Assessing Temperature-Dependent Deltamethrin Toxicity in Various kdr Genotypes of Aedes aegypti Mosquitoes
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Mosquito Genotypes and Rearing Procedures
2.2. Climate Boxes
2.3. Paper Impregnation
2.4. WHO Tube Test
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Vector-Borne Diseases [Fact Sheet]. Available online: https://www.who.int/news-room/fact-sheets/detail/vector-borne-diseases (accessed on 17 December 2024).
- Moyes, C.L.; Vontas, J.; Martins, A.J.; Ng, L.C.; Koou, S.Y.; Dusfour, I.; Raghavendra, K.; Pinto, J.; Corbel, V.; David, J.P.; et al. Contemporary Status of Insecticide Resistance in the Major Aedes Vectors of Arboviruses Infecting Humans. PLoS Neglected Trop. Dis. 2017, 11, e0005625. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Report on Insecticide Resistance in Malaria Vectors: 2010–2016; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- World Health Organization. Malaria Threats Map. Available online: https://apps.who.int/malaria/maps/threats (accessed on 6 August 2022).
- World Health Organization. World Malaria Report 2022; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Fouet, C.; Ashu, F.A.; Ambadiang, M.M.; Tchapga, W.; Wondji, C.S.; Kamdem, C. Clothianidin-Resistant Anopheles Gambiae Adult Mosquitoes from Yaoundé, Cameroon, Display Reduced Susceptibility to SumiShield® 50WG, a Neonicotinoid Formulation for Indoor Residual Spraying. BMC Infect. Dis. 2024, 24, 133. [Google Scholar] [CrossRef]
- World Health Organization Guidelines for Laboratory and Field Testing of Mosquito Larvicides. Available online: https://www.who.int/iris/handle/10665/69101 (accessed on 3 January 2019).
- World Health Organization. Standard Operating Procedure for Testing Insecticide Susceptibility of Adult Mosquitoes in WHO Tube Tests; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Centers for Disease Control and Prevention. Global Manual for Evaluating Insecticide Resistance Using the CDC Bottle Bioassay; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2024. [Google Scholar]
- Namias, A.; Jobe, N.B.; Paaijmans, K.P.; Huijben, S. The Need for Practical Insecticide-Resistance Guidelines to Effectively Inform Mosquito-Borne Disease Control Programs. eLife 2021, 10, e65655. [Google Scholar] [CrossRef]
- Lehane, Á.; Parker-Crockett, C.; Norris, E.; Wheeler, S.S.; Harrington, L.C. Measuring Insecticide Resistance in a Vacuum: Exploring next Steps to Link Resistance Data with Mosquito Control Efficacy. J. Med. Entomol. 2024, 61, 584–594. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, M.U.G.; Sinka, M.E.; Duda, K.A.; Mylne, A.; Shearer, F.M.; Brady, O.J.; Messina, J.P.; Barker, C.M.; Moore, C.G.; Carvalho, R.G.; et al. The Global Compendium of Aedes Aegypti and Ae. Albopictus Occurrence. Sci. Data 2015, 2, 150035. [Google Scholar] [CrossRef]
