Use of Sugar Dispensers at Lower Density Can Decrease Mealybug (Hemiptera: Pseudococcidae) Infestation in Vineyards by Disrupting Ants
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Sampling Sites and Experimental Design
2.2. Assessment of Mealybug Infestations, Natural Enemies, and Ants
2.3. Statistical Analysis
3. Results
3.1. Ant Fauna Analysis
3.2. Mealybug Infestation in Sugar Dispenser Versus Control Plots
3.3. Evaluation of the Range of Sugar Dispensers on Mealybug Infestation
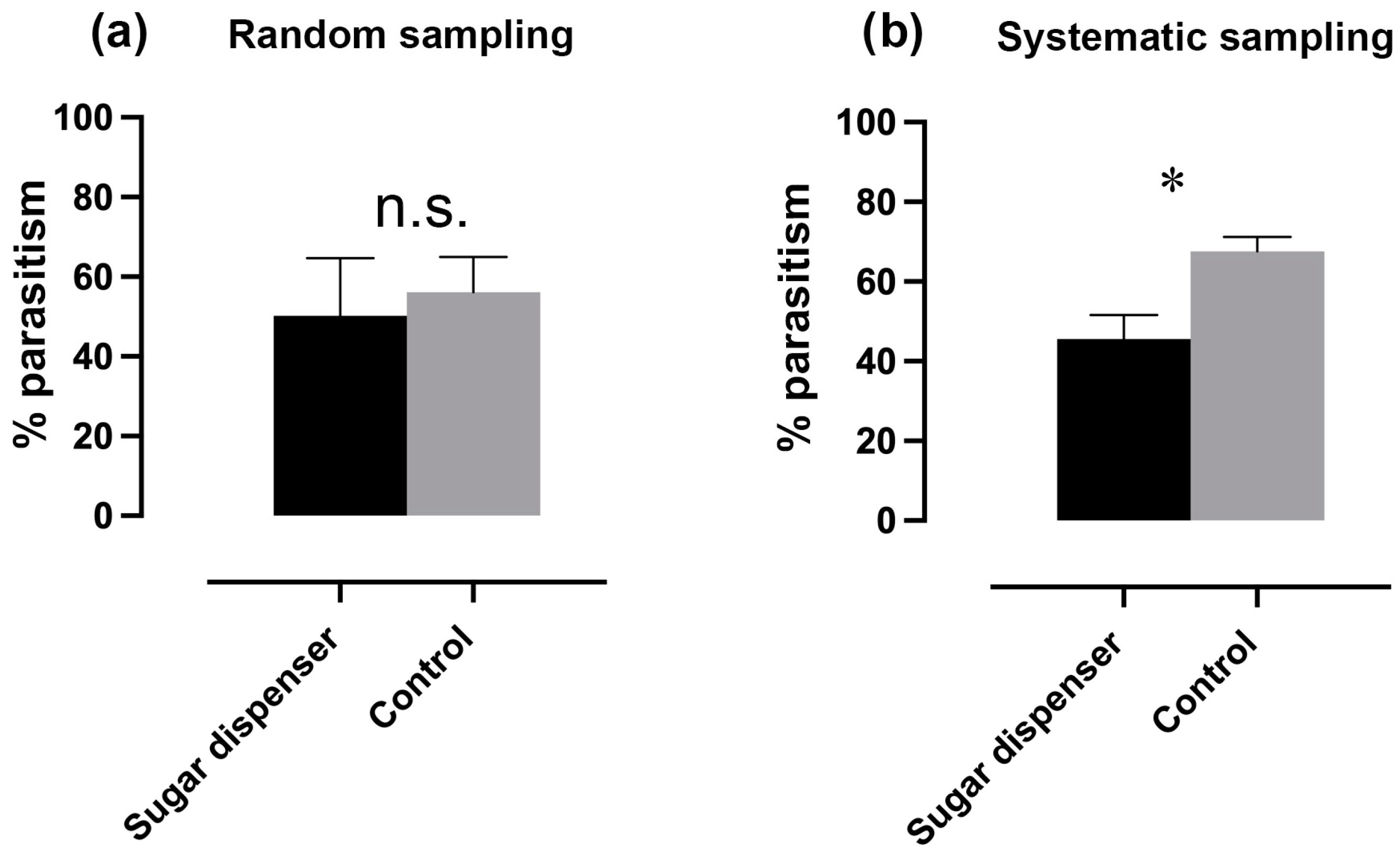
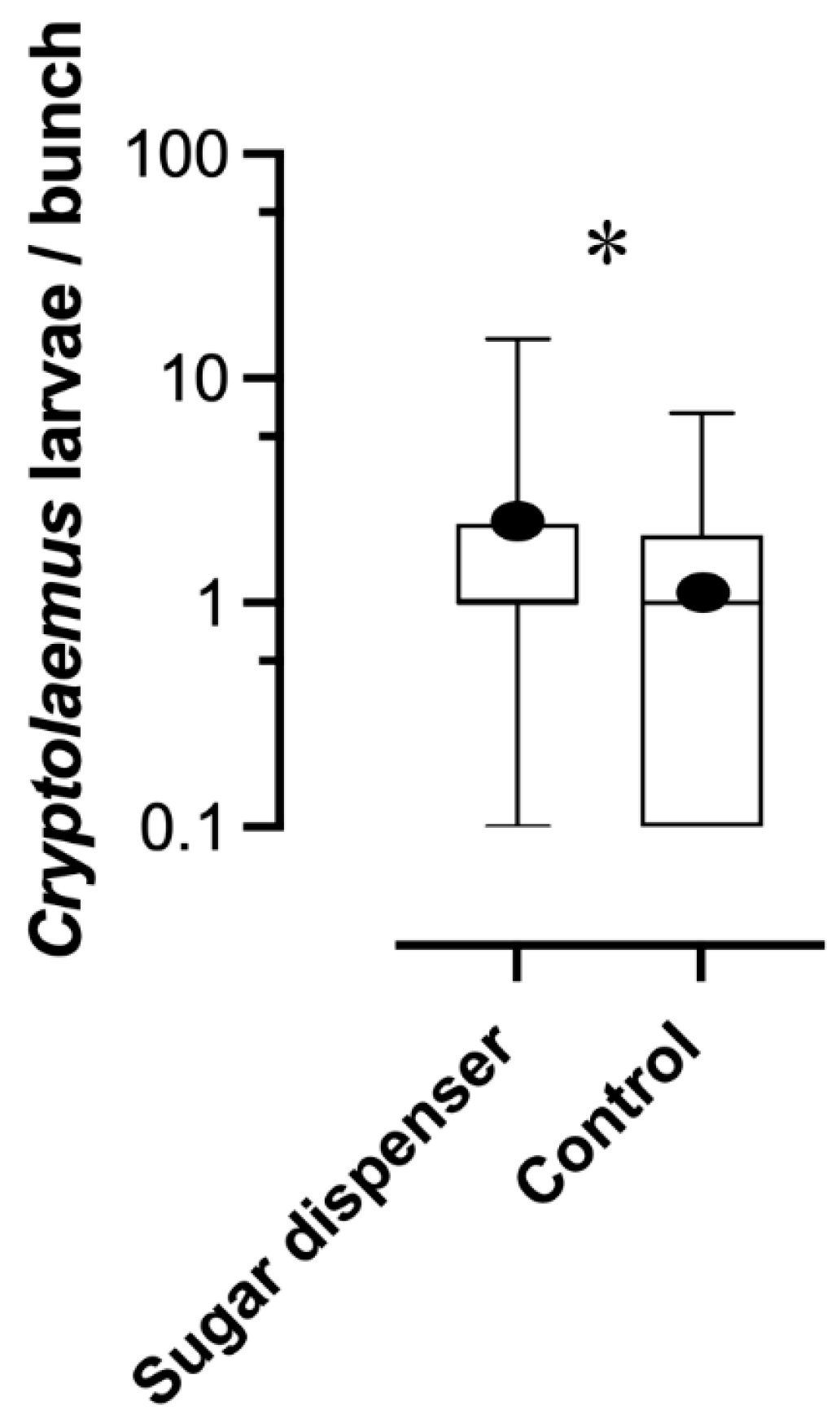
3.4. Effect of Sugar Dispensers on Parasitism Rate and Predator Abundance
3.5. Effect of the Distance from Sugar Dispensers on Parasitism and Predator Abundance
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Daane, K.M.; Almeida, R.P.; Bell, V.A.; Walker, J.T.S.; Botton, M.; Fallahzadeh, M.; Mani, M.; Miano, J.L.; Sforza, R.; Walton, V.M.; et al. Biology and management of mealybugs in vineyards. In Arthropod Management in Vineyards: Pests, Approaches and Future Directions; Bostanian, N.J., Vincent, C., Isaacs, R., Eds.; Springer: New York, NY, USA, 2012; pp. 271–307. [Google Scholar]
- Cocco, A.; Muscas, E.; Mura, A.; Iodice, A.; Savino, F.; Lentini, A. Influence of Mating Disruption on the Reproductive Biology of the Vine Mealybug, Planococcus Ficus (Hemiptera: Pseudococcidae), under Field Conditions. Pest Manag. Sci. 2018, 74, 2806–2816. [Google Scholar] [CrossRef] [PubMed]
- Lucchi, A.; Suma, P.; Ladurner, E.; Iodice, A.; Savino, F.; Ricciardi, R.; Cosci, F.; Marchesini, E.; Conte, G.; Benelli, G. Managing the Vine Mealybug, Planococcus Ficus, through Pheromone-Mediated Mating Disruption. Environ. Sci. Pollut. Res. 2019, 26, 10708–10718. [Google Scholar] [CrossRef] [PubMed]
- Pellizzari, G.; Duso, C.; Rainato, A.; Pozzebon, A.; Zanini, G. Phenology, Ethology and Distribution of Pseudococcus Comstocki, an Invasive Pest in Northeastern Italy. Bull. Insectol. 2012, 62, 209–215. [Google Scholar]
- Marchesini, E.; Duso, C.; Pellizzari, G. Pseudococcus comstocki Colpisce i Vigneti Del Veneto. L’INFORMATORE Agrar. 2018, 33, 61–63. [Google Scholar]
