Development of Attractive Toxic Sugar Baits (ATSBs) System and Its Effectiveness in Mosquito Control
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Mosquito Strains
2.2. Preparation of ATSB
2.2.1. Preparation of Laboratory Attractants
2.2.2. Preparation of Laboratory Preservative Concentrations
2.2.3. Preparation of Laboratory Insecticides
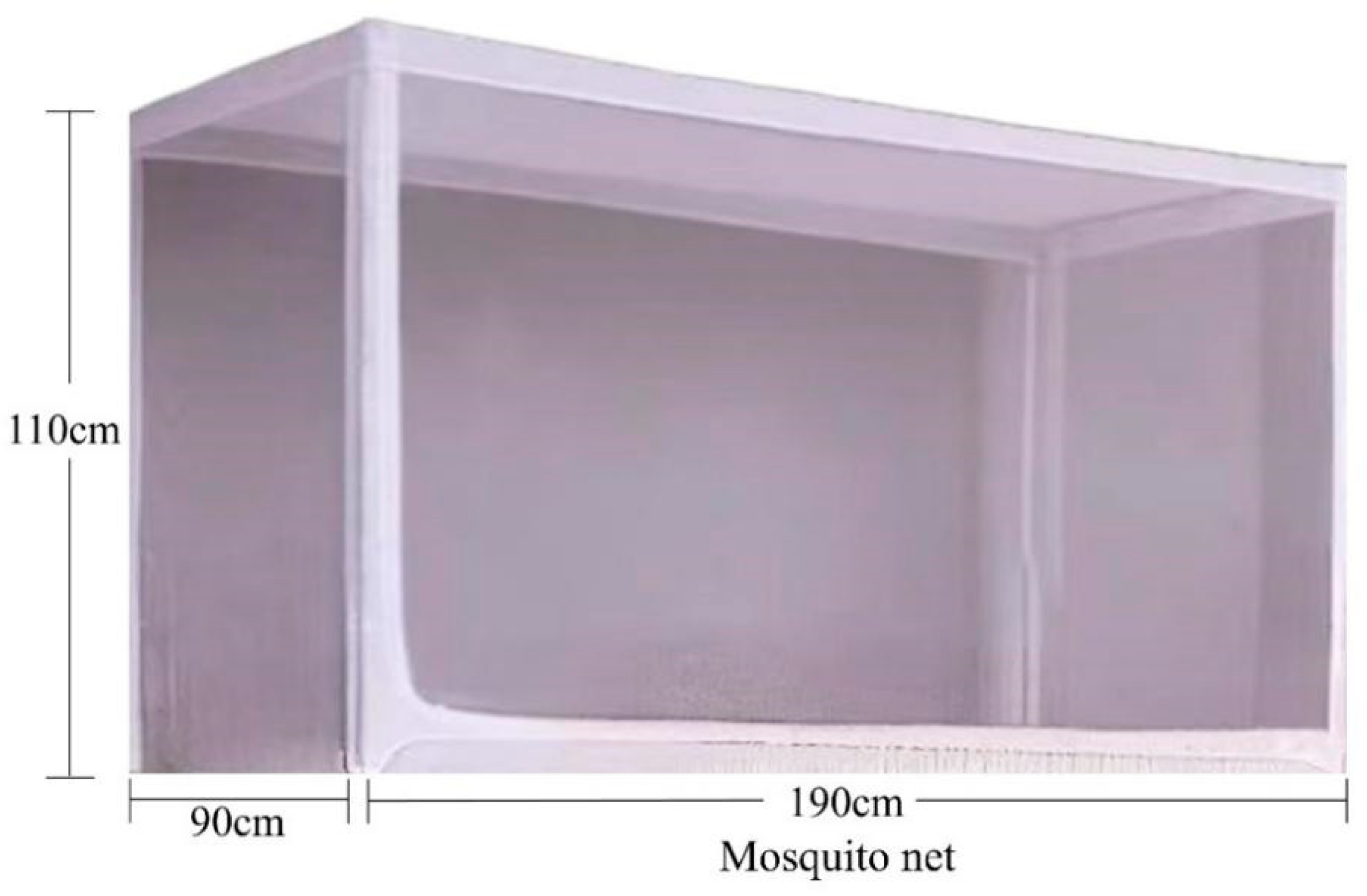
2.2.4. Laboratory Trapping Device
2.3. Laboratory ATSB Screening Method
2.3.1. Laboratory ATSB Attractant Component Screening Method
2.3.2. Laboratory ATSB Preservative Component Screening Method
2.3.3. Laboratory ATSB Insecticidal Component and Concentration Screening Method
2.3.4. Laboratory ATSB Trap Screening Method
2.3.5. Mortality Test of Experimental Mosquitoes in Semi-Field Cage with ATSB
2.4. Statistical Analysis
3. Results
3.1. Screening of Attractants in the ATSB System
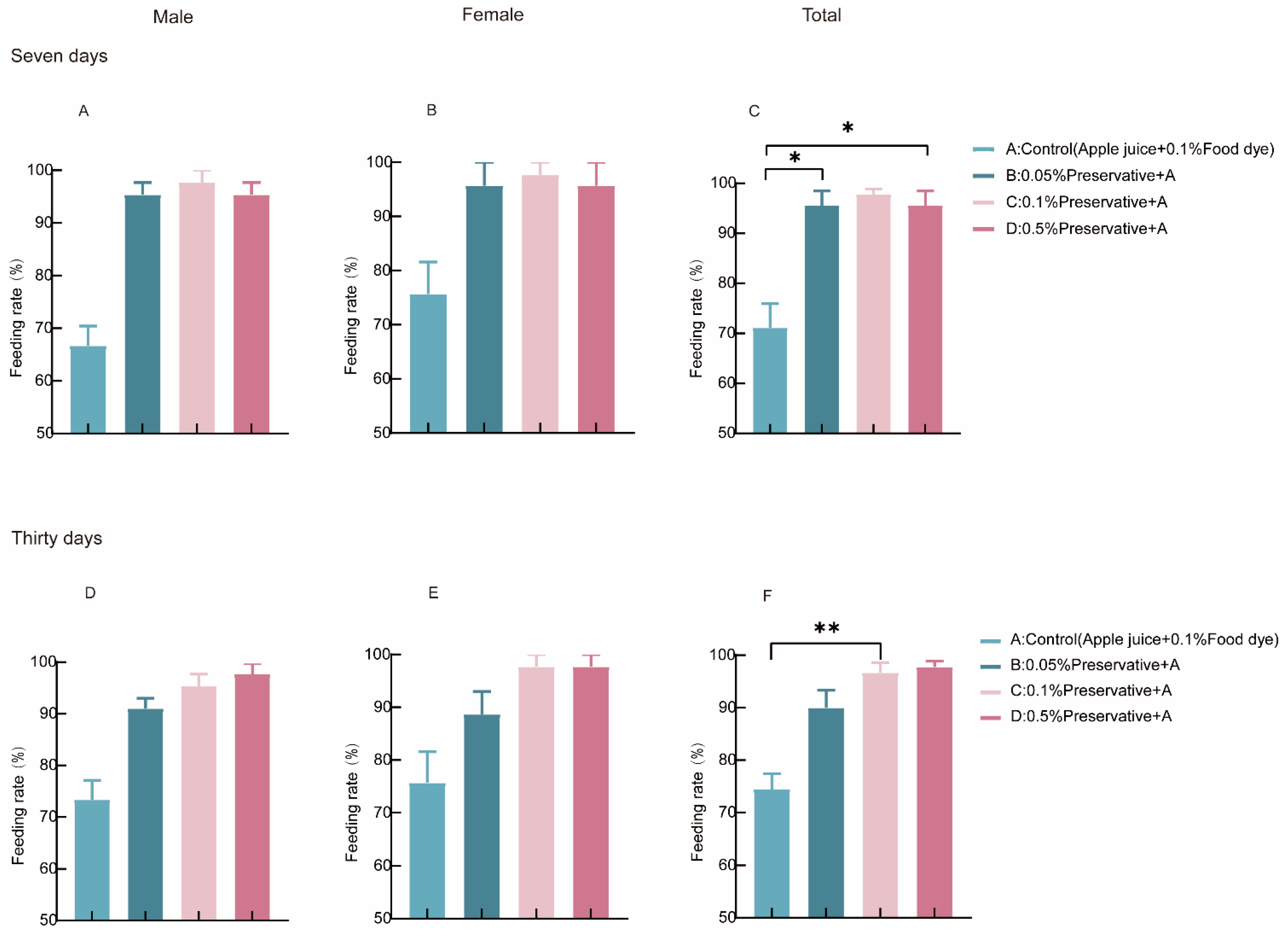
3.2. Screening of Preservative Concentration in the ATSBs System
3.3. Screening of Insecticide Types and Concentrations in the ATSBs System
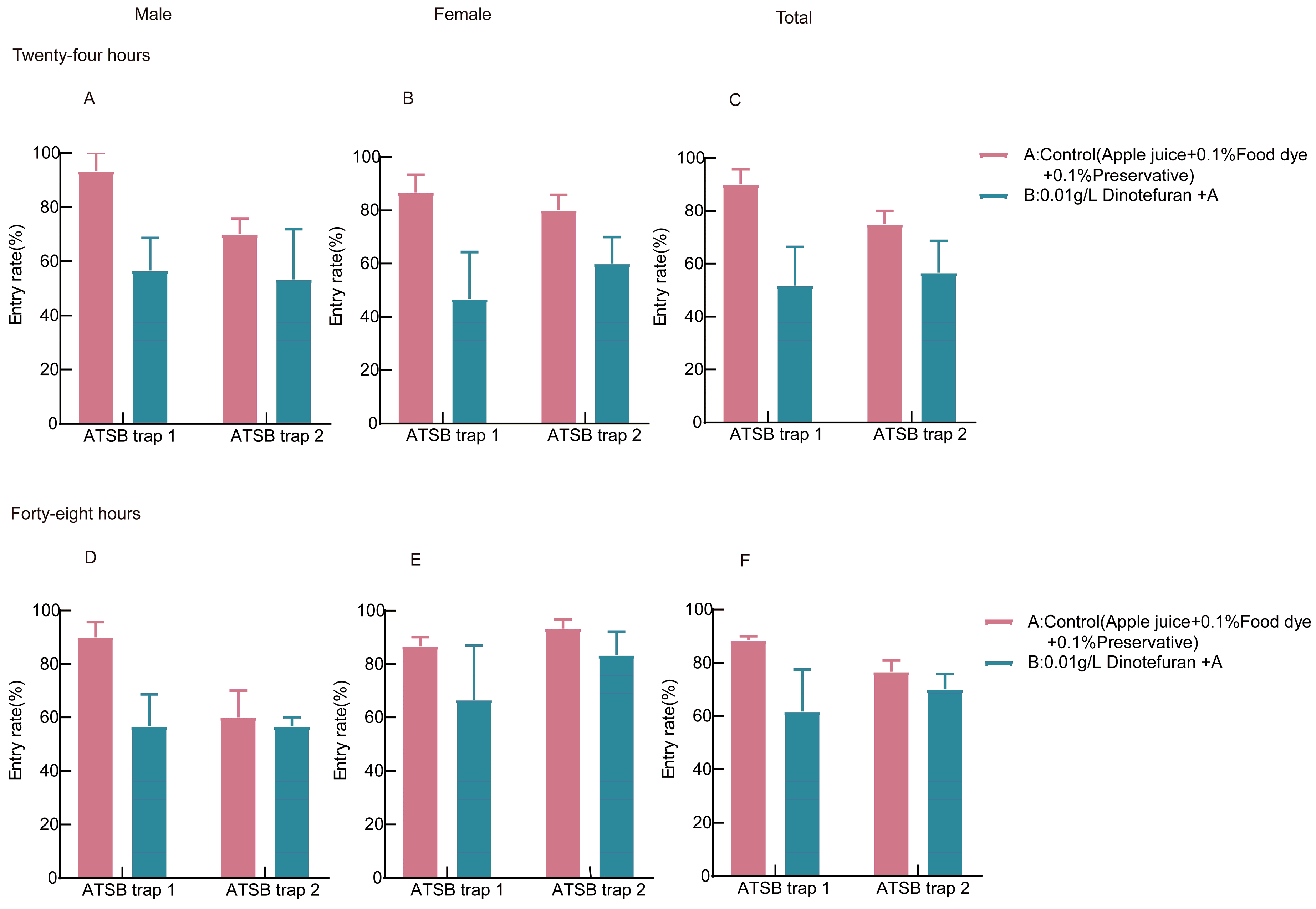
3.4. Screening of Trap Devices in the ATSBs System
3.5. Insecticidal Efficacy of the ATSB System Against Culex quinquefasciatus and Aedes albopictus in Semi-Field Cage
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATSBs | Attractive Toxic Sugar Baits |
| AI | Attraction index |
| SIT | Sterile Insect Technique |
| IIT | Incompatible Insect Technique |
| RIDL | Release of Insects carrying Dominant Lethal genes |
References
- Lambrechts, L. Does arbovirus emergence in humans require adaptation to domestic mosquitoes? Curr. Opin. Virol. 2023, 60, 101315. [Google Scholar] [CrossRef]
- Graff, S.L.; Eibner, G.J.; Ochieng, J.R.; Jones, T.C.; Nsubuga, A.M.; Lutwama, J.J.; Rwego, I.B.; Junglen, S. Detection of two alphaviruses: Middelburg virus and Sindbis virus from enzootic amplification cycles in southwestern Uganda. Front. Microbiol. 2024, 15, 1394661. [Google Scholar] [CrossRef]
- Shaw, W.R.; Catteruccia, F. Vector biology meets disease control: Using basic research to fight vector-borne diseases. Nat. Microbiol. 2019, 4, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Yu, X.; Cheng, G. Impact of the microbiome on mosquito-borne diseases. Protein Cell 2023, 14, 743–761. [Google Scholar] [CrossRef]
