GSTD1 Mediates the Tolerance to Abamectin and Beta-Cypermethrin in the Fall Armyworm Spodoptera frugiperda
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects and Insecticides
2.2. RNA Isolation and cDNA Synthesis
2.3. Sequence Analysis and Phylogenetic Tree Construction
2.4. Real-Time Quantitative PCR Analyses (RT-qPCR)
2.5. Preparation of dsRNA and RNA Interference
2.6. Construction of Sf9 Cell Lines Stably Expressing SfGSTD1
2.7. Cell Viability Assay
2.8. Construction of Transgenic D. melanogaster and Bioassay
2.9. Expression and Purification of Recombinant Proteins
2.10. Enzyme Kinetic Properties and Enzyme Inhibition Assays
2.11. Statistics
3. Results
3.1. Molecular Characterization of SfGSTD1

3.2. Temporal and Spatial Expression of SfGSTD1
3.3. Silencing of SfGSTD1 Increased Larval Susceptibility of S. frugiperda to Insecticides
3.4. Overexpression of SfGSTD1 Decreased Toxicity of Insecticides to Sf9 Cells
3.5. Expression of SfGSTD1 in D. melanogaster Leads to Decreased Susceptibility to Insecticides
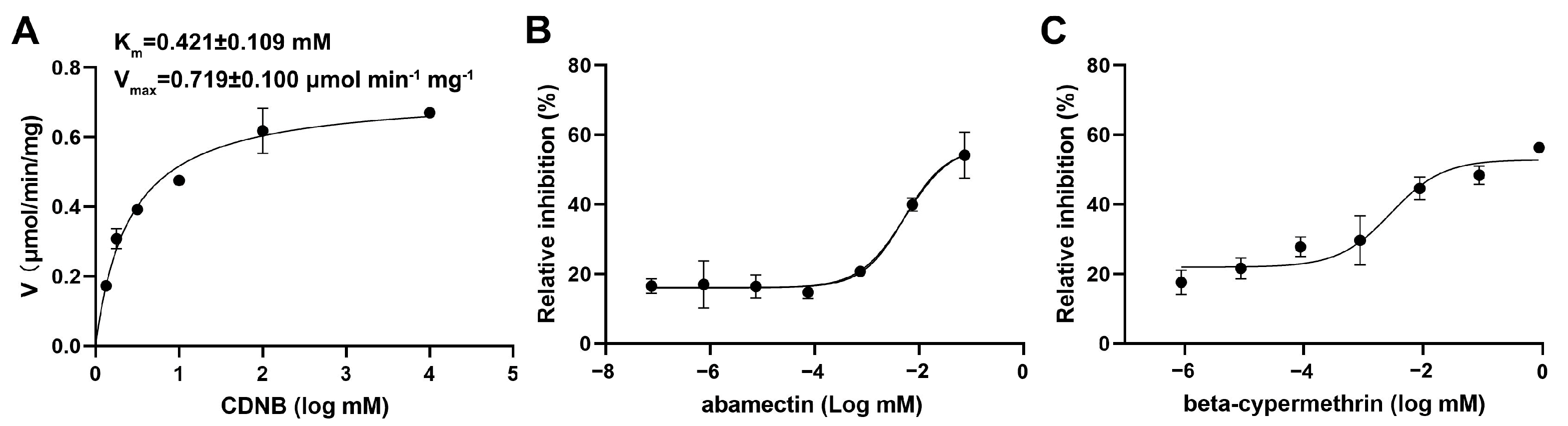
3.6. Enzymatic Properties and Inhibitions of Insecticides on Recombinant SfGSTD1
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wahba, T.F.; El-Bassyouni, G.T.; El-Shamy, A.M.; Wahba, M.N. Nanoinert diatomaceous and emamectin benzoate: Enhancing wheat protection against fall armyworms for sustainable management. S Afr. J. Bot. 2024, 169, 413–425. [Google Scholar] [CrossRef]
- Montezano, D.G.; Sosa-Gómez, D.R.; Specht, A.; Roque-Specht, V.F.; Sousa-Silva, J.C.; Paula-Moraes, S.V.d.; Peterson, J.A.; Hunt, T.E. Host plants of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas. Afr. Entomol. 2018, 26, 286–300. [Google Scholar] [CrossRef]
- Pantoja, A.; Smith, C.M.; Robinson, J.F. Effects of the fall armyworm (Lepidoptera: Noctuidae) on rice yields. J. Econ. Entomol. 1986, 79, 1324–1329. [Google Scholar] [CrossRef]
- Pitre, H.N.; Hogg, D.B. Development of the fall armyworm on cotton, soybean and corn. J. Ga. Entomol. Soc. 1983, 18, 182–187. [Google Scholar]
- Young, J.R.; McMillian, W.W. Differential feeding by two strains of fall armyworm larvae on carbaryl treated surfaces. J. Econ. Entomol. 1979, 72, 202–203. [Google Scholar] [CrossRef]
- Yu, S.J. Insecticide resistance in the fall armyworm, Spodoptera frugiperda (J. E. Smith). Pestic. Biochem. Physiol. 1991, 39, 84–91. [Google Scholar] [CrossRef]
- Wood, K.A.; Wilson, B.H.; Graves, J.B. Influence of host plant on the susceptibility of the fall armyworm to insecticides. J. Econ. Entomol. 1981, 74, 96–98. [Google Scholar] [CrossRef]
- Gutiérrez-Moreno, R.; Mota-Sanchez, D.; Blanco, C.A.; Whalon, M.E.; Terán-Santofimio, H.; Rodriguez-Maciel, J.C.; DiFonzo, C. Field-evolved resistance of the fall armyworm (Lepidoptera: Noctuidae) to synthetic insecticides in Puerto Rico and Mexico. J. Econ. Entomol. 2019, 112, 792–802. [Google Scholar] [CrossRef]
- Hayes, J.D.; Pulford, D.J. The glutathione S-transferase supergene family: Regulation of GST and the contribution of the lsoenzymes to cancer chemoprotection and drug resistance part II. Crit. Rev. Biochem. Mol. Biol. 1995, 30, 521–600. [Google Scholar] [CrossRef]