- Gan, S.J.; Leong, Y.Q.; bin Barhanuddin, M.F.H.; Wong, S.T.; Wong, S.F.; Mak, J.W.; Ahmad, R.B. Dengue Fever and Insecticide Resistance in Aedes Mosquitoes in Southeast Asia: A Review. Parasites Vectors 2021, 14, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Kawada, H.; Higa, Y.; Futami, K.; Muranami, Y.; Kawashima, E.; Osei, J.H.N.; Sakyi, K.Y.; Dadzie, S.; de Souza, D.K.; Appawu, M.; et al. Discovery of Point Mutations in the Voltage-Gated Sodium Channel from African Aedes Aegypti Populations: Potential Phylogenetic Reasons for Gene Introgression. PLoS Neglected Trop. Dis. 2016, 10, e0004780. [Google Scholar] [CrossRef]
- Vera-Maloof, F.Z.; Saavedra-Rodriguez, K.; Elizondo-Quiroga, A.E.; Lozano-Fuentes, S.; Black IV, W.C. Coevolution of the Ile1,016 and Cys1,534 Mutations in the Voltage Gated Sodium Channel Gene of Aedes Aegypti in Mexico. PLoS Neglected Trop. Dis. 2015, 9, e0004263. [Google Scholar] [CrossRef]
- Alvarez, L.C.; Ponce, G.; Saavedra-Rodriguez, K.; Lopez, B.; Flores, A.E. Frequency of V1016I and F1534C Mutations in the Voltage-Gated Sodium Channel Gene in Aedes Aegypti in Venezuela. Pest Manag. Sci. 2015, 71, 863–869. [Google Scholar] [CrossRef]
- Cosme, L.V.; Gloria-Soriaid, A.; Caccone, A.; Powell, J.R.; Martins, A.J. Evolution of Kdr Haplotypes in Worldwide Populations of Aedes Aegypti: Independent Origins of the F1534C Kdr Mutation. PLoS Neglected Trop. Dis. 2020, 14, e0008219. [Google Scholar] [CrossRef]
- Linss, J.G.B.; Brito, L.P.; Garcia, G.A.; Araki, A.S.; Bruno, R.V.; Lima, J.B.P.; Valle, D.; Martins, A.J. Distribution and Dissemination of the Val1016Ile and Phe1534Cys Kdr Mutations in Aedes Aegypti Brazilian Natural Populations. Parasites Vectors 2014, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Yanola, J.; Somboon, P.; Walton, C.; Nachaiwieng, W.; Somwang, P.; Prapanthadara, L. High-Throughput Assays for Detection of the F1534C Mutation in the Voltage-Gated Sodium Channel Gene in Permethrin-Resistant Aedes Aegypti and the Distribution of This Mutation throughout Thailand. Trop. Med. Int. Health 2011, 16, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Endersby-Harshman, N.M.; Wuliandari, J.R.; Harshman, L.G.; Frohn, V.; Johnson, B.J.; Ritchie, S.A.; Hoffmann, A.A. Pyrethroid Susceptibility Has Been Maintained in the Dengue Vector, Aedes Aegypti (Diptera: Culicidae), in Queensland, Australia. J. Med. Entomol. 2017, 54, 1649–1658. [Google Scholar] [CrossRef] [PubMed]