- Njoroge, T.M.; Hamid-Adiamoh, M.; Duman-Scheel, M. Maximizing the Potential of Attractive Targeted Sugar Baits (ATSBs) for Integrated Vector Management. Insects 2023, 14, 585. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Sharma, A.; Samal, R.R.; Kumar, M.; Verma, V.; Sagar, R.K.; Singh, S.; Raghavendra, K. Attractive Sugar Bait Formulation for Development of Attractive Toxic Sugar Bait for Control of Aedes aegypti (Linnaeus). J. Trop. Med. 2022, 2022, 2977454. [Google Scholar] [CrossRef] [PubMed]
- Müller, G.C.; Beier, J.C.; Traore, S.F.; Toure, M.B.; Traore, M.M.; Bah, S.; Doumbia, S.; Schlein, Y. Successful field trial of attractive toxic sugar bait (ATSB) plant-spraying methods against malaria vectors in the Anopheles gambiae complex in Mali, West Africa. Malar. J. 2010, 9, 210. [Google Scholar] [CrossRef]
- Xue, R.D.; Kline, D.L.; Ali, A.; Barnard, D.R. Application of boric acid baits to plant foliage for adult mosquito control. J. Am. Mosq. Control Assoc. 2006, 22, 497–500. [Google Scholar] [CrossRef]
- Allan, S.A. Susceptibility of adult mosquitoes to insecticides in aqueous sucrose baits. J. Vector Ecol. 2011, 36, 59–67. [Google Scholar] [CrossRef]
- Aguilera-Alcala, N.; Morales-Reyes, Z.; Martin-Lopez, B.; Moleon, M.; Sanchez-Zapata, J.A. Role of scavengers in providing non-material contributions to people. Ecol. Indic. 2020, 117, 106643. [Google Scholar] [CrossRef]
- Lea, A.O. Sugar-Baited Insecticide Residues against Mosquitoes. Mosq. News 1965, 25, 65–66. [Google Scholar]
- Nayar, J.K.; Sauerman, D.M., Jr. The effects of nutrition on survival and fecundity in Florida mosquitoes. Part 3. Utilization of blood and sugar for fecundity. J. Med. Entomol. 1975, 12, 220–225. [Google Scholar] [CrossRef]
- Gary, R.E., Jr.; Foster, W.A. Diel timing and frequency of sugar feeding in the mosquito Anopheles gambiae, depending on sex, gonotrophic state and resource availability. Med. Vet. Entomol. 2006, 20, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Foster, W.A. Mosquito sugar feeding and reproductive energetics. Annu. Rev. Entomol. 1995, 40, 443–474. [Google Scholar] [CrossRef] [PubMed]
- Müller, G.; Schlein, Y. Sugar questing mosquitoes in arid areas gather on scarce blossoms that can be used for control. Int. J. Parasitol. 2006, 36, 1077–1080. [Google Scholar] [CrossRef]
