Integrated mRNA and miRNA Omics Analyses Reveal Transcriptional Regulation of the Tolerance Traits by Aquatica leii in Response to High Temperature
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Materials and Rearing Conditions
2.2. High-Temperature Treatment and Sample Collection
2.3. RNA Isolation
2.4. mRNA Sequencing and Genomes Align
2.5. Screening of Differentially Expressed Genes (DEGs) and KEGG Enrichment Analysis
2.6. Small RNA Sequencing and Genomes Align
2.7. Screening of Differentially Expressed miRNAs (DEMs)
2.8. Prediction and Enrichment Analysis of DEMs Targets
2.9. Quantitative PCR (qPCR) Analysis
3. Results
3.1. Overview of A. leii mRNA Sequencing Data
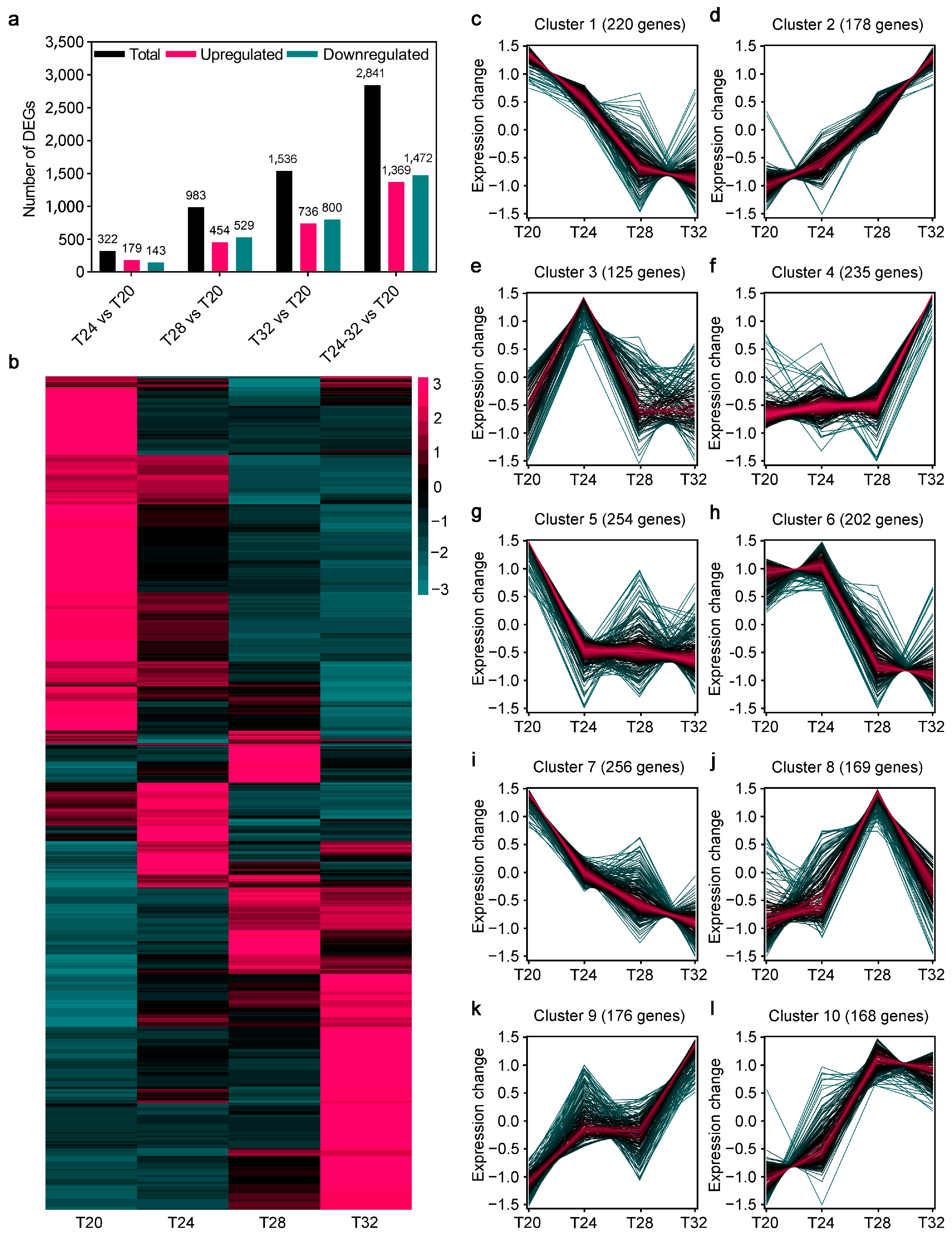
3.2. Analysis of DEGs of A. leii Under Different Temperature Treatments
3.3. KEGG Enrichment Analysis of Temperature-Responsive DEGs of A. leii
3.4. Overview of A. leii miRNA Sequencing Data
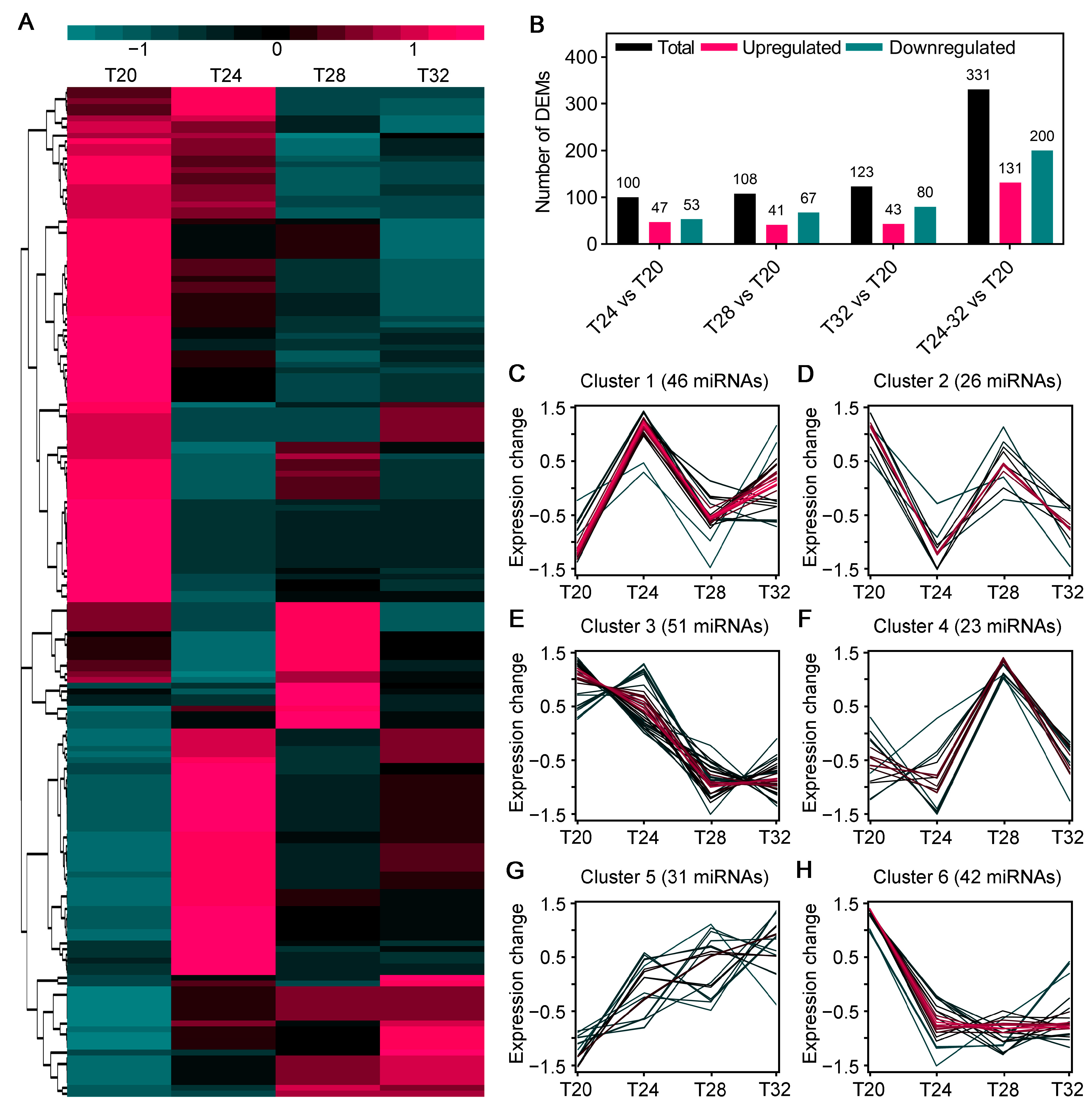
3.5. Analysis of DEMs of A. leii Under Different Temperature Treatments
3.6. Functional Annotation of DEM Targets of A. leii
3.7. Analysis of Key Temperature-Responsive DEMs in A. leii
3.8. Negatively Correlated of Key DEMs-DEGs Modules
3.9. Validation of the 70-Kilodalton Heat Shock Protein (HSP70) Gene by qPCR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gamboa, M.; Gotoh, Y.; Doloiras-Laraño, A.; Watanabe, K. Response of wild aquatic insect communities to thermal variation through comparative landscape transcriptomics. Arch. Insect Biochem. Physiol. 2024, 116, e22137. [Google Scholar] [CrossRef] [PubMed]
- Waldvogel, A.M.; Feldmeyer, B.; Rolshausen, G.; Exposito-Alonso, M.; Rellstab, C.; Kofler, R.; Mock, T.; Schmid, K.; Schmitt, I.; Bataillon, T.; et al. Evolutionary genomics can improve prediction of species’ responses to climate change. Evol. Lett. 2020, 4, 4–18. [Google Scholar] [CrossRef]
- González-Tokman, D.; Córdoba-Aguilar, A.; Dáttilo, W.; Lira-Noriega, A.; Sánchez-Guillén, R.A.; Villalobos, F. Insect responses to heat: Physiological mechanisms, evolution and ecological implications in a warming world. Biol. Rev. Camb. Philos. Soc. 2020, 95, 802–821. [Google Scholar] [CrossRef]
- Jensen, A.J.; Hagen, I.J.; Czorlich, Y.; Bolstad, G.H.; Bremset, G.; Finstad, B.; Hindar, K.; Skaala, Ø.; Karlsson, S. Large-effect loci mediate rapid adaptation of salmon body size after river regulation. Proc. Natl. Acad. Sci. USA 2022, 119, e2207634119. [Google Scholar] [CrossRef] [PubMed]
