Temporal Variation in Target Site Mutations Is Associated with Diamide Cross-Resistance in Diamondback Moth Populations (Lepidoptera: Plutellidae) from Florida and Georgia, USA
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Toxicological Bioassays
2.3. mRNA Extraction and cDNA Synthesis
2.4. cDNA Library Preparation and Illumina Sequencing
2.5. Statistical Analysis
3. Results
3.1. Dose–Response Assays
3.2. Maximum Dose Bioassays
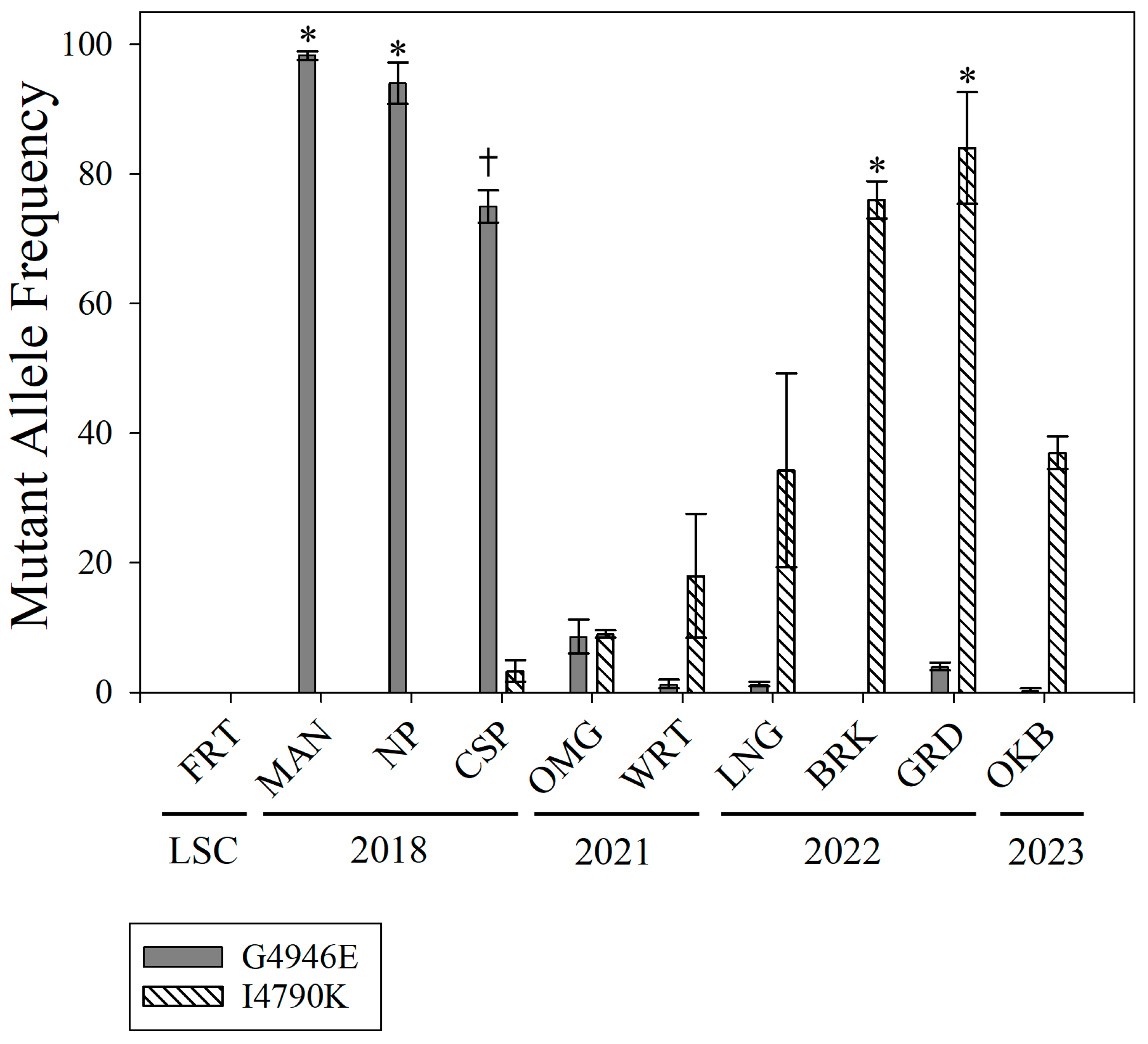
3.3. Mutation Frequency Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Talekar, N.; Shelton, A. Biology, ecology, and management of the diamondback moth. Annu. Rev. Entomol. 1993, 38, 275–301. [Google Scholar] [CrossRef]
- Furlong, M.J.; Wright, D.J.; Dosdall, L.M. Diamondback moth ecology and management: Problems, progress, and prospects. Annu. Rev. Entomol. 2013, 58, 517–541. [Google Scholar] [CrossRef] [PubMed]
- Zalucki, M.P.; Shabbir, A.; Silva, R.; Adamson, D.; Shu-Sheng, L.; Furlong, M.J. Estimating the economic cost of one of the world’s major insect pests, Plutella xylostella (Lepidoptera: Plutellidae): Just how long is a piece of string? J. Econ. Entomol. 2012, 105, 1115–1129. [Google Scholar] [CrossRef] [PubMed]
- Onstad, D.W.; Knolhoff, L.M. Insecticide Resistance Management: Biology, Economics, and Prediction; Academic Press: London, UK, 2022; p. 15. [Google Scholar]
- Wang, X.; Wu, Y. High levels of resistance to chlorantraniliprole evolved in field populations of Plutella xylostella. J. Econ. Entomol. 2012, 105, 1019–1023. [Google Scholar] [CrossRef]
- Bhandari, K.B.; Torrance, P.; Huffman, E.; Bennett, J.; Riley, D.G. Insecticide Resistance in Diamondback Moth (Lepidoptera: Plutellidae) in Georgia. J. Entomol. Sci. 2020, 55, 416–420. [Google Scholar] [CrossRef]
- Riley, D.; Smith, H.; Bennett, J.; Torrance, P.; Huffman, E.; Sparks, A., Jr.; Gruver, C.; Dunn, T.; Champagne, D. Regional survey of diamondback moth (Lepidoptera: Plutellidae) response to maximum dosages of insecticides in Georgia and Florida. J. Econ. Entomol. 2020, 113, 2458–2464. [Google Scholar] [CrossRef]
- Dunn, T.P.; Cremonez, P.S.G.; Furuya, A.; Brown, W.S.; Nagaoka, M.M.; Powell, C.B.; Sparks, A.N.S., Jr.; Smith, H.; Riley, D.G.; Champagne, D.E. Regional Changes in Insecticide Resistance in Diamondback Moth, Plutella xylostella (Lepidoptera: Plutellidae) Populations from Georgia and Florida, USA. J. Econ. Entomol. 2024, 117, 2628–2635. [Google Scholar] [CrossRef]
- Lahm, G.P.; Selby, T.P.; Freudenberger, J.H.; Stevenson, T.M.; Myers, B.J.; Seburyamo, G.; Smith, B.K.; Flexner, L.; Clark, C.E.; Cordova, D. Insecticidal anthranilic diamides: A new class of potent ryanodine receptor activators. Bioorg. Med. Chem. Lett. 2005, 15, 4898–4906. [Google Scholar] [CrossRef]