- Fiorenzano, J.M.; Koehler, P.G.; Xue, R.-D. Attractive Toxic Sugar Bait (ATSB) For Control of Mosquitoes and Its Impact on Non-Target Organisms: A Review. Int. J. Environ. Res. Public Health 2017, 14, 398. [Google Scholar] [CrossRef] [PubMed]
- Sparks, T.C.; Storer, N.; Porter, A.; Slater, R.; Nauen, R. Insecticide resistance management and industry: The origins and evolution of the Insecticide Resistance Action Committee (IRAC) and the mode of action classification scheme. Pest. Manag. Sci. 2021, 77, 2609–2619. [Google Scholar] [CrossRef]
- Diarra, R.A.; Traore, M.M.; Junnila, A.; Traore, S.F.; Doumbia, S.; Revay, E.E.; Kravchenko, V.D.; Schlein, Y.; Arheart, K.L.; Gergely, P.; et al. Testing configurations of attractive toxic sugar bait (ATSB) stations in Mali, West Africa, for improving the control of malaria parasite transmission by vector mosquitoes and minimizing their effect on non-target insects. Malar. J. 2021, 20, 184. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, A.; Samal, R.R.; Kumar, M.; Verma, V.; Sagar, R.K.; Singh, S.P.; Raghavendra, K. Laboratory evaluation of the efficacy of deltamethrin-laced attractive toxic sugar bait formulation on Anopheles stephensi. Malar. J. 2023, 22, 92. [Google Scholar] [CrossRef]
- Stewart, Z.P.; Oxborough, R.M.; Tungu, P.K.; Kirby, M.J.; Rowland, M.W.; Irish, S.R. Indoor application of attractive toxic sugar bait (ATSB) in combination with mosquito nets for control of pyrethroid-resistant mosquitoes. PLoS ONE 2013, 8, e84168. [Google Scholar] [CrossRef]
- Traore, M.M.; Junnila, A.; Traore, S.F.; Doumbia, S.; Revay, E.E.; Kravchenko, V.D.; Schlein, Y.; Arheart, K.L.; Gergely, P.; Xue, R.-D.; et al. Large-scale field trial of attractive toxic sugar baits (ATSB) for the control of malaria vector mosquitoes in Mali, West Africa. Malar. J. 2020, 19, 72. [Google Scholar] [CrossRef]
- Kumar, G.; Sharma, A.; Dhiman, R.C. Laboratory evaluation of the efficacy of boric acid containing toxic sugar baits against Anopheles culicifacies, An. stephensi and Aedes aegypti mosquitoes. J. Vector Borne Dis. 2022, 59, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.; Bibbs, C.S.; Müller, G.C.; Xue, R.D. Evaluation of Bacillus thuringiensis israelensis as toxic sugar bait against adult Aedes aegypti, Aedes albopictus, and