- You, S.; Lei, G.; Zhou, H.; Li, J.; Chen, S.; Huang, J.; Vasseur, L.; Gurr, G.M.; You, M.; Chen, Y. Thermal acclimation uncovers a simple genetic basis of adaptation to high temperature in a cosmopolitan pest. iScience 2024, 27, 109242. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.S.; Ma, G.; Pincebourde, S. Survive a warming climate: Insect responses to extreme high temperatures. Annu. Rev. Entomol. 2021, 66, 163–184. [Google Scholar] [CrossRef]
- Liu, Z.; Li, C.; Yang, W.; Wu, Q.; Xiao, W.; Zhu, Y.; Wei, Q.; Dong, Z.; Zhang, G.; Lu, C.; et al. The Bombyx mori singed gene is involved in the high-temperature resistance of silkworms. Insects 2024, 15, 264. [Google Scholar] [CrossRef]
- Weaving, H.; Terblanche, J.S.; Pottier, P.; English, S. Meta-analysis reveals weak but pervasive plasticity in insect thermal limits. Nat. Commun. 2022, 13, 5292. [Google Scholar] [CrossRef]
- Esperk, T.; Kjaersgaard, A.; Walters, R.J.; Berger, D.; Blanckenhorn, W.U. Plastic and evolutionary responses to heat stress in a temperate dung fly: Negative correlation between basal and induced heat tolerance? J. Evol. Biol. 2016, 29, 900–915. [Google Scholar] [CrossRef]
- Shah, A.A.; Woods, H.A.; Havird, J.C.; Encalada, A.C.; Flecker, A.S.; Funk, W.C.; Guayasamin, J.M.; Kondratieff, B.C.; Poff, N.L.; Thomas, S.A.; et al. Temperature dependence of metabolic rate in tropical and temperate aquatic insects: Support for the Climate Variability Hypothesis in mayflies but not stoneflies. Glob. Chang. Biol. 2021, 27, 297–311. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Tada, A.; Musolin, D.L.; Hari, N.; Hosokawa, T.; Fujisaki, K.; Fukatsu, T. Collapse of insect gut symbiosis under simulated climate change. mBio 2016, 7, e01578-16. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Zhang, X.; Yang, F.; Jocelin, N.F.; Shang, Y.; Wang, Q.; Liu, Z.; Guo, Y. Effects of heat tolerance on the gut microbiota of Sarcophaga peregrina (Diptera: Sarcophagidae) and impacts on the life history traits. Parasites Vectors 2023, 16, 364. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.L.; Meng, J.Y.; Zhou, J.Y.; Zhang, J.S.; Zhang, C.Y. Integrated transcriptomic and proteomic analyses reveal the molecular mechanism underlying the thermotolerant response of Spodoptera frugiperda. Int. J. Biol. Macromol. 2024, 264, 130578. [Google Scholar] [CrossRef] [PubMed]