- Stenhouse, S.A.; Plernsub, S.; Yanola, J.; Lumjuan, N.; Dantrakool, A.; Choochote, W.; Somboon, P. Detection of the V1016G Mutation in the Voltage-Gated Sodium Channel Gene of Aedes Aegypti (Diptera: Culicidae) by Allele-Specific PCR Assay, and Its Distribution and Effect on Deltamethrin Resistance in Thailand. Parasites Vectors 2013, 6, 253. [Google Scholar] [CrossRef]
- Haddi, K.; Tomé, H.V.V.; Du, Y.; Valbon, W.R.; Nomura, Y.; Martins, G.F.; Dong, K.; Oliveira, E.E. Detection of a New Pyrethroid Resistance Mutation (V410L) in the Sodium Channel of Aedes Aegypti: A Potential Challenge for Mosquito Control. Sci. Rep. 2017, 7, 46549. [Google Scholar] [CrossRef]
- Saavedra-Rodriguez, K.; Maloof, F.V.; Campbell, C.L.; Garcia-Rejon, J.; Lenhart, A.; Penilla, P.; Rodriguez, A.; Sandoval, A.A.; Flores, A.E.; Ponce, G.; et al. Parallel Evolution of Vgsc Mutations at Domains IS6, IIS6 and IIIS6 in Pyrethroid Resistant Aedes Aegypti from Mexico. Sci. Rep. 2018, 8, 16747. [Google Scholar] [CrossRef]
- Silver, K.S.; Du, Y.; Nomura, Y.; Oliveira, E.E.; Salgado, V.L.; Zhorov, B.S.; Dong, K. Voltage-Gated Sodium Channels as Insecticide Targets. Adv. In Insect Phys. 2014, 46, 389–433. [Google Scholar] [CrossRef]
- Soderlund, D.M.; Knipple, D.C. The Molecular Biology of Knockdown Resistance to Pyrethroid Insecticides. Insect Biochem. Mol. Biol. 2003, 33, 563–577. [Google Scholar] [CrossRef]
- Dickens, B.L.; Sun, H.; Jit, M.; Cook, A.R.; Carrasco, L.R.; Borame, D.; Dickens, L. Determining Environmental and Anthropogenic Factors Which Explain the Global Distribution of Aedes Aegypti and Ae. albopictus. BMJ Glob. Health 2018, 3, 801. [Google Scholar] [CrossRef]
- Kramer, I.M.; Kreß, A.; Klingelhöfer, D.; Scherer, C.; Phuyal, P.; Kuch, U.; Ahrens, B.; Groneberg, D.A.; Dhimal, M.; Müller, R. Does Winter Cold Really Limit the Dengue Vector Aedes Aegypti in Europe? Parasites Vectors 2020, 13, 178. [Google Scholar] [CrossRef]
- Wilke, A.B.B.; Damian, D.; Litvinova, M.; Byrne, T.; Zardini, A.; Poletti, P.; Merler, S.; Mutebi, J.P.; Townsend, J.; Ajelli, M. Spatiotemporal Distribution of Vector Mosquito Species and Areas at Risk for Arbovirus Transmission in Maricopa County, Arizona. Acta Trop. 2023, 240, 106833. [Google Scholar] [CrossRef] [PubMed]
- Brady, O.J.; Johansson, M.A.; Guerra, C.A.; Bhatt, S.; Golding, N.; Pigott, D.M.; Delatte, H.; Grech, M.G.; Leisnham, P.T.; Maciel-De-Freitas, R.; et al. Modelling Adult Aedes Aegypti and Aedes Albopictus Survival at Different Temperatures in Laboratory and Field Settings. Parasites Vectors 2013, 6, 351. [Google Scholar] [CrossRef] [PubMed]
- Hooper, M.J.; Ankley, G.T.; Cristol, D.A.; Maryoung, L.A.; Noyes, P.D.; Pinkerton, K.E. Interactions between Chemical and Climate Stressors: A Role for Mechanistic Toxicology in Assessing Climate Change Risks. Environ. Toxicol. Chem. 2013, 32, 32–48. [Google Scholar] [CrossRef]