Culex quinquefasciatus mosquitoes. J. Vector Ecol. 2021, 46, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Chiu, M.C.; Neoh, K.B.; Hwang, S.Y. The effect of attractive toxic sugar bait on the Asian tiger mosquito, Aedes albopictus (Diptera: Culicidae) in community farms in Northern Taiwan. Acta Trop. 2024, 250, 107102. [Google Scholar] [CrossRef] [PubMed]
- Fryzlewicz, L.; VanWinkle, A.; Lahondère, C. Development of an Attractive Toxic Sugar Bait for the Control of Aedes j. japonicus (Diptera: Culicidae). J. Med. Entomol. 2022, 59, 308–313. [Google Scholar] [CrossRef]
- Revay, E.E.; Müller, G.C.; Qualls, W.A.; Kline, D.L.; Naranjo, D.P.; Arheart, K.L.; Kravchenko, V.D.; Yefremova, Z.; Hausmann, A.; Beier, J.C.; et al. Control of Aedes albopictus with attractive toxic sugar baits (ATSB) and potential impact on non-target organisms in St. Augustine, Florida. Parasitol. Res. 2014, 113, 73–79. [Google Scholar] [CrossRef]
- Qualls, W.A.; Müller, G.C.; Revay, E.E.; Allan, S.A.; Arheart, K.L.; Beier, J.C.; Smith, M.L.; Scott, J.M.; Kravchenko, V.D.; Hausmann, A.; et al. Evaluation of attractive toxic sugar bait (ATSB)-Barrier for control of vector and nuisance mosquitoes and its effect on non-target organisms in sub-tropical environments in Florida. Acta Trop. 2014, 131, 104–110. [Google Scholar] [CrossRef]
- Xue, R.D.; Ali, A.; Kline, D.L.; Barnard, D.R. Field evaluation of boric acid- and fipronil-based bait stations against adult mosquitoes. J. Am. Mosq. Control Assoc. 2008, 24, 415–418. [Google Scholar] [CrossRef]
- Fikrig, K.; Johnson, B.J.; Fish, D.; Ritchie, S.A. Assessment of synthetic floral-based attractants and sugar baits to capture male and female Aedes aegypti (Diptera: Culicidae). Parasites Vectors 2017, 10, 32. [Google Scholar] [CrossRef]
- Furnival-Adams, J.E.C.; Camara, S.; Rowland, M.; Koffi, A.A.; Ahoua Alou, L.P.; Oumbouke, W.A.; N’Guessan, R. Indoor use of attractive toxic sugar bait in combination with long-lasting insecticidal net against pyrethroid-resistant Anopheles gambiae: An experimental hut trial in Mbé, central Côte d’Ivoire. Malar. J. 2020, 19, 11. [Google Scholar] [CrossRef]
- Tenywa, F.C.; Kambagha, A.; Saddler, A.; Maia, M.F. The development of an ivermectin-based attractive toxic sugar bait (ATSB) to target Anopheles arabiensis. Malar. J. 2017, 16, 338. [Google Scholar] [CrossRef]
