Screening the Resistance of Male Aedes aegypti and Anopheles coluzzii to Insecticides in the Context of Using Genetic Control Tools in Burkina Faso
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Biological Material
2.2. Susceptibility Bioassay
2.3. Mosquito Wing Length
2.4. Statistical Analyses
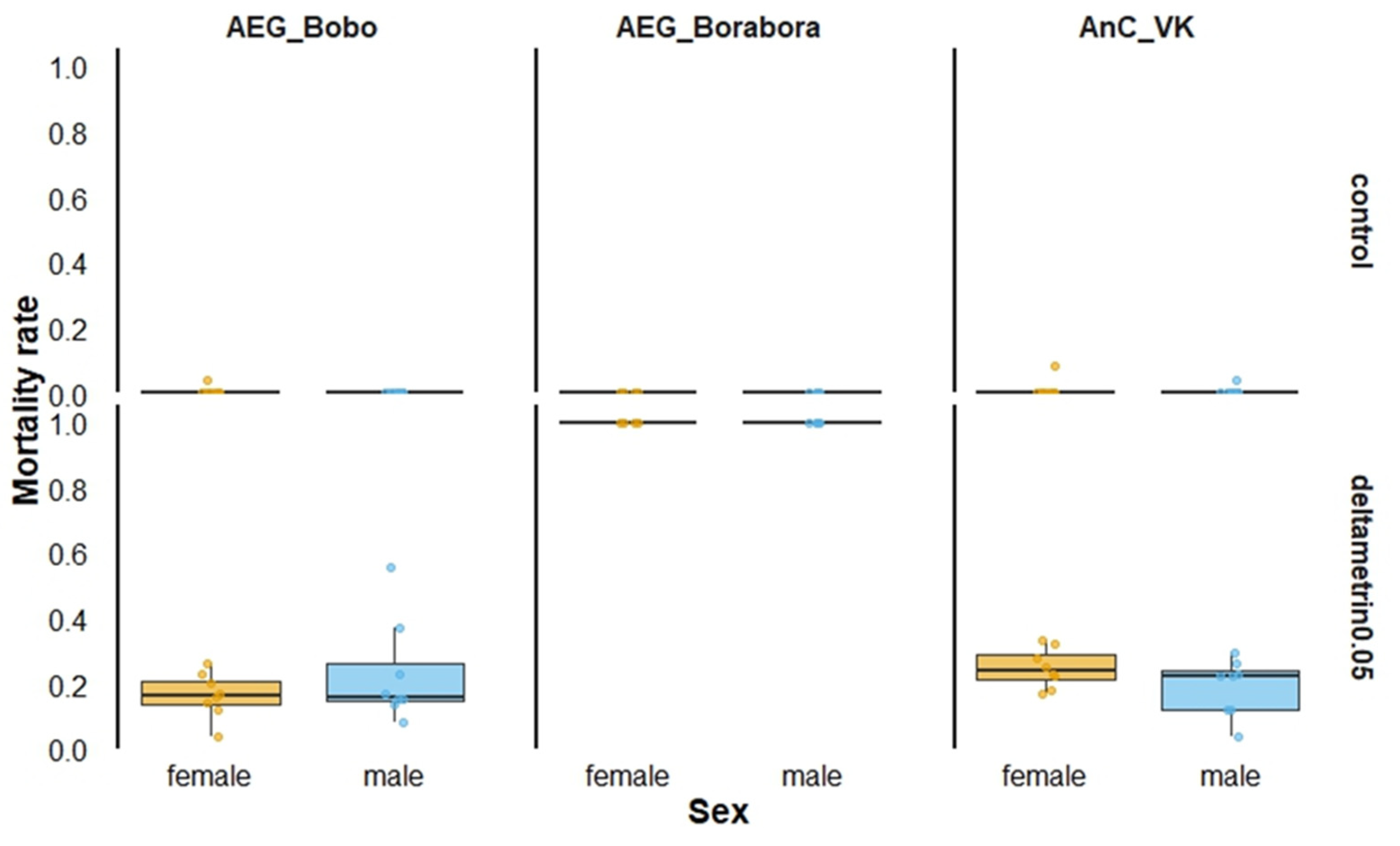