- Ebbinghaus-Kintscher, U.; Luemmen, P.; Lobitz, N.; Schulte, T.; Funke, C.; Fischer, R.; Masaki, T.; Yasokawa, N.; Tohnishi, M. Phthalic acid diamides activate ryanodine-sensitive Ca2+ release channels in insects. Cell Calcium 2006, 39, 21–33. [Google Scholar] [CrossRef]
- Cordova, D.; Benner, E.A.; Sacher, M.D.; Rauh, J.J.; Sopa, J.S.; Lahm, G.P.; Selby, T.P.; Stevenson, T.M.; Flexner, L.; Gutteridge, S.; et al. Anthranilic diamides: A new class of insecticides with a novel mode of action, ryanodine receptor activation. Pestic. Biochem. Physiol. 2006, 84, 196–214. [Google Scholar] [CrossRef]
- Lahm, G.P.; Cordova, D.; Barry, J.D. New and selective ryanodine receptor activators for insect control. Bioorg. Med. Chem. 2009, 17, 4127–4133. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, L.A.F.; Andaloro, J.T. Diamide insecticides: Global efforts to address insect resistance stewardship challenges. Pestic. Biochem. Physiol. 2013, 106, 76–78. [Google Scholar] [CrossRef]
- Troczka, B.J.; Williamson, M.S.; Field, L.M.; Davies, T.E. Rapid selection for resistance to diamide insecticides in Plutella xylostella via specific amino acid polymorphisms in the ryanodine receptor. Neurotoxicology 2017, 60, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Jouraku, A.; Kuwazaki, S.; Miyamoto, K.; Uchiyama, M.; Kurokawa, T.; Mori, E.; Mori, M.X.; Mori, Y.; Sonoda, S. Ryanodine receptor mutations (G4946E and I4790K) differentially responsible for diamide insecticide resistance in diamondback moth, Plutella xylostella L. Insect Biochem. Mol. Biol. 2020, 118, 103308. [Google Scholar] [CrossRef]
- Jiang, D.; Qian, C.; Wang, D.; Wang, F.; Zhao, S.; Yang, Y.; Baxter, S.W.; Wang, X.; Wu, Y. Varying contributions of three ryanodine receptor point mutations to diamide insecticide resistance in Plutella xylostella. Pest Manag. Sci. 2021, 77, 4874–4883. [Google Scholar] [CrossRef] [PubMed]
- Pudasaini, R.; Chang, C.; Chen, M.H.; Dai, S.M. The I4790K mutation of the ryanodine receptor is responsible for anthranilic diamide resistance in field populations of Plutella xylostella (Lepidoptera: Plutellidae). J. Econ. Entomol. 2024, 117, 2081–2092. [Google Scholar] [CrossRef]
- Troczka, B.; Zimmer, C.T.; Elias, J.; Schorn, C.; Bass, C.; Davies, T.E.; Field, L.M.; Williamson, M.S.; Slater, R.; Nauen, R. Resistance to diamide insecticides in diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae) is associated with a mutation in the membrane-spanning domain of the ryanodine receptor. Insect Biochem. Mol. Biol. 2012, 42, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Liang, P.; Zhou, X.; Gao, X. Novel mutations and mutation combinations of ryanodine receptor in a chlorantraniliprole resistant population of Plutella xylostella (L.). Sci. Rep. 2014, 4, 6924. [Google Scholar] [CrossRef] [PubMed]