- Kumar, G.; Pasi, S.; Yadav, C.P.; Kaur, J.; Sharma, A. Potential of ivermectin as an active ingredient of the attractive toxic sugar baits against the Indian malaria vectors Anopheles culicifacies and Anopheles stephensi. Pest. Manag. Sci. 2023, 79, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.C.A.; Teixeira, A.V.; Lima Bezerra, F.; Andriolo, A.; Silva, A.A. Sugar Bait Composition Containing Ivermectin Affect Engorgement and Mortality of the Mosquito Aedes aegypti (Diptera: Culicidae). J. Med. Entomol. 2023, 60, 159–164. [Google Scholar] [CrossRef] [PubMed]
- McDermott, E.G.; Morris, E.K.; Garver, L.S. Sodium Ascorbate as a Potential Toxicant in Attractive Sugar Baits for Control of Adult Mosquitoes (Diptera: Culicidae) and Sand Flies (Diptera: Psychodidae). J. Med. Entomol. 2019, 56, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.Y.; He, J.; Teng, X.D.; Lan, C.J.; Shen, R.X.; Wang, Y.T.; Zhang, N.; Dong, Y.D.; Zhao, T.Y.; Li, C.X. Efficacy of orally toxic sugar baits against contact-insecticide resistant Culex quinquefasciatus. Acta Trop. 2020, 202, 105256. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, A.; Samal, R.R.; Verma, V.; Sagar, R.K.; Singh, S.P.; Raghavendra, K. Development of Deltamethrin-Laced Attractive Toxic Sugar Bait to Control Aedes aegypti (Linnaeus) Population. J. Trop. Med. 2024, 2024, 6966205. [Google Scholar] [CrossRef]
- Junnila, A.; Revay, E.E.; Müller, G.C.; Kravchenko, V.; Qualls, W.A.; Xue, R.D.; Allen, S.A.; Beier, J.C.; Schlein, Y. Efficacy of attractive toxic sugar baits (ATSB) against Aedes albopictus with garlic oil encapsulated in beta-cyclodextrin as the active ingredient. Acta Trop. 2015, 152, 195–200. [Google Scholar] [CrossRef]
- Revay, E.E.; Schlein, Y.; Tsabari, O.; Kravchenko, V.; Qualls, W.; De-Xue, R.; Beier, J.C.; Traore, S.F.; Doumbia, S.; Hausmann, A.; et al. Formulation of attractive toxic sugar bait (ATSB) with safe EPA-exempt substance significantly diminishes the Anopheles sergentii population in a desert oasis. Acta Trop. 2015, 150, 29–34. [Google Scholar] [CrossRef]
- World Health Organization. New Frontiers in Vector Control. Available online: https://www.who.int/news-room/feature-stories/detail/new-frontiers-in-vector-control (accessed on 10 December 2024).