- Pigliucci, M.; Murren, C.J.; Schlichting, C.D. Phenotypic plasticity and evolution by genetic assimilation. J. Exp. Biol. 2006, 209, 2362–2367. [Google Scholar] [CrossRef]
- Craig Stillwell, R.; Fox, C.W. Geographic variation in body size, sexual size dimorphism and fitness components of a seed beetle: Local adaptation versus phenotypic plasticity. Oikos 2009, 118, 703–712. [Google Scholar] [CrossRef]
- Bodlah, M.A.; Iqbal, J.; Ashiq, A.; Bodlah, I.; Jiang, S.; Mudassir, M.A.; Fareen, A.G.E. Insect behavioral restraint and adaptation strategies under heat stress: An inclusive review. J. Saudi Soc. Agric. Sci. 2023, 22, 327–350. [Google Scholar] [CrossRef]
- Tian, C.; Li, Y.; Wu, Y.; Chu, W.; Liu, H. Sustaining induced heat shock protein 70 confers biological thermotolerance in a high-temperature adapted predatory mite Neoseiulus barkeri (Hughes). Pest Manag. Sci. 2021, 77, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, G.A.; Xiao, C.; Krill, J.L.; Seroude, L.; Dawson-Scully, K.; Robertson, R.M. Glial Hsp70 protects K+ homeostasis in the Drosophila brain during repetitive anoxic depolarization. PLoS ONE 2011, 6, e28994. [Google Scholar] [CrossRef] [PubMed]
- Verberk, W.C.; Buchwalter, D.B.; Kefford, B.J. Energetics as a lens to understanding aquatic insect’s responses to changing temperature, dissolved oxygen and salinity regimes. Curr. Opin. Insect Sci. 2020, 41, 46–53. [Google Scholar] [CrossRef]
- Horne, C.R.; Hirst, A.G.; Atkinson, D. Temperature-size responses match latitudinal-size clines in arthropods, revealing critical differences between aquatic and terrestrial species. Ecol. Lett. 2015, 18, 327–335. [Google Scholar] [CrossRef]
- Nair, P.; Gibson, J.R.; Schwartz, B.F.; Nowlin, W.H. Temperature responses vary between riffle beetles from contrasting aquatic environments. J. Therm. Biol. 2023, 112, 103485. [Google Scholar] [CrossRef] [PubMed]
- Oba, Y.; Schultz, D.T. Firefly genomes illuminate the evolution of beetle bioluminescent systems. Curr. Opin. Insect Sci. 2022, 50, 100879. [Google Scholar] [CrossRef] [PubMed]
- Fukuta, K.; Kato, D.I.; Maeda, J.; Tsuruta, A.; Suzuki, H.; Nagano, Y.; Tsukamoto, H.; Niwa, K.; Terauchi, M.; Toyoda, A.; et al. Genome assembly of Genji firefly (Nipponoluciola cruciata) reveals novel luciferase-like luminescent proteins without peroxisome targeting signal. DNA Res. 2024, 31, dsae006. [Google Scholar] [CrossRef]