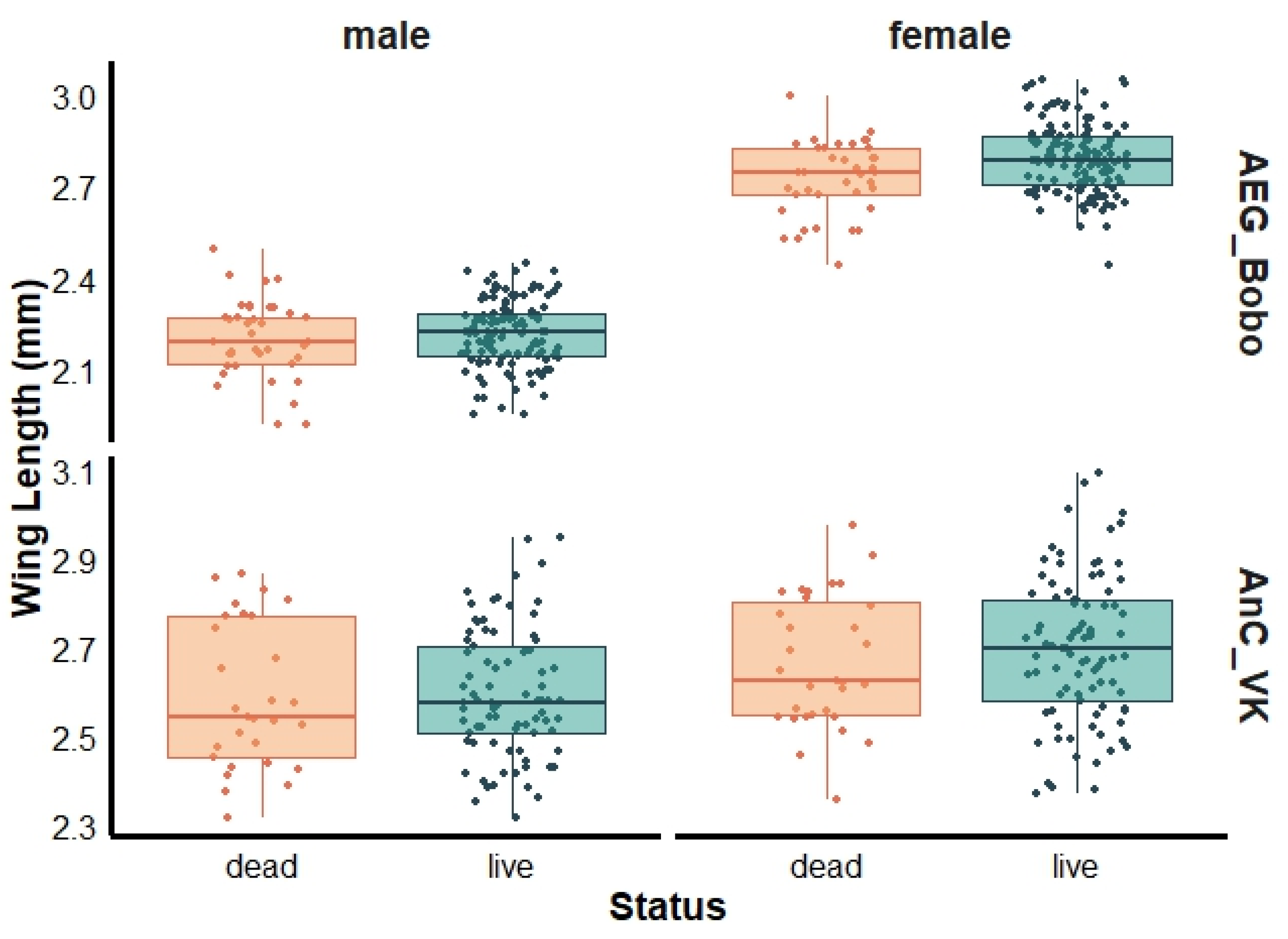
3. Results
3.1. Susceptibility Bioassays
3.2. Mosquito Wing Length
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Guidelines for Malaria Vector Control; World Health Organization: Geneva, Switzerland, 2019; Available online: https://iris.who.int/handle/10665/310862 (accessed on 17 January 2025).