- Bagni, T.; Siaussat, D.; Maria, A.; Couzi, P.; Maïbèche, M.; Massot, M. The Impact of Temperature on Insecticide Sensitivity Depends on Transgenerational Effects. Sci. Total Environ. 2022, 851, 158140. [Google Scholar] [CrossRef] [PubMed]
- Almog, M.; Degani-Katzav, N.; Korngreen, A. Kinetic and Thermodynamic Modeling of a Voltage-Gated Sodium Channel. Eur. Biophys. J. 2022, 51, 241–256. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Laboratory and Field-Testing of Long-Lasting Insecticidal Nets; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Glunt, K.D.; Paaijmans, K.P.; Read, A.F.; Thomas, M.B. Environmental Temperatures Significantly Change the Impact of Insecticides Measured Using WHOPES Protocols. Malar. J. 2014, 13, 350. [Google Scholar] [CrossRef]
- Tran, T.T.; Janssens, L.; Dinh, K.V.; Stoks, R. Transgenerational Interactions between Pesticide Exposure and Warming in a Vector Mosquito. Evol. Appl. 2018, 11, 906–917. [Google Scholar] [CrossRef]
- Muturi, E.J.; Lampman, R.; Costanzo, K.; Alto, B.W. Effect of Temperature and Insecticide Stress on Life-History Traits of Culex Restuans and Aedes Albopictus (Diptera: Culicidae). J. Med. Entomol. 2011, 48, 243–250. [Google Scholar] [CrossRef]
- Kalmouni, J.; Will, J.B.; Townsend, J.; Paaijmans, K.P. Temperature and Time of Host-Seeking Activity Impact the Efficacy of Chemical Control Interventions Targeting the West Nile Virus Vector, Culex tarsalis. PLoS Neglected Trop. Dis. 2024, 18, e0012460. [Google Scholar] [CrossRef]
- Oliver, S.V.; Brooke, B.D. The Effect of Elevated Temperatures on the Life History and Insecticide Resistance Phenotype of the Major Malaria Vector Anopheles Arabiensis (Diptera: Culicidae). Malar. J. 2017, 16, 73. [Google Scholar] [CrossRef]
- Polson, K.A.; Brogdon, W.G.; Rawlins, S.C.; Chadee, D.D. Impact of Environmental Temperatures on Resistance to Organophosphate Insecticides in Aedes Aegypti from Trinidad. Rev. Panam. Salud Publica 2012, 32, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Muturi, E.J. Larval Rearing Temperature Influences the Effect of Malathion on Aedes Aegypti Life History Traits and Immune Responses. Chemosphere 2013, 92, 1111–1116. [Google Scholar] [CrossRef]
- Mseti, J.J.; Maasayi, M.S.; Lugenge, A.G.; Mpelepele, A.B.; Kibondo, U.A.; Tenywa, F.C.; Odufuwa, O.G.; Tambwe, M.M.; Moore, S.J. Temperature, Mosquito Feeding Status and Mosquito Density Influence the Measured Bio-Efficacy of Insecticide-Treated Nets in Cone Assays. Parasites Vectors 2024, 17, 159. [Google Scholar] [CrossRef]
- Hodjati, M.H.; Curtis, C.F. Effects of Permethrin at Different Temperatures on Pyrethroid-Resistant and Susceptible Strains of Anopheles. Med. Vet. Entomol. 1999, 13, 415–422. [Google Scholar] [CrossRef]