- Fu, X.; Zhu, X. Key homeobox transcription factors regulate the development of the firefly’s adult light organ and bioluminescence. Nat. Commun. 2024, 15, 1736. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.M.; Jusoh, W.F.A.; Walker, A.C.; Fallon, C.E.; Joyce, R.; Yiu, V. Illuminating firefly diversity: Trends, threats and conservation strategies. Insects 2024, 15, 71. [Google Scholar] [CrossRef]
- Lewis, S.M.; Wong, C.H.; Owens, A.C.; Fallon, C.; Jepsen, S.; Thancharoen, A.; Reed, J.M. A global perspective on firefly extinction threats. BioScience 2020, 70, 157–167. [Google Scholar] [CrossRef]
- Zhao, Z.S.; Yang, L.Y.; Li, F.X.; Cun, W.; Wang, X.Y.; Cao, C.Q.; Zhang, Q.L. Gut flora alterations among aquatic firefly Aquatica leii inhabiting various dissolved oxygen in fresh water. iScience 2023, 26, 107809. [Google Scholar] [CrossRef]
- Idris, N.S.; Mustapha, M.A.; Sulaiman, N.; Khamis, S.; Husin, S.M.; Darbis, N.D.A. The dynamics of landscape changes surrounding a firefly ecotourism area. Glob. Ecol. Conserv. 2021, 29, e01741. [Google Scholar] [CrossRef]
- Fu, X.; Ballantyne, L. An Overview of Aquatica Fu et al., a phylogeny of aquatic fireflies using mitochondrial genomes, a description of two new species, and a new record of aquatic fireflies in China (Coleoptera: Lampyridae: Luciolinae). Insects 2024, 15, 31. [Google Scholar] [CrossRef]
- Catalán, A.; Gygax, D.; Rodríguez-Montes, L.; Hinzke, T.; Hoff, K.J.; Duchen, P. Two novel genomes of fireflies with different degrees of sexual dimorphism reveal insights into sex-biased gene expression and dosage compensation. Commun. Biol. 2024, 7, 906. [Google Scholar] [CrossRef]
- Fu, X.; Ballantyne, L. Luciola leii sp. nov.; a new species of aquatic firefly (Coleoptera: Lampyridae: Luciolinae) from mainland China. Can. Entomol. 2006, 138, 339–347. [Google Scholar] [CrossRef]
- Fu, X.; Ballantyne, L.; Lambkin, C.L. Aquatica gen. nov. from mainland China with a description of Aquatica wuhana sp. nov. (Coleoptera: Lampyridae: Luciolinae). Zootaxa 2010, 2530, 1–18. [Google Scholar] [CrossRef]
- Zhang, Q.L.; Guo, J.; Deng, X.Y.; Wang, F.; Chen, J.Y.; Lin, L.B. Comparative transcriptomic analysis provides insights into the response to the benzo(a)pyrene stress in aquatic firefly (Luciola leii). Sci. Total Environ. 2019, 661, 226–234. [Google Scholar] [CrossRef]