- Sippy, R.; Rivera, G.E.; Sanchez, V.; Heras, F.; Morejón, B.; Beltrán, E.; Hikida, R.S.; López-Latorre, M.A.; Aguirre, A.; Stewart-Ibarra, A.M.; et al. Ingested insecticide to control Aedes aegypti: Developing a novel dried attractive toxic sugar bait device for intra-domiciliary control. Parasites Vectors 2020, 13, 78. [Google Scholar] [CrossRef]
- McMeniman, C.J.; Corfas, R.A.; Matthews, B.J.; Ritchie, S.A.; Vosshall, L.B. Multimodal integration of carbon dioxide and other sensory cues drives mosquito attraction to humans. Cell 2014, 156, 1060–1071. [Google Scholar] [CrossRef]
- Naranjo, D.P.; Qualls, W.A.; Müller, G.C.; Samson, D.M.; Roque, D.; Alimi, T.; Arheart, K.; Beier, J.C.; Xue, R.D. Evaluation of boric acid sugar baits against Aedes albopictus (Diptera: Culicidae) in tropical environments. Parasitol. Res. 2013, 112, 1583–1587. [Google Scholar] [CrossRef] [PubMed]
- Owino, E.A. Human and plant volatiles; lures for mosquito, vectors of dengue virus and malaria. J. Vector Borne Dis. 2021, 58, 1–11. [Google Scholar] [CrossRef]
- van Loon, J.J.A.; Smallegange, R.C.; Bukovinszkiné-Kiss, G.; Jacobs, F.; De Rijk, M.; Mukabana, W.R.; Verhulst, N.O.; Menger, D.J.; Takken, W. Mosquito Attraction: Crucial Role of Carbon Dioxide in Formulation of a Five-Component Blend of Human-Derived Volatiles. J. Chem. Ecol. 2015, 41, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Müller, G.C.; Beier, J.C.; Traore, S.F.; Toure, M.B.; Traore, M.M.; Bah, S.; Doumbia, S.; Schlein, Y. Field experiments of Anopheles gambiae attraction to local fruits/seedpods and flowering plants in Mali to optimize strategies for malaria vector control in Africa using attractive toxic sugar bait methods. Malar. J. 2010, 9, 262. [Google Scholar] [CrossRef]
- Kline, D.L. Traps and trapping techniques for adult mosquito control. J. Am. Mosq. Control Assoc. 2006, 22, 490–496. [Google Scholar] [CrossRef]
- Beier, J.C.; Müller, G.C.; Gu, W.; Arheart, K.L.; Schlein, Y. Attractive toxic sugar bait (ATSB) methods decimate populations of Anopheles malaria vectors in arid environments regardless of the local availability of favoured sugar-source blossoms. Malar. J. 2012, 11, 31. [Google Scholar] [CrossRef] [PubMed]
- Müller, G.C.; Junnila, A.; Qualls, W.; Revay, E.E.; Kline, D.L.; Allan, S.; Schlein, Y.; Xue, R.D. Control of Culex quinquefasciatus in a storm drain system in Florida using attractive toxic sugar baits. Med. Vet. Entomol. 2010, 24, 346–351. [Google Scholar] [CrossRef]
- An, X.; Hu, Y.; Wang, N.; Zhou, Z.; Liu, Z. Continuous juice concentration by integrating forward osmosis with membrane distillation using potassium sorbate preservative as a draw solute. J. Membr. Sci. 2019, 573, 192–199. [Google Scholar] [CrossRef]
- Pylypiw, H.M.; Grether, M.T. Rapid high-performance liquid chromatography method for the analysis of sodium benzoate and potassium sorbate in foods. J. Chromatogr. A 2000, 883, 299–304. [Google Scholar] [CrossRef]
- Magomya, A.; Yebpella, G.; Okpaegbe, U.; Oko, O.; Gambo, S. Analysis and Health Risk Assessment of Sodium Benzoate and Potassium Sorbate in Selected Fruit Juice and Soft Drink Brands in Nigeria. Int. J. Pharm. Chem. 2020, 6, 54–59. [Google Scholar] [CrossRef]
- Ayub, M.; Ullah, J.; Muhammad, A.; Zeb, A. Evaluation of strawberry juice preserved with chemical preservatives at refrigeration temperature. Int. J. Nutr. Metab. 2010, 2, 27–32. [Google Scholar]