- Younus, F.; Chertemps, T.; Pearce, S.L.; Pandey, G.; Bozzolan, F.; Coppin, C.W.; Russell, R.J.; Maïbèche-Coisne, M.; Oakeshott, J.G. Identification of candidate odorant degrading gene/enzyme systems in the antennal transcriptome of Drosophila melanogaster. Insect Biochem. Mol. Biol. 2014, 53, 30–43. [Google Scholar] [CrossRef]
- Terrier, P.; Townsend, A.J.; Coindre, J.M.; Triche, T.J.; Cowan, K.H. An immunohistochemical study of pi class glutathione S-transferase expression in normal human tissue. Am. J. Pathol. 1990, 137, 845–853. [Google Scholar] [PubMed]
- Enya, S.; Yamamoto, C.; Mizuno, H.; Esaki, T.; Lin, H.-K.; Iga, M.; Morohashi, K.; Hirano, Y.; Kataoka, H.; Masujima, T.; et al. Dual roles of glutathione in ecdysone biosynthesis and antioxidant function during larval development in Drosophila. Genetics 2017, 207, 1519–1532. [Google Scholar] [CrossRef] [PubMed]
- Dasari, S.; Ganjayi, M.S.; Yellanurkonda, P.; Basha, S.; Meriga, B. Role of glutathione S-transferases in detoxification of a polycyclic aromatic hydrocarbon, methylcholanthrene. Chem. Biol. Interact. 2018, 294, 81–90. [Google Scholar] [CrossRef]
- Jin, M.; Peng, Y.; Peng, J.; Zhang, H.; Shan, Y.; Liu, K.; Xiao, Y. Transcriptional regulation and overexpression of GST cluster enhances pesticide resistance in the cotton bollworm, Helicoverpa armigera (Lepidoptera: Noctuidae). Commun. Biol. 2023, 6, 1064. [Google Scholar] [CrossRef]
- Wang, Q.; Rui, C.; Wang, L.; Nahiyoon, S.A.; Huang, W.; Zhu, J.; Ji, X.; Yang, Q.; Yuan, H.; Cui, L. Field-evolved resistance to 11 insecticides and the mechanisms involved in Helicoverpa armigera (Lepidoptera: Noctuidae). Pest. Manag. Sci. 2021, 77, 5086–5095. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Liu, Y.; Zhang, Z.P.; Wu, D.D.; Zhuang, L.X.; Algradi, A.M.; Kuang, H.X.; Yang, B.Y. Schisandraceae triterpenoids: A review of phytochemistry, bioactivities and synthesis. Fitoterapia 2022, 161, 105230. [Google Scholar] [CrossRef]
- Lu, X.P.; Xu, L.; Meng, L.W.; Wang, L.L.; Niu, J.; Wang, J.J. Divergent molecular evolution in glutathione S-transferase conferring malathion resistance in the oriental fruit fly, Bactrocera dorsalis (Hendel). Chemosphere 2020, 242, 125203. [Google Scholar] [CrossRef]
- Tang, B.; Dai, W.; Qi, L.; Zhang, Q.; Zhang, C. Identification and functional analysis of a delta class glutathione S-transferase gene associated with insecticide detoxification in Bradysia odoriphaga. J. Agric. Food Chem. 2019, 67, 9979–9988. [Google Scholar] [CrossRef]
- Liu, J.; Yang, X.; Zhang, Y. Characterization of a lambda-cyhalothrin metabolizing glutathione S-transferase CpGSTd1 from Cydia pomonella (L.). Appl. Microbiol. Biotechnol. 2014, 98, 8947–8962. [Google Scholar] [CrossRef]
- Daneshian, L.; Schlachter, C.; Timmers, L.F.S.M.; Radford, T.; Kapingidza, B.; Dias, T.; Liese, J.; Sperotto, R.A.; Grbic, V.; Grbic, M.; et al. Delta class glutathione S-transferase (TuGSTd01) from the two-spotted spider mite Tetranychus urticae is inhibited by abamectin. Pestic. Biochem. Physiol. 2021, 176, 104873. [Google Scholar] [CrossRef]