- Zhang, Q.L.; Jiang, Y.H.; Dong, Z.X.; Li, H.W.; Lin, L.B. Exposure to benzo[a]pyrene triggers distinct patterns of microRNA transcriptional profiles in aquatic firefly Aquatica wuhana (Coleoptera: Lampyridae). J. Hazard. Mater. 2021, 401, 123409. [Google Scholar] [CrossRef]
- Yang, L.; Zhao, Z.; Luo, D.; Liang, M.; Zhang, Q. Global metabolomics of fireflies (Coleoptera: Lampyridae) explore metabolic adaptation to fresh water in insects. Insects 2022, 13, 823. [Google Scholar] [CrossRef]
- Zhang, Q.L.; Li, H.W.; Dong, Z.X.; Yang, X.J.; Lin, L.B.; Chen, J.Y.; Yuan, M.L. Comparative transcriptomic analysis of fireflies (Coleoptera: Lampyridae) to explore the molecular adaptations to fresh water. Mol. Ecol. 2020, 29, 2676–2691. [Google Scholar] [CrossRef] [PubMed]
- Salo, T.; Stamm, C.; Burdon, F.J.; Räsänen, K.; Seppälä, O. Resilience to heat waves in the aquatic snail Lymnaea stagnalis: Additive and interactive effects with micropollutants. Freshw. Biol. 2017, 62, 1831–1846. [Google Scholar] [CrossRef]
- Hermann, M.; Peeters, E.T.H.M.; Van den Brink, P.J. Heatwaves, elevated temperatures, and a pesticide cause interactive effects on multi-trophic levels of a freshwater ecosystem. Environ. Pollut. 2023, 327, 121498. [Google Scholar] [CrossRef] [PubMed]
- Hermann, M.; Polazzo, F.; Cherta, L.; Crettaz-Minaglia, M.; García-Astillero, A.; Peeters, E.T.H.M.; Rico, A.; Van den Brink, P.J. Combined stress of an insecticide and heatwaves or elevated temperature induce community and food web effects in a Mediterranean freshwater ecosystem. Water Res. 2024, 260, 121903. [Google Scholar] [CrossRef]
- Lee, H.; Calvin, K.; Dasgupta, D.; Krinner, G.; Mukherji, A.; Thorne, P.; Park, Y. IPCC, 2023: Climate Change 2023: Synthesis Report, Summary for Policymakers. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Lee, H., Romero, J., Eds.; IPCC: Geneva, Switzerland, 19 March 2023. [Google Scholar]
- Woolway, R.I.; Jennings, E.; Shatwell, T.; Golub, M.; Pierson, D.C.; Maberly, S.C. Lake heatwaves under climate change. Nature 2021, 589, 402–407. [Google Scholar] [CrossRef]
- Fu, X.; Meyer-Rochow, V.B. Selection and validation of suitable reference genes for rt-qpcr analysis in the rare aquatic firefly Aquatica leii (Coleoptera: Lampyridae). Insects 2021, 12, 359. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, L.; Zhou, F.; Li, J.; Wu, X.; Zhong, X.; Lv, H.; Yi, S.; Gao, Q.; Yang, Z.; et al. Integrated comparative transcriptome and weighted gene co-expression network analysis provide valuable insights into the response mechanisms of crayfish (Procambarus clarkii) to copper stress. J. Hazard. Mater. 2023, 448, 130820. [Google Scholar] [CrossRef] [PubMed]