- Bortolotti, P.P.; Nannini, R.; Bonmbardini, E.; Pasqualini, E.; Masetti, A. Monitoraggio Cocciniglie Farinose per Interventi Mirati Su Vite. Inf. Agrar. 2020, 26, 56–59. [Google Scholar]
- Parrilli, M.; Profeta, M.; Casoli, L.; Gambirasio, F.; Masetti, A.; Burgio, G. Use of Sugar Dispensers to Disrupt Ant Attendance and Improve Biological Control of Mealybugs in Vineyard. Insects 2021, 12, 330. [Google Scholar] [CrossRef]
- Mgocheki, N.; Addison, P. The Sublethal Effects of a Systemic Insecticide on the Vine Mealybug Parasitoids Anagyrus Sp. near pseudococci (Girault) and Coccidoxenoides Perminutus (Timberlake)(Hymenoptera: Encyrtidae). South Afr. J. Enol. Vitic. 2015, 36, 175–179. [Google Scholar] [CrossRef][Green Version]
- Mgocheki, N.; Addison, P. Interference of Ants (Hymenoptera: Formicidae) with Biological Control of the Vine Mealybug Planococcus ficus (Signoret)(Hemiptera: Pseudococcidae). Biol. Control 2009, 49, 180–185. [Google Scholar] [CrossRef]
- Cocco, A.; Mercenaro, L.; Muscas, E.; Mura, A.; Nieddu, G.; Lentini, A. Multiple Effects of Nitrogen Fertilization on Grape Vegetative Growth, Berry Quality and Pest Development in Mediterranean Vineyards. Horticulturae 2021, 7, 530. [Google Scholar] [CrossRef]
- Mansour, R.; Suma, P.; Mazzeo, G.; La Pergola, A.; Pappalardo, V.; Grissa Lebdi, K.; Russo, A. Interactions between the Ant Tapinoma nigerrimum (Hymenoptera: Formicidae) and the Main Natural Enemies of the Vine and Citrus Mealybugs (Hemiptera: Pseudococcidae). Biocontrol Sci. Technol. 2012, 22, 527–537. [Google Scholar] [CrossRef]
- Ricciardi, R.; Zeni, V.; Michelotti, D.; Di Giovanni, F.; Cosci, F.; Canale, A.; Zang, L.-S.; Lucchi, A.; Benelli, G. Old Parasitoids for New Mealybugs: Host Location Behavior and Parasitization Efficacy of Anagyrus vladimiri on Pseudococcus comstocki. Insects 2021, 12, 257. [Google Scholar] [CrossRef] [PubMed]
- Castracani, C.; Giannetti, D.; Spotti, F.A.; Schifani, E.; Ghizzoni, M.; Delaiti, M.; Penner, F.; Leonardi, S.; Mori, A.; Ioriatti, C. Ants as Mealybug Detectors: A Novel Tool for Monitoring Planococcus ficus Infestation Based on Ant Behaviour. Agric. For. Entomol. 2023, 25, 237–250. [Google Scholar] [CrossRef]