- Ward, C.; Perry, K.; Baker, G.; Powis, K.; Heckel, D.G.; Baxter, S. A haploid diamondback moth (Plutella xylostella L.) genome assembly resolves 31 chromosomes and identifies a diamide resistance mutation. Insect Biochem. Mol. Biol. 2021, 138, 103622. [Google Scholar] [CrossRef]
- Steinbach, D.; Gutbrod, O.; Lümmen, P.; Matthiesen, S.; Schorn, C.; Nauen, R. Geographic spread, genetics and functional characteristics of ryanodine receptor based target-site resistance to diamide insecticides in diamondback moth, Plutella xylostella. Insect Biochem. Mol. Biol. 2015, 63, 14–22. [Google Scholar] [CrossRef]
- Chang, C.C.; Dai, S.M.; Chen, C.Y.; Huang, L.H.; Chen, Y.H.; Hsu, J.C. Insecticide resistance and characteristics of mutations related to target site insensitivity of diamondback moths in Taiwan. Pestic. Biochem. Physiol. 2024, 203, 106001. [Google Scholar] [CrossRef]
- Lin, L.; Hao, Z.; Cao, P.; Yuchi, Z. Homology modeling and docking study of diamondback moth ryanodine receptor reveals the mechanisms for channel activation, insecticide binding and resistance. Pest Manag. Sci. 2020, 76, 1291–1303. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, H.; Li, K.; Liu, J.; Zhu, H.; Zhou, Y.; Man, Y.; Zhou, X.; Liu, Z. Frequencies of insecticide resistance mutations detected by the amplicon sequencing in Plutella xylostella (Lepidoptera: Plutellidae) and Spodoptera exigua (Lepidoptera: Noctuidae) from China. J. Econ. Entomol. 2024, 117, 1648–1654. [Google Scholar] [CrossRef]
- Roditakis, E.; Steinbach, D.; Moritz, G.; Vasakis, E.; Stavrakaki, M.; Ilias, A.; García-Vidal, L.; del Rosario Martinez-Aguirre, M.; Bielza, P.; Morou, E.; et al. Ryanodine receptor point mutations confer diamide insecticide resistance in tomato leafminer, Tuta absoluta (Lepidoptera: Gelechiidae). Insect Biochem. Mol. Biol. 2017, 80, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Boaventura, D.; Martin, M.; Pozzebon, A.; Mota-Sanchez, D.; Nauen, R. Monitoring of target-site mutations conferring insecticide resistance in Spodoptera frugiperda. Insects 2020, 11, 545. [Google Scholar] [CrossRef]
- Huang, J.M.; Rao, C.; Wang, S.; He, L.F.; Zhao, S.Q.; Zhou, L.Q.; Zhao, Y.X.; Yang, F.X.; Gao, C.F.; Wu, S.F. Multiple target-site mutations occurring in lepidopterans confer resistance to diamide insecticides. Insect Biochem. Mol. Biol. 2020, 121, 103367. [Google Scholar] [CrossRef]