- Glunt, K.D.; Oliver, S.V.; Hunt, R.H.; Paaijmans, K.P. The Impact of Temperature on Insecticide Toxicity against the Malaria Vectors Anopheles Arabiensis and Anopheles funestus. Malar. J. 2018, 17, 131. [Google Scholar] [CrossRef] [PubMed]
- Stark, P.M.; Fredregill, C.L.; Nolan, M.S.; Debboun, M. Field Cage Insecticide Resistance Tests against Culex Quinquefasciatus Say (Diptera: Culicidae) in Harris County, Texas, U.S.A. J. Vector Ecol. 2017, 42, 279–288. [Google Scholar] [CrossRef]
- Whiten, S.R.; Peterson, R.K.D. The Influence of Ambient Temperature on the Susceptibility of Aedes Aegypti (Diptera: Culicidae) to the Pyrethroid Insecticide Permethrin. J. Med. Entomol. 2016, 53, 139–143. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Salinas, W.S.; Feria-Arroyo, T.P.; Vitek, C.J. Temperatures Influence Susceptibility to Insecticides in Aedes Aegypti and Aedes Albopictus (Diptera: Culicidae) Mosquitoes. Pathogens 2021, 10, 992. [Google Scholar] [CrossRef]
- Hurlbert, S.H. Pseudoreplication and the Design of Ecological Field Experiments. Ecol. Monogr. 1984, 54, 187–211. [Google Scholar] [CrossRef]
- Suzuki, D.T.; Grigliatti, T.; Williamson, R. Temperature-Sensitive Mutations in Drosophila Melanogaster. VII. A Mutation (Para-Ts) Causing Reversible Adult Paralysis. Proc. natn. Acad. Sci. USA 1971, 68, 890–893. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, Q.; Dong, H.; Yuan, H.; Bai, J.; Gao, J.; Tao, F.; Ma, H.; Li, X.; Peng, H.; et al. The Pattern of Kdr Mutations Correlated with the Temperature in Field Populations of Aedes Albopictus in China. Parasites Vectors 2021, 14, 406. [Google Scholar] [CrossRef] [PubMed]
- Estep, A.S.; Sanscrainte, N.D.; Waits, C.M.; Bernard, S.J.; Lloyd, A.M.; Lucas, K.J.; Buckner, E.A.; Vaidyanathan, R.; Morreale, R.; Conti, L.A.; et al. Quantification of Permethrin Resistance and Kdr Alleles in Florida Strains of Aedes Aegypti (L.) and Aedes Albopictus (Skuse). PLoS Neglected Trop. Dis. 2018, 12, e0006544. [Google Scholar] [CrossRef]
- Glunt, K.D.; Abílio, A.P.; Bassat, Q.; Bulo, H.; Gilbert, A.E.; Huijben, S.; Manaca, M.N.; Macete, E.; Alonso, P.; Paaijmans, K.P. Long-Lasting Insecticidal Nets No Longer Effectively Kill the Highly Resistant Anopheles Funestus of Southern Mozambique. Malar. J. 2015, 14, 298. [Google Scholar] [CrossRef]
- World Health Organization. Standard Operating Procedure for Impregnation of Filter Papers for Testing Insecticide Susceptibility of Adult Mosquitoes in WHO Tube Tests; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Mellanby, K. Low Temperature and Insect Activity. Proc. R. Soc. Lond. B Biol. Sci. 1939, 127, 473–487. [Google Scholar]