- Hölldobler, B.; Wilson, E.O. The Ants; Harvard University Press: Cambridge, MA, USA, 1990; ISBN 0-674-04075-9. [Google Scholar]
- Drummond, F.; Choate, B. Ants as Biological Control Agents in Agricultural Cropping Systems. Terr. Arthropod Rev. 2011, 4, 157–180. [Google Scholar] [CrossRef]
- Offenberg, J. Ants as Tools in Sustainable Agriculture. J. Appl. Ecol. 2015, 52, 1197–1205. [Google Scholar] [CrossRef]
- Castracani, C.; Bulgarini, G.; Giannetti, D.; Spotti, F.A.; Maistrello, L.; Mori, A.; Grasso, D.A. Predatory Ability of the Ant Crematogaster scutellaris on the Brown Marmorated Stink Bug Halyomorpha halys. J. Pest Sci. 2017, 90, 1181–1190. [Google Scholar] [CrossRef]
- Giannetti, D.; Castracani, C.; Spotti, F.A.; Mori, A.; Grasso, D.A. Gall-Colonizing Ants and Their Role as Plant Defenders: From’bad Job’to’useful Service’. Insects 2019, 10, 392. [Google Scholar] [CrossRef]
- Sanchez, J.A.; Carrasco-Ortiz, A.; López-Gallego, E.; Ramírez-Soria, M.J.; La Spina, M.; Ortín-Angulo, M.C.; Ibáñez-Martínez, H. Density Thresholds and the Incorporation of Biocontrol into Decision-making to Enhance the Control of Cacopsylla pyri in Pear (Cv. Ercolini) Orchards. Pest Manag. Sci. 2022, 78, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Schifani, E.; Castracani, C.; Giannetti, D.; Spotti, F.A.; Reggiani, R.; Leonardi, S.; Mori, A.; Grasso, D.A. New Tools for Conservation Biological Control: Testing Ant-Attracting Artificial Nectaries to Employ Ants as Plant Defenders. Insects 2020, 11, 129. [Google Scholar] [CrossRef] [PubMed]
- Penn, H.J.; Crist, T.O. From Dispersal to Predation: A Global Synthesis of Ant–Seed Interactions. Ecol. Evol. 2018, 8, 9122–9138. [Google Scholar] [CrossRef]
- Schulze-Sylvester, M.; Sylvester, F.; Torres, V.M.; Paris, C.I.; Corronca, J.A. Alien vs. Herbivore: Ant-Mediated Plant Defense as an Option for Biological Control of Leafcutter Ants. Agron. Sustain. Dev. 2022, 42, 104. [Google Scholar] [CrossRef]
- Schifani, E.; Peri, E.; Giannetti, D.; Colazza, S.; Grasso, D.A. Ant Attendance Does Not Necessarily Imply Protection of Aphids from Their Arthropod Natural Enemies. Ecol. Entomol. 2023, 48, 384–388. [Google Scholar] [CrossRef]