- Chen, S. Ultrafast one-pass FASTQ data preprocessing, quality control, and deduplication using fastp. IMeta 2023, 2, e107. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef]
- Tang, D.; Chen, M.; Huang, X.; Zhang, G.; Zeng, L.; Zhang, G.; Wu, S.; Wang, Y. SRplot: A free online platform for data visualization and graphing. PLoS ONE 2023, 18, e0294236. [Google Scholar] [CrossRef]
- Wang, C.; Yin, S.; Wang, Q.; Jiang, M.; Li, S.; Zhen, W.; Duan, Y.; Gu, H. miR-409-3p Regulated by GATA2 Promotes Cardiac Fibrosis through Targeting Gpd1. Oxid. Med. Cell Longev. 2022, 2022, 8922246. [Google Scholar] [CrossRef]
- Kang, Y.; Yang, X.; Liu, Y.; Shi, M.; Zhang, W.; Fan, Y.; Yao, Y.; Zhang, J.; Qin, S. Integration of mRNA and miRNA analysis reveals the molecular mechanism of potato (Solanum tuberosum L.) response to alkali stress. Int. J. Biol. Macromol. 2021, 182, 938–949. [Google Scholar] [CrossRef] [PubMed]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Friedländer, M.R.; Mackowiak, S.D.; Li, N.; Chen, W.; Rajewsky, N. miRDeep2 accurately identifies known and hundreds of novel microRNA genes in seven animal clades. Nucleic Acids. Res. 2012, 40, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Kozomara, A.; Griffiths-Jones, S. miRBase: Annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res. 2014, 42, D68–D73. [Google Scholar] [CrossRef]
- Wang, X.; Chen, X.; Sun, X.; Ao, J. Comparative analysis of miRNAs and mRNAs in large yellow croaker head kidney cells (LYCK) provided novel insights into the redox regulation of fish. Sci. Total Environ. 2024, 918, 170503. [Google Scholar] [CrossRef]
- Enright, A.; John, B.; Gaul, U.; Tuschl, T.; Sander, C.; Marks, D. MicroRNA targets in Drosophila. Genome Biol. 2003, 4, R1. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Harvey, J.A.; Tougeron, K.; Gols, R.; Heinen, R.; Abarca, M.; Abram, P.K.; Chown, S.L. Scientists’ warning on climate change and insects. Ecol. Monogr. 2023, 93, e1553. [Google Scholar] [CrossRef]
- Mason, C.J.; Shikano, I. Hotter days, stronger immunity? Exploring the impact of rising temperatures on insect gut health and microbial relationships. Curr. Opin. Insect Sci. 2023, 59, 101096. [Google Scholar] [CrossRef]