- Song, X.; Pei, L.; Zhang, Y.; Chen, X.; Zhong, Q.; Ji, Y.; Tang, J.; Feng, F.; Li, B. Functional diversification of three delta-class glutathione S-transferases involved in development and detoxification in Tribolium castaneum. Insect Mol. Biol. 2020, 29, 320–336. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.L.; Chen, R.R.; Zhang, Y.; Ma, Y.; Cui, J.J.; Han, Z.J.; Mu, L.L.; Li, G.Q. A Spodoptera exigua cadherin serves as a putative receptor for Bacillus thuringiensis Cry1Ca toxin and shows differential enhancement of Cry1Ca and Cry1Ac toxicity. Appl. Environ. Microbiol. 2013, 79, 5576–5583. [Google Scholar] [CrossRef]
- Wang, L.X.; Wen, B.; Guo, S.Y.; Han, Y.J.; Deng, Z.Y.; Ding, Q.; Li, X.C. Identification and characterization of ATP-binding cassette transporters involved in chlorantraniliprole tolerance of model insect Drosophila melanogaster and agricultural pest Spodoptera frugiperda. Pestic. Biochem. Physiol. 2024, 206, 106212. [Google Scholar] [CrossRef]
- Chen, X.; Palli, S.R. Transgenic overexpression of P450 genes confers deltamethrin resistance in the fall armyworm, Spodoptera frugiperda. J. Pest. Sci. 2022, 95, 1197–1205. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Hayes, J.D.; Flanagan, J.U.; Jowsey, I.R. Glutathione transferases. Annu. Rev. Pharmacol. 2005, 45, 51–88. [Google Scholar] [CrossRef]
- Fang, S.M. Insect glutathione S-transferase: A review of comparative genomic studies and response to xenobiotics. Bull. Insectol. 2012, 65, 265–271. [Google Scholar]
- Enayati, A.A.; Ranson, H.; Hemingway, J. Insect glutathione transferases and insecticide resistance. Insect Mol. Biol. 2005, 14, 3–8. [Google Scholar] [CrossRef]
- Farina, P.; Bedini, S.; Conti, B. Multiple functions of malpighian tubules in insects: A review. Insects 2022, 13, 1001. [Google Scholar] [CrossRef]
- Meng, L.W.; Yuan, G.R.; Lu, X.P.; Jing, T.X.; Zheng, L.S.; Yong, H.X.; Wang, J.J. Two delta class glutathione S-transferases involved in the detoxification of malathion in Bactrocera dorsalis (Hendel). Pest. Manag. Sci. 2019, 75, 1527–1538. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.H.; Zhu, M.Y.; Xu, Z.H.; Li, X.J.; Peng, C.Y.; Fan, X.P.; Li, Y.Q. Functional characterization of an epsilon glutathione S-transferase (SfGSTe9) associated with insecticide detoxification in Spodoptera frugiperda. Pestic. Biochem. Physiol. 2025, 208, 106305. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Xu, Z.; Zou, H.; Liu, J.; Chen, S.; Feng, Q.; Zheng, S. Glutathione S-transferase SlGSTE1 in Spodoptera litura may be associated with feeding adaptation of host plants. Insect Biochem. Mol. Biol. 2016, 70, 32–43. [Google Scholar] [CrossRef]
- Hu, B.; Hu, S.; Huang, H.; Wei, Q.; Ren, M.; Huang, S.; Tian, X.; Su, J. Insecticides induce the co-expression of glutathione S-transferases through ROS/CncC pathway in Spodoptera exigua. Pestic. Biochem. Physiol. 2019, 155, 58–71. [Google Scholar] [CrossRef]