- Fan, Y.; Scott, J.G. The F1534C Voltage-Sensitive Sodium Channel Mutation Confers 7- to 16-Fold Resistance to Pyrethroid Insecticides in Aedes aegypti. Pest. Manag. Sci. 2020, 76, 2251–2259. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; R Core Team: Vienna, Austria, 2024. [Google Scholar]
- Boubidi, S.C.; Rossignol, M.; Chandre, F.; Tounsi, R.; Lagneau, C.; Fontenille, D.; Reiter, P. Gender Bias in Insecticide Susceptibility of Aedes Albopictus Is Solely Attributable to Size. J. Am. Mosq. Control Assoc. 2016, 32, 251–253. [Google Scholar] [CrossRef]
- Bjørge, J.D.; Overgaard, J.; Malte, H.; Gianotten, N.; Heckmann, L.H. Role of Temperature on Growth and Metabolic Rate in the Tenebrionid Beetles Alphitobius Diaperinus and Tenebrio molitor. J. Insect Physiol. 2018, 107, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Durak, R.; Durak, T. Metabolic Response of Aphid Cinara Tujafilina to Cold Stress. Biology 2021, 10, 1288. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.H.; Gillooly, J.F.; Allen, A.P.; Savage, V.M.; West, G.B. Toward a Metabolic Theory of Ecology. Ecology 2004, 85, 1771–1789. [Google Scholar] [CrossRef]
- Clarke, A.; Fraser, K.P.P. Why Does Metabolism Scale with Temperature? Funct. Ecol. 2004, 18, 243–251. [Google Scholar] [CrossRef]
- Agyekum, T.P.; Arko-Mensah, J.; Botwe, P.K.; Hogarh, J.N.; Issah, I.; Dadzie, S.K.; Dwomoh, D.; Billah, M.K.; Robins, T.; Fobil, J.N. Relationship between Temperature and Anopheles Gambiae Sensu Lato Mosquitoes’ Susceptibility to Pyrethroids and Expression of Metabolic Enzymes. Parasites Vectors 2022, 15, 163. [Google Scholar] [CrossRef] [PubMed]
- Harwood, A.D.; You, J.; Lydy, M.J. Temperature as a Toxicity Identification Evaluation Tool for Pyrethroid Insecticides: Toxicokinetic Confirmation. Environ. Toxicol. Chem. 2009, 28, 1051–1058. [Google Scholar] [CrossRef]
- Brito, L.P.; Carrara, L.; De Freitas, R.M.; Lima, J.B.P.; Martins, A.J. Levels of Resistance to Pyrethroid among Distinct Kdr Alleles in Aedes Aegypti Laboratory Lines and Frequency of Kdr Alleles in 27 Natural Populations from Rio de Janeiro, Brazil. Biomed. Res. Int. 2018, 2018, 2410819. [Google Scholar] [CrossRef]
- Martelli, F.; Alves, A.N.; Yang, Y.T.; Batterham, P.; Wedell, N. Genotype and Sex Affect the Combined Impact of Temperature and Low-Dose Insecticide Exposure on Insect Survival. Insect Sci. 2024; ahead of print. [Google Scholar] [CrossRef]
- Gloria-Soria, A.; Soghigian, J.; Kellner, D.; Powell, J.R. Genetic Diversity of Laboratory Strains and Implications for Research: The Case of Aedes aegypti. PLOS Neglected Trop. Dis. 2019, 13, e0007930. [Google Scholar] [CrossRef] [PubMed]
- Day, J.; Van Handel, E. Differences between the Nutritional Reserves of Laboratory-Maintained and Field-Collected Adult Mosquitoes. J. Am. Mosq. Control Assoc. 1986, 2, 154–157. [Google Scholar]