- Li, Y.; Zhou, G.; Zhong, S.; Wang, X.; Zhong, D.; Hemming-Schroeder, E.; Yi, G.; Fu, F.; Fu, F.; Cui, L.; et al. Spatial heterogeneity and temporal dynamics of mosquito population density and community structure in Hainan Island, China. Parasites Vectors 2020, 13, 444. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Triana, L.M.; Folly, A.J.; Sewgobind, S.; Lean, F.Z.X.; Ackroyd, S.; Nuñez, A.; Delacour, S.; Drago, A.; Visentin, P.; Mansfield, K.L.; et al. Susceptibility of Aedes albopictus and Culex quinquefasciatus to Japanese encephalitis virus. Parasites Vectors 2022, 15, 210. [Google Scholar] [CrossRef]
- Asid Alanazi, N. Boric Acid as a Safe Insecticide for Controlling the Mediterranean Fruit Fly Ceratitis Capitata Wiedemann (Diptera: Tephritidae). Eng. Technol. Appl. Sci. Res. 2023, 13, 11860–11864. [Google Scholar] [CrossRef]
- World Health Organization. The WHO Recommended Classification of Pesticides by Hazard and Guidelines to Classification 2019; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Tosi, S.; Burgio, G.; Nieh, J.C. A common neonicotinoid pesticide, thiamethoxam, impairs honey bee flight ability. Sci. Rep. 2017, 7, 1201. [Google Scholar] [CrossRef]
- Braunbeck, T.; Kais, B.; Lammer, E.; Otte, J.; Schneider, K.; Stengel, D.; Strecker, R. The fish embryo test (FET): Origin, applications, and future. Environ. Sci. Pollut. Res. Int. 2015, 22, 16247–16261. [Google Scholar] [CrossRef]
- Zheng, Q.; Wu, J.; Yan, W.; Zhu, S.; Miao, X.; Wang, R.; Huang, S.; Cheng, D.; Zhang, P.; Zhang, Z. Green synthesis of a chlorfenapyr chitosan nanopesticide for maize root application: Reducing environmental pollution and risks to nontarget organisms. Int. J. Biol. Macromol. 2023, 253, 126988. [Google Scholar] [CrossRef]
- Isman, M.B. Botanical Insecticides in the Twenty-First Century-Fulfilling Their Promise? Annu. Rev. Entomol. 2020, 65, 233–249. [Google Scholar] [CrossRef]
- Pretty, J.; Bharucha, Z.P. Integrated Pest Management for Sustainable Intensification of Agriculture in Asia and Africa. Insects 2015, 6, 152–182. [Google Scholar] [CrossRef]
- Qualls, W.A.; Müller, G.C.; Traore, S.F.; Traore, M.M.; Arheart, K.L.; Doumbia, S.; Schlein, Y.; Kravchenko, V.D.; Xue, R.D.; Beier, J.C. Indoor use of attractive toxic sugar bait (ATSB) to effectively control malaria vectors in Mali, West Africa. Malar. J. 2015, 14, 301. [Google Scholar] [CrossRef]
- Kumar, S.; Sahgal, A. Advances in Mosquito Control: A Comprehensive Review. In Advances in Diptera—Insight, Challenges and Management Tools, Kumar, S., Eds.; IntechOpen: Rijeka, Croatia, 2022. [Google Scholar] [CrossRef]
- Wang, G.H.; Hoffmann, A.; Champer, J. Gene Drive and Symbiont Technologies for Control of Mosquito-Borne Diseases. Annu. Rev. Entomol. 2025, 70, 229–249. [Google Scholar] [CrossRef] [PubMed]


| Apple Juice | Grape Juice | Orange Juice | Pear Juice | Peach Juice | ||
|---|---|---|---|---|---|---|
| Culex quinquefasciatus | ||||||
| Average mosquito feeding rate ± SEM | Male | 62.22 ± 5.88 ab | 51.11 ± 5.88 | 22.22 ± 8.01 ac | 53.33 ± 3.85 c | 31.11 ± 8.01 b |
| Female | 46.67 ± 11.55 | 48.89 ± 9.69 | 33.33 ± 17.64 | 31.11 ± 18.19 | 40.00 ± 10.18 | |
| Total | 54.44 ± 5.56 | 50.00 ± 6.94 | 27.78 ± 5.88 | 42.22 ± 7.29 | 35.56 ± 6.19 | |
| Control | Male | 15.56 ± 5.88 | 22.22 ± 8.89 | 31.11 ± 15.56 | 13.33 ± 7.70 | 15.56 ± 2.22 |
| Female | 33.33 ± 10.18 | 28.89 ± 4.44 | 37.78 ± 11.76 | 53.33 ± 19.25 | 24.44 ± 5.88 | |
| Total | 24.44 ± 7.78 | 25.56 ± 5.88 | 34.44 ± 12.81 | 33.33 ± 12.02 | 20.00 ± 1.92 | |
| Attraction index | Male | 4.00 | 2.30 | 0.71 | 4.00 | 2.00 |
| Female | 1.40 | 1.69 | 0.88 | 0.58 | 1.64 | |
| Total | 2.23 | 1.96 | 0.81 | 1.27 | 1.78 | |
| Aedes albopictus | ||||||
| Average mosquito feeding rate ± SEM | Male | 31.11 ± 8.01 | 31.11 ± 5.88 | 22.22 ± 4.44 | 35.56 ± 2.22 | 28.89 ± 5.88 |
| Female | 42.22 ± 5.88 ad | 22.22 ± 2.22 de | 20.00 ± 3.85 ac | 46.67 ± 3.85 ce | 33.33 ± 3.85 | |