- Hayward, S.A. Application of functional ‘Omics’ in environmental stress physiology: Insights, limitations, and future challenges. Curr. Opin. Insect Sci. 2014, 4, 35–41. [Google Scholar] [CrossRef]
- Zhang, X.; Shao, W.; Huo, Y.; Kong, Y.; Zhang, W.; Li, S.; Zhou, W.; Wu, X.; Qin, F.; Hu, X. The effects of short-term dietary exposure to SiO2 nanoparticle on the domesticated lepidopteran insect model silkworm (Bombyx mori): Evidence from the perspective of multi-omics. Chemosphere 2023, 323, 138257. [Google Scholar] [CrossRef]
- Turan, M. Genome-wide analysis and characterization of HSP gene families (HSP20, HSP40, HSP60, HSP70, HSP90) in the yellow fever mosquito (Aedes aegypti) (Diptera: Culicidae). J. Insect Sci. 2023, 23, 27. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Su, H.; Li, R.; Li, X.; Xu, Y.; Dai, X.; Zhou, Y.; Wang, H. Comparative transcriptome analysis of Glyphodes pyloalis Walker (Lepidoptera: Pyralidae) reveals novel insights into heat stress tolerance in insects. BMC Genom. 2017, 18, 974. [Google Scholar] [CrossRef]
- Sørensen, J.G.; Schou, M.F.; Kristensen, T.N.; Loeschcke, V. Thermal fluctuations affect the transcriptome through mechanisms independent of average temperature. Sci. Rep. 2016, 6, 30975. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Wan, K.; Lü, Y.; Ouyang, W.; Huang, J.; Zheng, L.; Miao, L.; Su, S.; Li, Z. Comparison of brain gene expression profiles associated with auto-grooming behavior between Apis cerana and Apis mellifera infested by Varroa destructor. Genes 2024, 15, 763. [Google Scholar] [CrossRef]
- Wei, J.; Liu, J.; Liang, S.; Sun, M.; Duan, J. Low-dose exposure of silica nanoparticles induces neurotoxicity via neuroactive ligand-receptor interaction signaling pathway in Zebrafish embryos. Int. J. Nanomed. 2020, 15, 4407–4415. [Google Scholar] [CrossRef] [PubMed]
- Li, S.S.; Fang, S.M.; Chen, J.; Zhang, Z.; Yu, Q.Y. Effects of short-term exposure to volatile pesticide dichlorvos on the olfactory systems in Spodoptera litura: Calcium homeostasis, synaptic plasticity and apoptosis. Sci. Total Environ. 2023, 864, 161050. [Google Scholar] [CrossRef]
- Wei, F.; Wang, D.; Li, H.; Xia, P.; Ran, Y.; You, J. Toxicogenomics provides insights to toxicity pathways of neonicotinoids to aquatic insect, Chironomus dilutus. Environ. Pollut. 2020, 260, 114011. [Google Scholar] [CrossRef]