- Aguilar, R.; Simard, F.; Kamdem, C.; Shields, T.; Glass, G.E.; Garver, L.S.; Dimopoulos, G. Genome-Wide Analysis of Transcriptomic Divergence between Laboratory Colony and Field Anopheles Gambiae Mosquitoes of the M and S Molecular Forms. Insect Mol. Biol. 2010, 19, 695. [Google Scholar] [CrossRef]
- Dennington, N.L.; Grossman, M.K.; Ware-Gilmore, F.; Teeple, J.L.; Johnson, L.R.; Shocket, M.S.; McGraw, E.A.; Thomas, M.B. Phenotypic Adaptation to Temperature in the Mosquito Vector, Aedes aegypti. Glob. Chang. Biol. 2024, 30, e17041. [Google Scholar] [CrossRef]
- Ponlawat, A.; Harwood, J.F.; Putnam, J.L.; Nitatsukprasert, C.; Pongsiri, A.; Kijchalao, U.; Linthicum, K.J.; Kline, D.L.; Clark, G.G.; Obenauer, P.J.; et al. Field Evaluation of Indoor Thermal Fog and Ultra-Low Volume Applications for Control of Aedes Aegypti in Thailand. J. Am. Mosq. Control Assoc. 2017, 33, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Dzul-Manzanilla, F.; Correa-Morales, F.; Medina-Barreiro, A.; Bibiano-Marín, W.; Vadillo-Sanchez, J.; Riestra-Morales, M.; Del Castillocenteno, L.F.; Morales-Rios, E.; Martin-Park, A.; Gonzalez-Olvera, G.; et al. Field Efficacy Trials of Aerial Ultra-Low-Volume Application of Insecticides against Caged Aedes Aegypti in Mexico. J. Am. Mosq. Control Assoc. 2019, 35, 140–146. [Google Scholar] [CrossRef]
- Mack, L.K.; Attardo, G.M. Heat Shock Proteins, Thermotolerance, and Insecticide Resistance in Mosquitoes. Front. Insect Sci. 2024, 4, 1309941. [Google Scholar] [CrossRef]
- Paulo Brito, L.; B Linss, J.G.; Lima-Camara, T.N.; Belinato, T.A.; Peixoto, A.A.; Bento Lima, J.P.; Valle, D.; Martins, A.J. Assessing the Effects of Aedes Aegypti Kdr Mutations on Pyrethroid Resistance and Its Fitness Cost. PLoS ONE 2013, 8, e60878. [Google Scholar] [CrossRef]
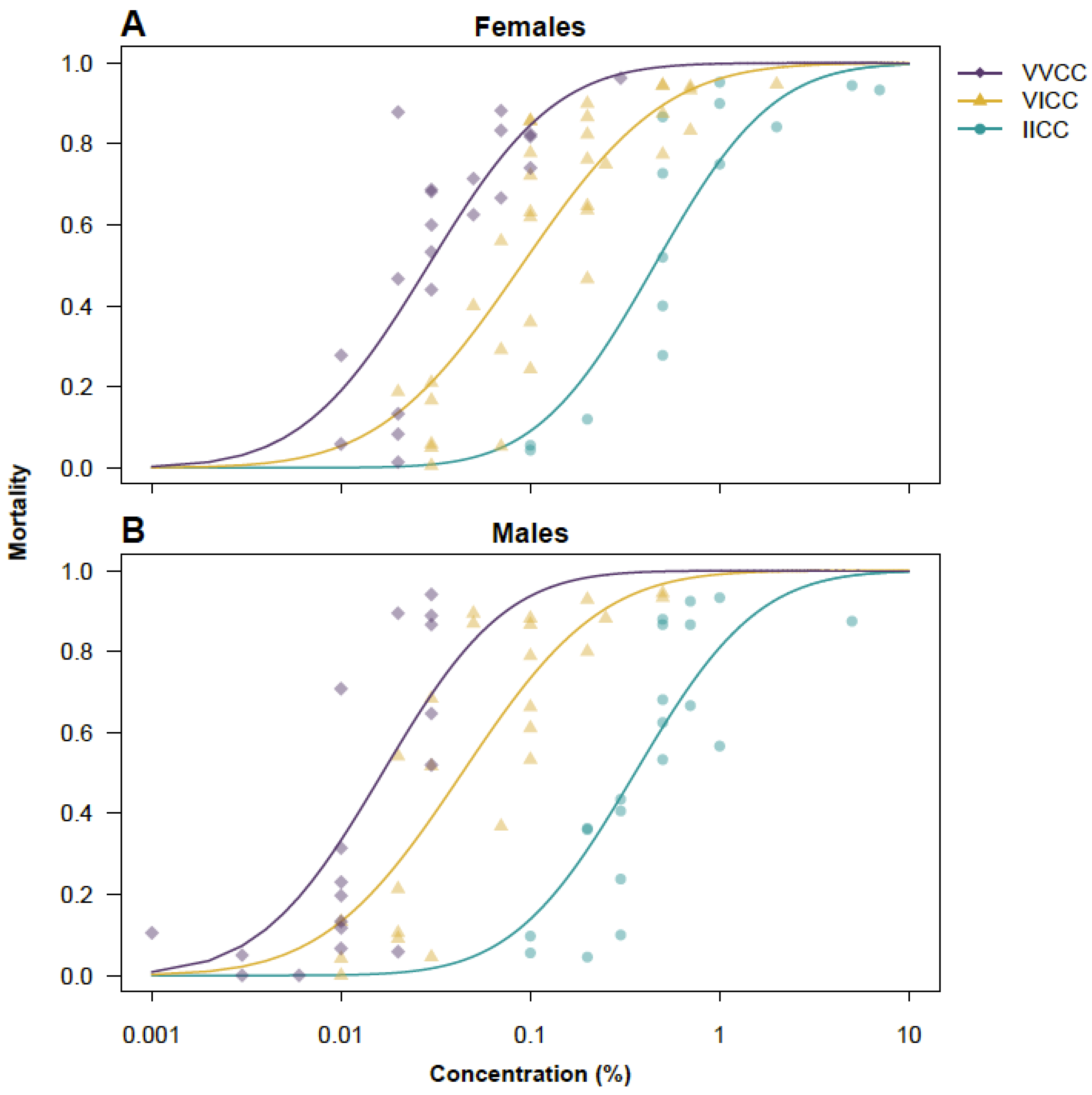
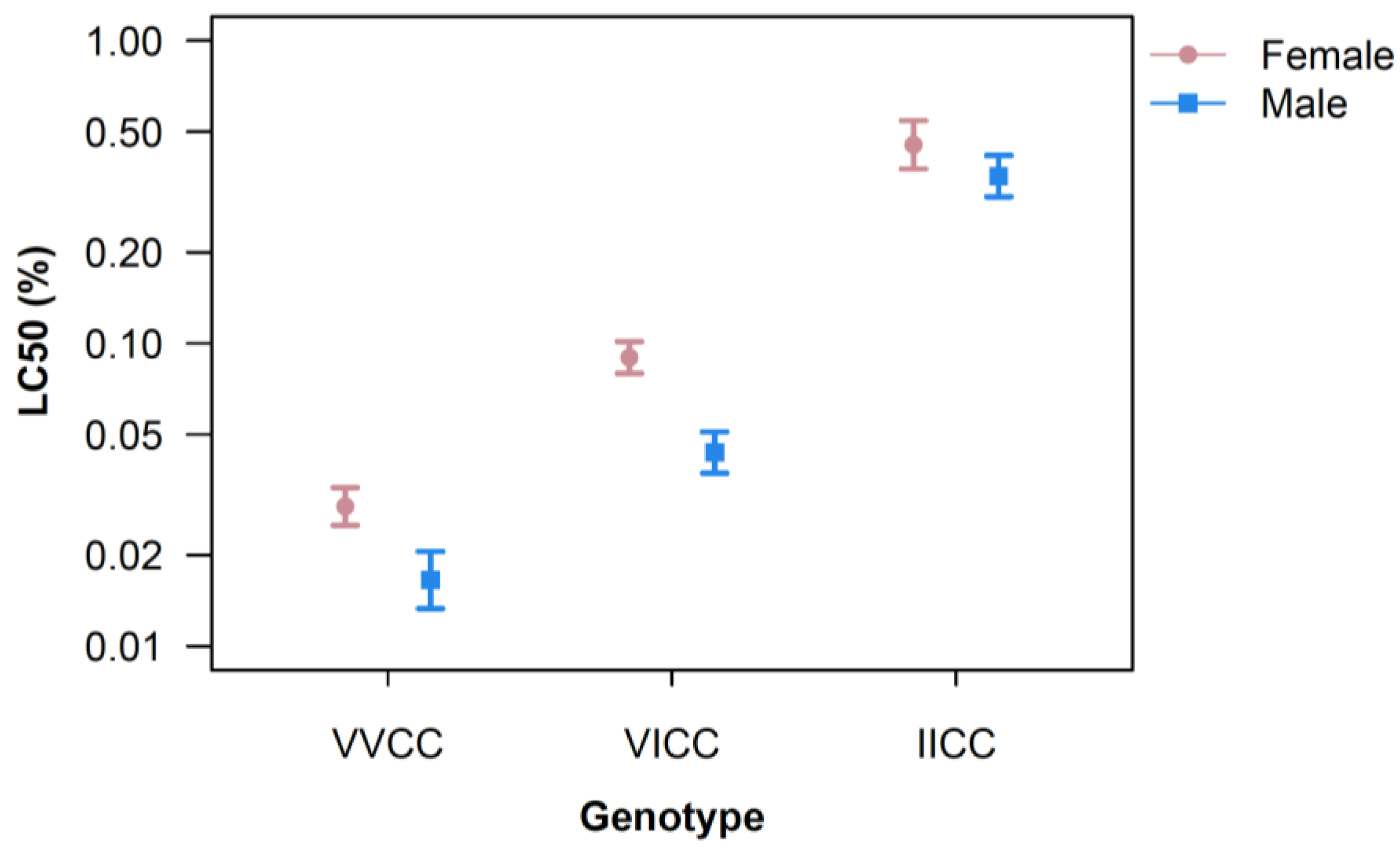

| VVCC | VICC | IICC | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ♀ | ♂ | ♀ | ♂ | ♀ | ♂ | |||||||
| Conc. | n | Conc. | n | Conc. | n | Conc. | n | Conc. | n | Conc. | n | |
| 22 °C | control | 5 | control | 2 | control | 5 | control | 5 | control | 5 | control | 3 |
| 0.005 | 2 | 0.003 | 1 | 0.02 | 2 | 0.01 | 2 * | 0.1 | 1 | 0.1 | 1 | |
| 0.01 | 2 * | 0.006 | 1 | 0.03 | 2 | 0.02 | 2 * | 0.2 | 1 | 0.3 | 2 | |
| 0.02 | 2 | 0.01 | 2 | 0.05 | 1 | 0.03 | 2 * | 0.5 | 1 | 0.5 | 1 | |
| 0.03 | 3 ** | 0.02 | 1 | 0.07 | 1 | 0.05 | 1 | 1 | 2 | 0.7 | 1 | |
| 0.05 | 1 | 0.03 | 1 | 0.1 | 3 | 0.07 | 1 | 2 | 1 | 1 | 3 ** | |
| 0.07 | 1 | 0.1 | 1 | 0.2 | 3 * | 0.1 | 4 | 3 | 1 | 2 | 1 | |
| 0.25 | 1 | 0.2 | 1 | 4 | 1 | |||||||
| 0.3 | 1 | 0.25 | 1 | 7 | 1 | |||||||
| 0.5 | 2 | 0.5 | 2 | |||||||||
| 0.7 | 1 | |||||||||||
| 1 | 1 | |||||||||||