- Huang, J.M.; Sun, H.; He, L.F.; Liu, C.; Ge, W.C.; Ni, H.; Gao, C.F.; Wu, S.F. Double ryanodine receptor mutations confer higher diamide resistance in rice stem borer, Chilo suppressalis. Pest Manag. Sci. 2021, 77, 4971–4979. [Google Scholar] [CrossRef]
- Zuo, Y.Y.; Ma, H.H.; Lu, W.J.; Wang, X.L.; Wu, S.W.; Nauen, R.; Wu, Y.D.; Yang, Y.H. Identification of the ryanodine receptor mutation I4743M and its contribution to diamide insecticide resistance in Spodoptera exigua (Lepidoptera: Noctuidae). Insect Sci. 2020, 27, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Okuma, D.M.; Cuenca, A.; Nauen, R.; Omoto, C. Large-scale monitoring of the frequency of ryanodine receptor target-site mutations conferring diamide resistance in Brazilian field populations of fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae). Insects 2022, 13, 626. [Google Scholar] [CrossRef]
- Ren, H.; Zhang, H.; Ni, R.; Li, Y.; Li, L.; Wang, W.; Tian, Y.; Pang, B.; Tan, Y. Detection of ryanodine receptor G4911E and I4754M mutation sites and analysis of binding modes of diamide insecticides with RyR on Galeruca daurica (Coleoptera: Chrysomelidae). Front. Physiol. 2022, 13, 1107045. [Google Scholar] [CrossRef] [PubMed]
- Mei, W.; Zuo, Y.; Su, T.; Yuan, J.; Wu, Y.; Yang, Y. The ryanodine receptor mutation I4728M confers moderate—Level resistance to diamide insecticides in Spodoptera litura. Pest Manag. Sci. 2023, 79, 3693–3699. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, S.T.; Ling, Y.; Wang, L.; Ni, H.; Guo, D.; Dong, B.B.; Huang, Q.; Long, L.P.; Zhang, S.; et al. Insecticide resistance monitoring of Cnaphalocrocis medinalis (Lepidoptera: Pyralidae) and its mechanism to chlorantraniliprole. Pest Manag. Sci. 2023, 79, 3290–3299. [Google Scholar] [CrossRef]
- Dunn, T.P.S.; Champagne, D.E.; Riley, D.G.; Sparks, A.N., Jr. A Beet Armyworm (Lepidoptera: Noctuidae) Ryanodine Receptor Mutation Associated with Diamide Resistance is Present in a Chlorantraniliprole-Resistant Population from South Georgia, USA. J. Entomol. Sci. 2024, 60, 481–496. [Google Scholar] [CrossRef]
- Dunn, T.P.S.; Champagne, D.E.; Riley, D.G.; Smith, H.; Bennett, J.E. A target site mutation associated with diamide insecticide resistance in the diamondback moth, Plutella xylostella, (Lepidoptera: Plutellidae) is widespread in South Georgia and Florida populations. J. Econ. Entomol. 2022, 115, 289–296. [Google Scholar] [CrossRef]
- Dunn, T.P.; Cremonez, P.S.; Brown, W.S.; Gruver, C.L.; Riley, D.G. Evaluation of insecticide treatments in cabbage, 2021. Arthropod Manag. Tests 2023, 48, tsad091. [Google Scholar] [CrossRef]