- Moyes, C.L.; Vontas, J.; Martins, A.J.; Ng, L.C.; Koou, S.Y.; Dusfour, I.; Raghavendra, K.; Pinto, J.; Corbel, V.; David, J.-P.; et al. Contemporary status of insecticide resistance in the major Aedes vectors of arboviruses infecting humans. PLoS Negl. Trop. Dis. 2017, 11, e0005625. [Google Scholar] [CrossRef] [PubMed]
- Kamgang, B.; Yougang, A.P.; Tchoupo, M.; Riveron, J.M.; Wondji, C. Temporal distribution and insecticide resistance profile of two major arbovirus vectors Aedes aegypti and Aedes albopictus in Yaoundé, the capital city of Cameroon. Parasit. Vectors 2017, 10, 469. [Google Scholar] [CrossRef] [PubMed]
- Badolo, A.; Sombié, A.; Pignatelli, P.M.; Sanon, A.; Yaméogo, F.; Wangrawa, D.W.; Sanon, A.; Kanuka, H.; McCall, P.J.; Weetman, D. Insecticide resistance levels and mechanisms in Aedes aegypti populations in and around Ouagadougou, Burkina Faso. PLoS Negl. Trop. Dis. 2019, 13, e0007439. [Google Scholar] [CrossRef] [PubMed]
- Sene, N.M.; Mavridis, K.; Ndiaye, E.H.; Diagne, C.T.; Gaye, A.; Ngom, E.H.M.; Ba, Y.; Diallo, D.; Vontas, J.; Dia, I.; et al. Insecticide resistance status and mechanisms in Aedes aegypti populations from Senegal. PLoS Negl. Trop. Dis. 2021, 15, e0009393. [Google Scholar] [CrossRef]
- Ranson, H.; Burhani, J.; Lumjuan, N.; William, C., IV. Insecticide Resistance in Dengue Vectors. Available online: https://archive.lstmed.ac.uk/999/ (accessed on 5 January 2021).
- WHO. Global Vector Control Response 2017–2030: A Strategic Approach to Tackle Vector-Borne Diseases. 2017. Available online: https://www.who.int/publications/i/item/WHO-HTM-GVCR-2017.01 (accessed on 17 January 2025).
- Carvalho, D.O.; Nimmo, D.; Naish, N.; McKemey, A.R.; Gray, P.; Wilke, A.B.B.; Marrelli, M.T.; Virginio, J.F.; Alphey, L.; Capurro, M.L. Mass production of genetically modified Aedes aegypti for field releases in Brazil. J. Vis. Exp. 2014, 83, e3579. [Google Scholar] [CrossRef]
- Bouyer, J.; Yamada, H.; Pereira, R.; Bourtzis, K.; Vreysen, M.J.B. Phased Conditional Approach for Mosquito Management Using Sterile Insect Technique. Trends Parasitol. 2020, 36, 325–336. [Google Scholar] [CrossRef]
- Burt, A.; Crisanti, A. Gene Drive: Evolved and Synthetic. ACS Chem. Biol. 2018, 13, 343–346. [Google Scholar] [CrossRef]
- Dyck, V.A.; Hendrichs, J.; Robinson, A.S. (Eds.) Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2021; p. 1216. [Google Scholar]
- Munhenga, G.; Brooke, B.D.; Gilles, J.R.L.; Slabbert, K.; Kemp, A.; Dandalo, L.C.; Wood, O.R.; Lobb, L.N.; Govender, D.; Renke, M.; et al. Mating competitiveness of sterile genetic sexing strain males (GAMA) under laboratory and semi-field conditions: Steps towards the use of the Sterile Insect Technique to control the major malaria vector Anopheles arabiensis in South Africa. Parasit. Vectors 2016, 9, 122. [Google Scholar] [CrossRef]
- Carvalho, D.O.; McKemey, A.R.; Garziera, L.; Lacroix, R.; Donnelly, C.A.; Alphey, L.; Malavasi, A.; Capurro, M.L. Suppression of a Field Population of Aedes aegypti in Brazil by Sustained Release of Transgenic Male Mosquitoes. PLoS Negl. Trop. Dis. 2015, 9, e0003864. [Google Scholar] [CrossRef]
- Nazni, W.A.; Teoh, G.-N.; Shaikh Norman Hakimi, S.I.; Muhammad Arif, M.A.; Tanusshni, M.; Nuradila, M.A.; Nurfarahin Hanini, A.; Shazia, I.A.; Tan, A.-M.; Rabizah, H.; et al. Aedes Control Using Sterile Insect Technique (SIT) in Malaysia. In Genetically Modified and Other Innovative Vector Control Technologies: Eco-Bio-Social Considerations for Safe Application; Tyagi, B.K., Ed.; Springer: Singapore, 2021; pp. 143–162. [Google Scholar] [CrossRef]
- Ranathunge, T.; Harishchandra, J.; Maiga, H.; Bouyer, J.; Gunawardena, Y.I.N.S.; Hapugoda, M. Development of the Sterile Insect Technique to control the dengue vector Aedes aegypti (Linnaeus) in Sri Lanka. PLoS ONE 2022, 17, e0265244. [Google Scholar] [CrossRef]
- Ong, J.; Ho, S.H.; Soh, S.X.H.; Wong, Y.; Ng, Y.; Vasquez, K.; Lai, Y.L.; Setoh, Y.X.; Chong, C.-S.; Lee, V.; et al. Assessing the efficacy of male Wolbachia-infected mosquito deployments to reduce dengue incidence in Singapore: Study protocol for a cluster-randomized controlled trial. Trials 2022, 23, 1023. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zhang, D.; Li, Y.; Yang, C.; Wu, Y.; Liang, X.; Liang, Y.; Pan, X.; Hu, L.; Sun, Q.; et al. Incompatible and sterile insect techniques combined eliminate mosquitoes. Nature 2019, 572, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Mating Harassment May Boost the Effectiveness of the Sterile Insect Technique for Aedes mosquitoes|Nature Communications. Available online: https://www.nature.com/articles/s41467-024-46268-x (accessed on 17 January 2025).