- Li, D.; He, C.; Xie, L.; Ge, X.; Deng, T.; Li, S.; Li, G.; Xu, L. SlGSTE9 participates in the stability of chlorpyrifos resistance in Spodoptera litura. Pest. Manag. Sci. 2021, 77, 5430–5438. [Google Scholar] [CrossRef]
- Li, D.; Xu, L.; Liu, H.; Chen, X.; Zhou, L. Metabolism and antioxidant activity of SlGSTD1 in Spodoptera litura as a detoxification enzyme to pyrethroids. Sci. Rep. 2022, 12, 10108. [Google Scholar] [CrossRef]
- Sun, H.; Li, X.; Yuan, X.; Tian, Z.; Li, Y.; Zhang, Y.; Liu, J. Elucidating the detoxification efficacy of Periplaneta americana delta glutathione S-transferase 1 (PaGSTd1) against organophosphates. Pestic. Biochem. Physiol. 2024, 203, 106013. [Google Scholar] [CrossRef]
- Qian, K.; Guan, D.; Wu, Z.; Zhuang, A.; Wang, J.; Meng, X. Functional analysis of insecticide inhibition and metabolism of six glutathione S-transferases in the rice stem borer, Chilo suppressalis. J. Agric. Food Chem. 2024, 72, 12489–12497. [Google Scholar] [CrossRef]
- Sun, Z.X.; Lu, Z.H.; Xiao, T.X.; Chen, Y.P.; Fu, P.F.; Lu, K.; Gui, F.R. Genome-wide scanning loci and differentially expressed gene analysis unveils the molecular mechanism of chlorantraniliprole resistance in Spodoptera frugiperda. J. Agric. Food Chem. 2023, 71, 14092–14107. [Google Scholar] [CrossRef]
- Xu, P.; Han, N.; Kang, T.; Zhan, S.; Lee, K.S.; Jin, B.R.; Li, J.; Wan, H. SeGSTo, a novel glutathione S-transferase from the beet armyworm (Spodoptera exigua), involved in detoxification and oxidative stress. Cell Stress. Chaperones 2016, 21, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Liu, J.; Chen, S.N.; Huang, L.H.; Feng, Q.L.; Zheng, S.C. Expression profiles of glutathione S-transferase superfamily in Spodoptera litura tolerated to sublethal doses of chlorpyrifos. Insect Sci. 2016, 23, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Liu, J.Y.; Wang, W.; Mota-Sanchez, D.; He, S.; Shi, Y.; Yang, X.Q. Glutathione S-transferase genes are involved in lambda-cyhalothrin resistance in Cydia pomonella via sequestration. J. Agric. Food Chem. 2022, 70, 2265–2279. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, Q.; Liu, Y.; Dai, B.; Han, Y.; Zhang, Y.; Deng, Z.; Wang, L.; Li, X. GSTD1 Mediates the Tolerance to Abamectin and Beta-Cypermethrin in the Fall Armyworm Spodoptera frugiperda. Insects 2025, 16, 299. https://doi.org/10.3390/insects16030299
Ding Q, Liu Y, Dai B, Han Y, Zhang Y, Deng Z, Wang L, Li X. GSTD1 Mediates the Tolerance to Abamectin and Beta-Cypermethrin in the Fall Armyworm Spodoptera frugiperda. Insects. 2025; 16(3):299. https://doi.org/10.3390/insects16030299
Chicago/Turabian StyleDing, Qian, Yangyang Liu, Baoxiang Dai, Yujie Han, Yan Zhang, Zhongyuan Deng, Lixiang Wang, and Xianchun Li. 2025. "GSTD1 Mediates the Tolerance to Abamectin and Beta-Cypermethrin in the Fall Armyworm Spodoptera frugiperda" Insects 16, no. 3: 299. https://doi.org/10.3390/insects16030299
APA StyleDing, Q., Liu, Y., Dai, B., Han, Y., Zhang, Y., Deng, Z., Wang, L., & Li, X. (2025). GSTD1 Mediates the Tolerance to Abamectin and Beta-Cypermethrin in the Fall Armyworm Spodoptera frugiperda. Insects, 16(3), 299. https://doi.org/10.3390/insects16030299