- Schifani, E.; Giannetti, D.; Grasso, D.A. Toward Sustainable Management of Ant-Hemipteran Mutualism in Agricultural Settings: A Comparison of Different Approaches. Crop Prot. 2024, 175, 106468. [Google Scholar] [CrossRef]
- Beltrà, A.; Navarro-Campos, C.; Calabuig, A.; Estopà, L.; Wäckers, F.L.; Pekas, A.; Soto, A. Association between Ants (Hymenoptera: Formicidae) and the Vine Mealybug (Hemiptera: Pseudococcidae) in Table-grape Vineyards in Eastern Spain. Pest Manag. Sci. 2017, 73, 2473–2480. [Google Scholar] [CrossRef]
- Jensen, I.C.; Hansen, R.R.; Damgaard, C.; Offenberg, J. Implementing Wood Ants in Biocontrol: Suppression of Apple Scab and Reduced Aphid Tending. Pest Manag. Sci. 2023, 79, 2415–2422. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Rodríguez, J.; Pekas, A.; Tena, A.; Wäckers, F. Sugar Provisioning for Ants Enhances Biological Control of Mealybugs in Citrus. Biol. Control 2021, 157, 104573. [Google Scholar] [CrossRef]
- Mogensen, A.L.; Andersen, L.B.; Sørensen, J.G.; Offenberg, J. Manipulated Ants: Inducing Loyalty to Sugar Feeders with an Alkaloid. Pest Manag. Sci. 2024, 80, 3445–3450. [Google Scholar] [CrossRef]
- Daane, K.M.; Sime, K.R.; Hogg, B.N.; Bianchi, M.L.; Cooper, M.L.; Rust, M.K.; Klotz, J.H. Effects of Liquid Insecticide Baits on Argentine Ants in California’s Coastal Vineyards. Crop Prot. 2006, 25, 592–603. [Google Scholar] [CrossRef]
- Daane, K.M.; Cooper, M.L.; Sime, K.R.; Nelson, E.H.; Battany, M.C.; Rust, M.K. Testing Baits to Control Argentine Ants (Hymenoptera: Formicidae) in Vineyards. J. Econ. Entomol. 2008, 101, 699–709. [Google Scholar] [CrossRef]
- Tay, J.; Hoddle, M.S.; Mulchandani, A.; Choe, D. Development of an Alginate Hydrogel to Deliver Aqueous Bait for Pest Ant Management. Pest Manag. Sci. 2017, 73, 2028–2038. [Google Scholar] [CrossRef]
- Buczkowski, G.; Roper, E.; Chin, D.; Mothapo, N.; Wossler, T. Hydrogel Baits with Low-dose Thiamethoxam for Sustainable Argentine Ant Management in Commercial Orchards. Entomol. Exp. Appl. 2014, 153, 183–190. [Google Scholar] [CrossRef]