- Yao, F.A.; Millogo, A.A.; Epopa, P.S.; North, A.; Noulin, F.; Dao, K.; Drabo, M.; Guissou, C.; Kekele, S.; Namountougou, M.; et al. Mark-release-recapture experiment in Burkina Faso demonstrates reduced fitness and dispersal of genetically-modified sterile malaria mosquitoes. Nat. Commun. 2022, 13, 796. [Google Scholar] [CrossRef]
- Doyle, M.A.; Kline, D.L.; Allan, S.A.; Kaufman, P.E. Efficacy of Residual Bifenthrin Applied to Landscape Vegetation Against Aedes albopictus. J. Am. Mosq. Control. Assoc. 2009, 25, 179–183. Available online: http://www.bioone.org/doi/abs/10.2987/08-5804.1 (accessed on 17 January 2025). [CrossRef]
- Sawadogo, P.S.; Namountougou, M.; Toé, K.H.; Rouamba, J.; Maïga, H.; Ouédraogo, K.R.; Baldet, T.; Gouagna, L.C.; Kengne, P.; Simard, F.; et al. Swarming behaviour in natural populations of Anopheles gambiae and An. coluzzii: Review of 4 years survey in rural areas of sympatry, Burkina Faso (West Africa). Acta Trop. 2014, 132, S42–S52. [Google Scholar] [CrossRef]
- Maïga, H.; Niang, A.; Sawadogo, S.P.; Dabiré, R.K.; Lees, R.S.; Gilles, J.R.L.; Tripet, F.; Diabaté, A. Role of nutritional reserves and body size in Anopheles gambiae males mating success. Acta Trop. 2014, 132, S102–S107. [Google Scholar] [CrossRef]
- Smidler, A.L.; Scott, S.N.; Mameli, E.; Shaw, W.R.; Catteruccia, F. A transgenic tool to assess Anopheles mating competitiveness in the field. Parasit. Vectors 2018, 11, 651. [Google Scholar] [CrossRef]
- Bhatt, S.; Weiss, D.J.; Cameron, E.; Bisanzio, D.; Mappin, B.; Dalrymple, U.; Battle, K.; Moyes, C.L.; Henry, A.; Eckhoff, P.A.; et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 2015, 526, 207–211. [Google Scholar] [CrossRef]
- Boubidi, S.C.; Rossignol, M.; Chandre, F.; Tounsi, R.; Lagneau, C.; Fontenille, D.; Reiter, P. Gender Bias in Insecticide Susceptibility of Aedes albopictus is Solely Attributable to Size. J. Am. Mosq. Control Assoc. 2016, 32, 251–253. [Google Scholar] [CrossRef]
- da Silva, E.B.; Florêncio, S.G.L.; Amaral, A.; de Melo-Santos, M.A.V. Assessing radiation-induced enzyme activation in Aedes aegypti: Potential challenges for SIT-based vector management. Acta Trop. 2025, 261, 107518. [Google Scholar] [CrossRef]
- Namountougou, M.; Soma, D.D.; Kientega, M.; Balboné, M.; Kaboré, D.P.A.; Drabo, S.F.; Coulibaly, A.Y.; Fournet, F.; Baldet, T.; Diabaté, A.; et al. Insecticide resistance mechanisms in Anopheles gambiae complex populations from Burkina Faso, West Africa. Acta Trop. 2019, 197, 105054. [Google Scholar] [CrossRef] [PubMed]
- WHO. Manual for Monitoring Insecticide Resistance in Mosquito Vectors and Selecting Appropriate Interventions. 2022. Available online: https://www.who.int/publications/i/item/9789240051089 (accessed on 17 January 2025).
