; Chen, J.; Zhang, W. Feeding-Based Rna Interference of a Trehalose Phosphate Synthase Gene in the Brown Planthopper, Nilaparvata lugens. Insect Mol. Biol. 2010, 19, 777–786. [Google Scholar] [CrossRef]
- Ashraf, H.; Qamar, A. Silkworm Bombyx mori as a Model Organism: A Review. Physiol. Entomol. 2023, 484, 107–121. [Google Scholar] [CrossRef]
- Kikawada, T.; Saito, A.; Kanamori, Y.; Nakahara, Y.; Iwata, K.; Tanaka, D.; Watanabe, M.; Okuda, T. Trehalose Transporter 1, a Facilitated and High-Capacity Trehalose Transporter, Allows Exogenous Trehalose Uptake into Cells. Proc. Natl. Acad. Sci. USA 2007, 104, 11585–11590. [Google Scholar] [CrossRef]
- Tellis, M.B.; Chaudhari, B.Y.; Deshpande, S.V.; Nikam, S.V.; Barvkar, V.T.; Kotkar, H.M.; Joshi, R.S. Trehalose Transporter-Like Gene Diversity and Dynamics Enhances Stress Response and Recovery in Helicoverpa armigera. Gene 2023, 862, 147259. [Google Scholar] [CrossRef]




| The Founds and Funtions of ERR in Insects | ||
|---|---|---|
| Order | Species | Funtions |
| Lepidoptera | Bombyx mori | |
| Agrotis ipsilon |
| |
| Diptera | Drosophila melanogaster | |
| Aedes aegypti |
| |
| Chironomus riparius | ||
| Hymenoptera | Polyrhachis vicina Roger | |
| Apis cerana cerana |
| |
| Hemiptera | Nilaparvata lugens |
|
| Orthoptera | Teleogryllus emma |
|
| Homoptera | Myzus persicae | |
| Metabolic Type | Gene_ID | Gene Name | Gene Description | Binding Motifs |
|---|---|---|---|---|
| Trehalose metabolism | KWMTBOMO07250 | GlyP | glycogen phosphorylase | CAACGTCA |
| KWMTBOMO07450 | Tps | trehalose-6-phosphate synthase | CAAGGTTA | |
| KWMTBOMO16046 | Tret1 | trehalose transporter | CTCACAAGGTCC | |
| Lipid metabolism | KWMTBOMO08103 | Atgl | adipose triglyceride lipase | CAATGTCA |
| KWMTBOMO00223 | GK | glycerol kinase | GAAGGTCA CAATGTCAATGTC | |
| KWMTBOMO03651 | Acyl | acyl-CoA | TGCCCTTG | |
| Amino acid metabolism | KWMTBOMO01118 | FAH | fumarylacetoacetate hydrolase | CCAAGGACGT |
| KWMTBOMO04345 | HPD | 4-hydroxyphenylpyruvate dioxygenase | AAAGGGCG ACGAGGTTAT AAACGTCA | |
| KWMTBOMO12353 | Chsy1 | chondroitin sulfate synthase 1 | CAAGGTTG | |
| Carbohydrate metabolism | KWMTBOMO00773 | G6pase | glucose-6-phosphatase | AAATGTCA |
| KWMTBOMO04913 | Ca7 | carbonic anhydrase 7 | CAAAGTCA CAAGGGCA CGAATGTCAT ATAGGTTA | |
| KWMTBOMO07147 | Aldh1b1 | aldehyde dehydrogenase 1 B1 | CTAACCTTAA | |
| KWMTBOMO00939 | akr2e | aldo-keto reductase family 2 member E4 | TTAAGGTTAC | |
| KWMTBOMO16421 | UGT33D8 | UDP-glycosyltransferase UGT33D8 | CAAGGTCA | |
| KWMTBOMO07079 | UGT46A2 | UDP-glycosyltransferase UGT46A2 | TTAAGGTTAC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, L.; Wu, J.; Liu, D.; Xu, H.; Yao, H.; Liang, Y.; Xia, Q.; Lin, P.; Shen, G. Estrogen-Related Receptor Potential Target Genes in Silkworm (Bombyx mori): Insights into Metabolic Regulation. Insects 2025, 16, 469. https://doi.org/10.3390/insects16050469
Hou L, Wu J, Liu D, Xu H, Yao H, Liang Y, Xia Q, Lin P, Shen G. Estrogen-Related Receptor Potential Target Genes in Silkworm (Bombyx mori): Insights into Metabolic Regulation. Insects. 2025; 16(5):469. https://doi.org/10.3390/insects16050469
Chicago/Turabian StyleHou, Luyu, Jinxin Wu, Die Liu, Haoran Xu, Hongbo Yao, Yiwen Liang, Qingyou Xia, Ping Lin, and Guanwang Shen. 2025. "Estrogen-Related Receptor Potential Target Genes in Silkworm (Bombyx mori): Insights into Metabolic Regulation" Insects 16, no. 5: 469. https://doi.org/10.3390/insects16050469
APA StyleHou, L., Wu, J., Liu, D., Xu, H., Yao, H., Liang, Y., Xia, Q., Lin, P., & Shen, G. (2025). Estrogen-Related Receptor Potential Target Genes in Silkworm (Bombyx mori): Insights into Metabolic Regulation. Insects, 16(5), 469. https://doi.org/10.3390/insects16050469