| Total | 36.67 ± 1.92 | 26.67 ± 3.33 | 21.11 ± 2.94 | 41.11 ± 1.11 | 31.11 ± 4.44 | |
| Control | Male | 37.78 ± 2.22 | 44.44 ± 5.88 | 42.22 ± 4.44 | 35.56 ± 5.88 | 28.89 ± 8.01 |
| Female | 42.22 ± 2.22 | 33.33 ± 7.70 | 44.44 ± 8.01 | 37.78 ± 5.88 | 46.67 ± 7.70 | |
| Total | 40.00 ± 1.92 | 38.89 ± 2.94 | 43.33 ± 1.92 | 36.67 ± 5.09 | 37.77 ± 7.78 | |
| Attraction index | Male | 0.82 | 0.70 | 0.53 | 1.00 | 1.00 |
| Female | 1.00 | 0.67 | 0.45 | 1.24 | 0.71 | |
| Total | 0.92 | 0.69 | 0.49 | 1.12 | 0.82 | |
| Anopheles sinensis | ||||||
| Average mosquito feeding rate ± SEM | Male | 48.89 ± 5.88 | 37.78 ± 8.01 | 33.33 ± 3.85 | 24.44 ± 4.44 | 28.89 ± 5.88 |
| Female | 57.78 ± 5.883 | 53.33 ± 3.85 | 40.00 ± 13.88 | 37.78 ± 14.57 | 37.78 ± 11.11 | |
| Total | 53.33 ± 3.85 | 45.56 ± 5.88 | 36.67 ± 7.70 | 31.11 ± 6.76 | 33.33 ± 5.77 | |
| Control | Male | 22.22 ± 8.89 | 28.89 ± 5.88 | 33.33 ± 10.18 | 33.33 ± 13.88 | 20.00 ± 6.67 |
| Female | 28.89 ± 9.69 | 31.11 ± 5.88 | 44.44 ± 5.88 | 31.11 ± 9.69 | 42.22 ± 9.69 | |
| Total | 25.56 ± 9.09 | 30.00 ± 0.00 | 38.89 ± 7.29 | 32.22 ± 11.60 | 31.11 ± 5.56 | |
| Attraction index | Male | 2.20 | 1.31 | 1.00 | 0.73 | 1.44 |
| Female | 2.00 | 1.71 | 0.90 | 1.21 | 0.89 | |
| Total | 2.09 | 1.52 | 0.94 | 0.97 | 1.07 | |
| Species and Sex | LC50 (95%CI a) g/L | LC90 (95%CI a) g/L |
|---|---|---|
| Culex quinquefasciatus | ||
| Total | ||
| Dinotefuran | 1.18 × 10−3 | 1.03 × 10−2 |
| Boric acid | 15.05 (13.52–16.90) | 29.70 (24.50–41.74) |
| Male | ||
| Dinotefuran | 8.13 × 10−4 (5.00 × 10−4–1.30 × 10−3) | 8.09 × 10−3 (4.34 × 10−3–2.16 × 10−2) |
| Boric acid | 12.22 (11.02–13.56) | 17.71 (15.61–21.85) |
| Female | ||
| Dinotefuran | 1.71 × 10−3 | 1.17 × 10−2 |
| Boric acid | 21.39 (16.80–45.38) | 61.14 (34.18–80.11) |
| Aedes albopictus | ||
| Total | ||
| Dinotefuran | 4.06 × 10−4 | 9.57 × 10−4 |
| Boric acid | 7.24 | 30.47 |
| male | ||
| Dinotefuran | 3.72 × 10−4 (2.94 × 10−4–4.54 × 10−4) | 9.17 × 10−4 (7.25 × 10−4–1.29 × 10−3) |
| Boric acid | 5.02 | 24.04 |
| Female | ||
| Dinotefuran | 4.45 × 10−4 | 9.76 × 10−4 |
| Boric acid | 11.49 (9.11–13.42) | 23.17 (18.62–38.53) |
| Anopheles sinensis | ||
| Total | ||
| Dinotefuran | 5.20 × 10−5 | 3.26 × 10−4 |
| Boric acid | 2.97 | 6.99 |
| Male | ||
| Dinotefuran | 3.20 × 10−5 (1.70 × 10−5–5.60 × 10−5) | 2.66 × 10−4 (1.34 × 10−4–9.53 × 10−4) |
| Boric acid | 2.72 (2.02–3.54) | 6.87 (5.13–10.64) |
| Female | ||
| Dinotefuran | 8.40 × 10−5 (5.40 × 10−5–1.32 × 10−4) | 3.26 × 10−4 (1.93 × 10−4–8.72 × 10−4) |
| Boric acid | 3.31 (2.45–4.17) | 7.03 (5.49–10.17) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Zhao, T.; Xing, D.; Zhou, X.; Yu, H.; Geng, D.; Fan, Z.; Wang, K.; Huang, X.; Li, C. Development of Attractive Toxic Sugar Baits (ATSBs) System and Its Effectiveness in Mosquito Control. Insects 2025, 16, 258. https://doi.org/10.3390/insects16030258
Zhang R, Zhao T, Xing D, Zhou X, Yu H, Geng D, Fan Z, Wang K, Huang X, Li C. Development of Attractive Toxic Sugar Baits (ATSBs) System and Its Effectiveness in Mosquito Control. Insects. 2025; 16(3):258. https://doi.org/10.3390/insects16030258
Chicago/Turabian StyleZhang, Ruixiang, Teng Zhao, Dan Xing, Xinyu Zhou, Haotian Yu, Dongfen Geng, Zhihua Fan, Kai Wang, Xinan Huang, and Chunxiao Li. 2025. "Development of Attractive Toxic Sugar Baits (ATSBs) System and Its Effectiveness in Mosquito Control" Insects 16, no. 3: 258. https://doi.org/10.3390/insects16030258
APA StyleZhang, R., Zhao, T., Xing, D., Zhou, X., Yu, H., Geng, D., Fan, Z., Wang, K., Huang, X., & Li, C. (2025). Development of Attractive Toxic Sugar Baits (ATSBs) System and Its Effectiveness in Mosquito Control. Insects, 16(3), 258. https://doi.org/10.3390/insects16030258