- Maïga, H.; Dabiré, R.K.; Lehmann, T.; Tripet, F.; Diabaté, A. Variation in energy reserves and role of body size in the mating system of Anopheles gambiae. J. Vector Ecol. 2012, 37, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Klowden, J.; Blackmer, L. Effects of larval nutrition on the host-seeking behavior of adult aedes aegypti mosquitoes. J. Am. Mosq. Control. Assoc. 1988, 4, 73–75. [Google Scholar] [PubMed]
- Hemingway, J. The role of vector control in stopping the transmission of malaria: Threats and opportunities. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2014, 369, 20130431. [Google Scholar] [CrossRef]
- Edi, C.V.A.; Koudou, B.G.; Jones, C.M.; Weetman, D.; Ranson, H. Multiple-insecticide resistance in Anopheles gambiae mosquitoes, Southern Côte d’Ivoire. Emerg. Infect. Dis. 2012, 18, 1508–1511. [Google Scholar] [CrossRef]
- Reiter, P. Surveillance and control of urban dengue vectors. In Dengue and Dengue Hemorrhagic Fever; Cabi: Wallingford, UK, 2014; pp. 481–518. [Google Scholar]
- Unlu, I.; Farajollahi, A.; Rochlin, I.; Crepaeau, T.N.; Strickman, D.; Gaugler, R. Differences in male-female ratios of Aedes albopictus (Diptera: Culicidae) following ultra-low volume adulticide applications. Acta Trop. 2014, 137, 201–205. [Google Scholar] [CrossRef]
- Vontas, J.; Kioulos, E.; Pavlidi, N.; Morou, E.; della Torre, A.; Ranson, H. Insecticide resistance in the major dengue vectors Aedes albopictus and Aedes aegypti. Pestic. Biochem. Physiol. 2012, 104, 126–131. [Google Scholar] [CrossRef]
- Smith, L.B.; Kasai, S.; Scott, J.G. Pyrethroid resistance in Aedes aegypti and Aedes albopictus: Important mosquito vectors of human diseases. Pestic. Biochem. Physiol. 2016, 133, 1–12. [Google Scholar] [CrossRef]
- Meng, L.-W.; Yuan, G.-R.; Chen, M.-L.; Zheng, L.-S.; Dou, W.; Peng, Y.; Bai, W.-J.; Li, Z.-Y.; Vontas, J.; Wang, J.-J. Cuticular competing endogenous RNAs regulate insecticide penetration and resistance in a major agricultural pest. BMC Biol. 2023, 21, 187. [Google Scholar] [CrossRef]
- Balabanidou, V.; Grigoraki, L.; Vontas, J. Insect cuticle: A critical determinant of insecticide resistance. Curr. Opin. Insect Sci. 2018, 27, 68–74. [Google Scholar] [CrossRef]
- Tsecouras, J.C.; Hung, K.Y.; Henke, J.A.; Gerry, A.C. Effect of sex and age on survival of adult culex tarsalis from a susceptible laboratory strain exposed to permethrin in the cdc bottle bioassay. J. Am. Mosq. Control Assoc. 2024, 40, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Hardstone, M.C.; Huang, X.; Harrington, L.C.; Scott, J.G. Differences in development, glycogen, and lipid content associated with cytochrome P450-mediated permethrin resistance in Culex pipiens quinquefasciatus (Diptera: Culicidae). J. Med. Entomol. 2010, 47, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Rivero, A.; Magaud, A.; Nicot, A.; Vézilier, J. Energetic cost of insecticide resistance in Culex pipiens mosquitoes. J. Med. Entomol. 2011, 48, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Owusu, H.F.; Jančáryová, D.; Malone, D.; Müller, P. Comparability between insecticide resistance bioassays for mosquito vectors: Time to review current methodology? Parasit. Vectors 2015, 8, 357. [Google Scholar] [CrossRef]
- Bamou, R.; Kopya, E.; Nkahe, L.D.; Menze, B.D.; Awono-Ambene, P.; Tchuinkam, T.; Njiokou, F.; Wondji, C.S.; Antonio-Nkondjio, C. Increased prevalence of insecticide resistance in Anopheles coluzzii populations in the city of Yaoundé, Cameroon and influence on pyrethroid-only treated bed net efficacy. Parasite Paris Fr. 2021, 28, 8. [Google Scholar] [CrossRef]
- Ouedraogo, C.O.W.; Some, F.A.; Sagna, A.B.; Sougue, E.; Soma, D.D.; Ndiaye, M.; Coulibaly, F.H.; Pooda, S.H.; Zela, L.; Roberge, C.; et al. Comparative Susceptibility of Wild and Laboratory-Reared Aedes and Anopheles larvae to Ivermectin®|Research Square. 2025. Available online: https://www.researchsquare.com/article/rs-5408919/v1 (accessed on 24 February 2025).