- Le, B.; Park, H.; Campbell, K.; Rust, M.K.; Lee, C.-Y.; Choe, D.-H. Laboratory Evaluations of Biodegradable Boric Acid Hydrogel Baits for the Control of Argentine Ant (Hymenoptera: Formicidae). J. Econ. Entomol. 2023, 116, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Mercer, N.H.; Haviland, D.R.; Daane, K.M. Mealybug, Planococcus ficus, Reduction through Pavement Ant, Tetramorium Immigrans, Management Using Polyacrylamide Hydrogel Baits in Vineyards. Crop Prot. 2025, 190, 107078. [Google Scholar] [CrossRef]
- Tay, J.-W.; Choe, D.-H.; Mulchandani, A.; Rust, M.K. Hydrogels: From Controlled Release to a New Bait Delivery for Insect Pest Management. J. Econ. Entomol. 2020, 113, 2061–2068. [Google Scholar] [CrossRef]
- Parrilli, M. The Use of Habitat Management, Elicitors and Augmentation to Improve Biological Control in Vineyard. Ph.D. Thesis, Alma Mater Studiorum Università, Bologna, Italy, June 2021. [Google Scholar]
- Seifert, B. A Taxonomic Revision of the Palaearctic Members of the Subgenus Lasius s. Str.(Hymenoptera, Formicidae):+ The Supplementary Informations SI1 and SI2 Are Downloadable as Digital Material. Soil Org. 2020, 92, 15–86. [Google Scholar]
- Mateos-Aparicio, G. Partial Least Squares (PLS) Methods: Origins, Evolution, and Application to Social Sciences. Commun. Stat.-Theory Methods 2011, 40, 2305–2317. [Google Scholar] [CrossRef]
- Abdi, H. Partial Least Square Regression (PLS Regression). Encycl. Res. Methods Soc. Sci. 2003, 6, 792–795. [Google Scholar]
- Fienberg, S.E.; Rinaldo, A. Three Centuries of Categorical Data Analysis: Log-Linear Models and Maximum Likelihood Estimation. J. Stat. Plan. Inference 2007, 137, 3430–3445. [Google Scholar] [CrossRef]
- Giannetti, D.; Schifani, E.; Castracani, C.; Ghizzoni, M.; Delaiti, M.; Penner, F.; Spotti, F.A.; Mori, A.; Ioriatti, C.; Grasso, D.A. Assessing Ant Diversity in Agroecosystems: The Case of Italian Vineyards of the Adige Valley. Redia 2021, 104, 97–109. [Google Scholar] [CrossRef]
- Plata, Á.; Gómez-Martínez, M.A.; Beitia, F.J.; Tena, A. Exclusion of Mediterranean Ant Species Enhances Biological Control of the Invasive Mealybug Delottococcus aberiae in Citrus. Pest Manag. Sci. 2023, 79, 2056–2065. [Google Scholar] [CrossRef]
- Schifani, E.; Giannetti, D.; Costi, E.; Franconi, G.; Campostrini, A.; Maistrello, L.; Grasso, D.A. Interactions between Egg Parasitoids and Predatory Ants for the Biocontrol of the Invasive Brown Marmorated Stink Bug Halyomorpha halys. J. Appl. Entomol. 2023, 147, 868–874. [Google Scholar] [CrossRef]