- Raza, S.H.A.; Abdelnour, S.A.; Alotaibi, M.A.; AlGabbani, Q.; Naiel, M.A.; Shokrollahi, B.; Zan, L. MicroRNAs mediated environmental stress responses and toxicity signs in teleost fish species. Aquaculture 2022, 546, 737310. [Google Scholar] [CrossRef]
- Zhang, B.; Zhao, L.; Ning, J.; Wickham, J.D.; Tian, H.; Zhang, X.; Yang, M.; Wang, X.; Sun, J. miR-31-5p regulates cold acclimation of the wood-boring beetle Monochamus alternatus via ascaroside signaling. BMC Biol. 2020, 18, 184. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, P.; Sun, L.; Cao, C. Integration of miRNA and mRNA expression profiles in Asian spongy moth Lymantria dispar in response to cyantraniliprole. Pestic. Biochem. Physiol. 2023, 191, 105364. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.K.; Sharp, P.A. MicroRNA functions in stress responses. Mol. Cell 2010, 40, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, S.A.; Beal, P.R.; Yao, S.Y.; King, A.E.; Cass, C.E.; Young, J.D. The equilibrative nucleoside transporter family, SLC29. Pflugers Arch. 2004, 447, 735–743. [Google Scholar] [PubMed]
- Lemos, F.J.; Cornel, A.J.; Jacobs-Lorena, M. Trypsin and aminopeptidase gene expression is affected by age and food composition in Anopheles gambiae. Insect Biochem. Mol. Biol. 1996, 26, 651–658. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Li, J.; Yan, L.; Zhu, Y.; Li, Z.; Cao, C.; Wang, Y. Integrated mRNA and miRNA Omics Analyses Reveal Transcriptional Regulation of the Tolerance Traits by Aquatica leii in Response to High Temperature. Insects 2025, 16, 316. https://doi.org/10.3390/insects16030316
Liu C, Li J, Yan L, Zhu Y, Li Z, Cao C, Wang Y. Integrated mRNA and miRNA Omics Analyses Reveal Transcriptional Regulation of the Tolerance Traits by Aquatica leii in Response to High Temperature. Insects. 2025; 16(3):316. https://doi.org/10.3390/insects16030316
Chicago/Turabian StyleLiu, Chao, Jiapeng Li, Lihong Yan, Yuting Zhu, Zikun Li, Chengquan Cao, and Yiping Wang. 2025. "Integrated mRNA and miRNA Omics Analyses Reveal Transcriptional Regulation of the Tolerance Traits by Aquatica leii in Response to High Temperature" Insects 16, no. 3: 316. https://doi.org/10.3390/insects16030316
APA StyleLiu, C., Li, J., Yan, L., Zhu, Y., Li, Z., Cao, C., & Wang, Y. (2025). Integrated mRNA and miRNA Omics Analyses Reveal Transcriptional Regulation of the Tolerance Traits by Aquatica leii in Response to High Temperature. Insects, 16(3), 316. https://doi.org/10.3390/insects16030316