- Ranson, H.; Lissenden, N. Insecticide Resistance in African Anopheles Mosquitoes: A Worsening Situation that Needs Urgent Action to Maintain Malaria Control. Trends Parasitol. 2016, 32, 187–196. [Google Scholar] [CrossRef]
- Achee, N.L.; Grieco, J.P.; Vatandoost, H.; Seixas, G.; Pinto, J.; Ching-Ng, L.; Martins, A.J.; Juntarajumnong, W.; Corbel, V.; Gouagna, C.; et al. Alternative strategies for mosquito-borne arbovirus control. PLoS Negl. Trop. Dis. 2019, 13, e0006822. [Google Scholar] [CrossRef]
- Douchet, L.; Haramboure, M.; Baldet, T.; L’Ambert, G.; Damiens, D.; Gouagna, L.C.; Bouyer, J.; Labbé, P.; Tran, A. Comparing sterile male releases and other methods for integrated control of the tiger mosquito in temperate and tropical climates. Sci. Rep. 2021, 11, 7354. [Google Scholar] [CrossRef]
- Pleydell, D.R.J.; Bouyer, J. Biopesticides improve efficiency of the sterile insect technique for controlling mosquito-driven dengue epidemics. Commun. Biol. 2019, 2, 201. [Google Scholar] [CrossRef]
- Selvaraj, P.; Wenger, E.A.; Bridenbecker, D.; Windbichler, N.; Russell, J.R.; Gerardin, J.; Bever, C.A.; Nikolov, M. Vector genetics, insecticide resistance and gene drives: An agent-based modeling approach to evaluate malaria transmission and elimination. PLoS Comput. Biol. 2020, 16, e1008121. [Google Scholar] [CrossRef]
- Hancock, P.A.; North, A.; Leach, A.W.; Winskill, P.; Ghani, A.C.; Godfray, H.C.J.; Burt, A.; Mumford, J.D. The potential of gene drives in malaria vector species to control malaria in African environments. Nat. Commun. 2024, 15, 8976. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maiga, H.; Millogo, A.S.; Bayili, K.; Bilgo, E.; Toe, I.; Dabiré, R.K.; Bouyer, J.; Diabaté, A. Screening the Resistance of Male Aedes aegypti and Anopheles coluzzii to Insecticides in the Context of Using Genetic Control Tools in Burkina Faso. Insects 2025, 16, 315. https://doi.org/10.3390/insects16030315
Maiga H, Millogo AS, Bayili K, Bilgo E, Toe I, Dabiré RK, Bouyer J, Diabaté A. Screening the Resistance of Male Aedes aegypti and Anopheles coluzzii to Insecticides in the Context of Using Genetic Control Tools in Burkina Faso. Insects. 2025; 16(3):315. https://doi.org/10.3390/insects16030315
Chicago/Turabian StyleMaiga, Hamidou, Abel Souro Millogo, Koama Bayili, Etienne Bilgo, Inoussa Toe, Roch Kounbobr Dabiré, Jeremy Bouyer, and Abdoulaye Diabaté. 2025. "Screening the Resistance of Male Aedes aegypti and Anopheles coluzzii to Insecticides in the Context of Using Genetic Control Tools in Burkina Faso" Insects 16, no. 3: 315. https://doi.org/10.3390/insects16030315
APA StyleMaiga, H., Millogo, A. S., Bayili, K., Bilgo, E., Toe, I., Dabiré, R. K., Bouyer, J., & Diabaté, A. (2025). Screening the Resistance of Male Aedes aegypti and Anopheles coluzzii to Insecticides in the Context of Using Genetic Control Tools in Burkina Faso. Insects, 16(3), 315. https://doi.org/10.3390/insects16030315