- Schifani, E.; Peri, E.; Giannetti, D.; Alınç, T.; Colazza, S.; Grasso, D.A. Mediterranean Ants Can Increase Nymph Mortality in the Stink Bug Nezara viridula without Interfering with Its Egg Parasitoid Trissolcus basalis. Entomol. Exp. Appl. 2023, 171, 739–744. [Google Scholar] [CrossRef]




| Site | Latitude | Longitude | Variety | Total Area of Farm | Plot Size (Control + Sugar Dispenser) |
|---|---|---|---|---|---|
| A | 44.83491° N | 10.72488° E | Lambrusco Marani | 8 ha | 0.52 ha |
| B | 44.71957° N | 10.61416° E | Ancellotta | 6.6 ha | 0.2855 ha |
| C | 44.79276° N | 10.79912° E | Lambrusco Salamino | 11 ha | 0.1225 ha |
| D | 44.79636° N | 10.82416° E | Ancellotta | 9 ha | 0.1872 ha |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burgio, G.; Magagnoli, S.; Casoli, L.; Profeta, M.; Grasso, D.A.; Schifani, E.; Giannetti, D.; Parrilli, M. Use of Sugar Dispensers at Lower Density Can Decrease Mealybug (Hemiptera: Pseudococcidae) Infestation in Vineyards by Disrupting Ants. Insects 2025, 16, 468. https://doi.org/10.3390/insects16050468
Burgio G, Magagnoli S, Casoli L, Profeta M, Grasso DA, Schifani E, Giannetti D, Parrilli M. Use of Sugar Dispensers at Lower Density Can Decrease Mealybug (Hemiptera: Pseudococcidae) Infestation in Vineyards by Disrupting Ants. Insects. 2025; 16(5):468. https://doi.org/10.3390/insects16050468
Chicago/Turabian StyleBurgio, Giovanni, Serena Magagnoli, Luca Casoli, Marco Profeta, Donato Antonio Grasso, Enrico Schifani, Daniele Giannetti, and Martina Parrilli. 2025. "Use of Sugar Dispensers at Lower Density Can Decrease Mealybug (Hemiptera: Pseudococcidae) Infestation in Vineyards by Disrupting Ants" Insects 16, no. 5: 468. https://doi.org/10.3390/insects16050468
APA StyleBurgio, G., Magagnoli, S., Casoli, L., Profeta, M., Grasso, D. A., Schifani, E., Giannetti, D., & Parrilli, M. (2025). Use of Sugar Dispensers at Lower Density Can Decrease Mealybug (Hemiptera: Pseudococcidae) Infestation in Vineyards by Disrupting Ants. Insects, 16(5), 468. https://doi.org/10.3390/insects16050468