| 2 | 1 | |||||||||||
| 27 °C | control | 2 | control | 3 | control | 3 | control | 3 | control | 4 | control | 3 |
| 0.006 | 1 | 0.001 | 1 | 0.03 | 1 | 0.01 | 1 | 0.1 | 1 | 0.1 | 1 | |
| 0.01 | 1 | 0.003 | 1 | 0.07 | 1 | 0.02 | 1 | 0.2 | 1 | 0.2 | 1 | |
| 0.02 | 1 | 0.01 | 2 | 0.1 | 2 | 0.03 | 2 | 0.5 | 1 | 0.3 | 2 | |
| 0.03 | 3 | 0.02 | 1 | 0.2 | 2 | 0.1 | 1 | 1 | 1 | 0.5 | 2 | |
| 0.05 | 1 | 0.03 | 2 | 0.5 | 2 * | 0.2 | 1 | 2 | 2 * | 0.7 | 1 | |
| 0.07 | 1 | 0.1 | 1 | 0.5 | 1 | 5 | 1 | 1 | 1 | |||
| 0.1 | 1 | 7 | 1 | 1.5 | 1 | |||||||
| 2 | 1 | |||||||||||
| 32 °C | control | 4 | control | 3 | control | 5 | control | 2 | control | 3 | control | 3 |
| 0.006 | 1 | 0.003 | 1 | 0.01 | 1 | 0.01 | 1 | 0.1 | 1 | 0.03 | 1 | |
| 0.01 | 1 | 0.006 | 1 | 0.03 | 2 | 0.02 | 2 | 0.2 | 1 | 0.1 | 1 | |
| 0.02 | 2 | 0.01 | 3 | 0.07 | 1 | 0.03 | 1 | 0.5 | 3 | 0.2 | 2 | |
| 0.05 | 1 | 0.03 | 2 | 0.1 | 3 | 0.05 | 1 | 5 | 2 * | 0.5 | 3 ** | |
| 0.07 | 1 | 0.3 | 1 | 0.2 | 3 | 0.1 | 1 | 7 | 1 | 0.7 | 1 | |
| 0.1 | 2 | 0.5 | 3 * | 0.2 | 1 | 5 | 1 | |||||
| 0.3 | 1 | 0.7 | 2 | 0.5 | 1 | |||||||
| LC50 (95% Confidence Interval) | |||
|---|---|---|---|
| Genotype | Temperature (°C) | Females | Males |
| VVCC | 22 | 0.016 (0.012–0.021) | 0.023 (0.016–0.033) |
| 27 | 0.032 (0.029–0.036) | 0.012 (0.008–0.018) | |
| 32 | 0.051 (0.047–0.055) | 0.017 (0.012–0.023) | |
| VICC | 22 | 0.077 (0.065–0.092) | 0.042 (0.031–0.056) |
| 27 | 0.094 (0.078–0.112) | 0.051 (0.040–0.066) | |
| 32 | 0.106 (0.086–0.312) | 0.035 (0.024–0.050) | |
| IICC | 22 | 0.543 (0.413–0.715) | 0.479 (0.370–0.619) |
| 27 | 0.426 (0.217–0.837) | 0.297 (0.269–0.328) | |
| 32 | 0.378 (0.280–0.512) | 0.361 (0.273–0.478) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalmouni, J.; Jensen, B.M.; Ain, J.; Paaijmans, K.P.; Huijben, S. Assessing Temperature-Dependent Deltamethrin Toxicity in Various kdr Genotypes of Aedes aegypti Mosquitoes. Insects 2025, 16, 254. https://doi.org/10.3390/insects16030254
Kalmouni J, Jensen BM, Ain J, Paaijmans KP, Huijben S. Assessing Temperature-Dependent Deltamethrin Toxicity in Various kdr Genotypes of Aedes aegypti Mosquitoes. Insects. 2025; 16(3):254. https://doi.org/10.3390/insects16030254
Chicago/Turabian StyleKalmouni, Joshua, Brook M. Jensen, Joshua Ain, Krijn P. Paaijmans, and Silvie Huijben. 2025. "Assessing Temperature-Dependent Deltamethrin Toxicity in Various kdr Genotypes of Aedes aegypti Mosquitoes" Insects 16, no. 3: 254. https://doi.org/10.3390/insects16030254
APA StyleKalmouni, J., Jensen, B. M., Ain, J., Paaijmans, K. P., & Huijben, S. (2025). Assessing Temperature-Dependent Deltamethrin Toxicity in Various kdr Genotypes of Aedes aegypti Mosquitoes. Insects, 16(3), 254. https://doi.org/10.3390/insects16030254