- Troczka, B.J.; Williams, A.J.; Williamson, M.S.; Field, L.M.; Lüemmen, P.; Davies, T.E. Stable expression and functional characterisation of the diamondback moth ryanodine receptor G4946E variant conferring resistance to diamide insecticides. Sci. Rep. 2015, 5, 14680. [Google Scholar] [CrossRef]
- Champagne, D.C.; Riley, D.G.; Brown, W.S.; Cremonez, P.S.G.; Nagaoka, M.M.; Dunn, T.P. Management of Diamondback Moth in Georgia, USA. In Proceedings of the IX International Conference on Management of the Diamondback Moth and Other Crucifer Insect Pests, Phnom Penh, Cambodia, 2–5 May 2023; Ramaswamy, S., Ed.; World Vegetable Center: Tainan, Taiwan, 2024; pp. 78–94, ISBN 92-9058-245-6. [Google Scholar]
- Jiang, D.; Yu, Z.; He, Y.; Wang, F.; Gu, Y.; Davies, T.E.; Fan, Z.; Wang, X.; Wu, Y. Key role of the ryanodine receptor I4790K mutation in mediating diamide resistance in Plutella xylostella. Insect Biochem. Mol. Biol. 2024, 168, 104107. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Kim, K.; Kwon, D.H.; Jeong, I.H.; Clark, J.M.; Lee, S.H. Transcriptome-based identification and characterization of genes commonly responding to five different insecticides in the diamondback moth, Plutella xylostella. Pestic. Biochem. Physiol. 2018, 144, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Shi, H.; Gao, X.; Liang, P. Characterization of UDP-glucuronosyltransferase genes and their possible roles in multi-insecticide resistance in Plutella xylostella (L.). Pest Manag. Sci. 2018, 74, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Mallott, M.; Hamm, S.; Troczka, B.J.; Randall, E.; Pym, A.; Grant, C.; Baxter, S.; Vogel, H.; Shelton, A.M.; Field, L.M.; et al. A flavin-dependent monooxygenase confers resistance to chlorantraniliprole in the diamondback moth, Plutella xylostella. Insect Biochem. Mol. Biol. 2019, 115, 103247. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, Y. Identification and characterisation of multiple glutathione S-transferase genes from the diamondback moth, Plutella xylostella. Pest Manag. Sci. 2015, 71, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Fukada, M.; Itagaki, Y.; Nagayoshi, A.; Sonoda, S. Field survey of ryanodine receptor mutations (G4946E and I4790K) and their effects on biotic performance in the diamondback moth. J. Pestic. Sci. 2020, 45, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Calvin, W.; Palumbo, J.C. Chlorantraniliprole resistance associated with diamondback moth (Lepidoptera: Plutellidae) outbreaks in Arizona Brassica crops. J. Econ. Entomol. 2024, 117, 2608–2617. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, J.C. Diamondback Moth Control with Harvanta in Cauliflower, Spring 2021. Arthropod Manag. Tests. 2022, 47, tsac034. [Google Scholar] [CrossRef]
- Palumbo, J.C. Diamondback Moth Control with Plinazolin in Green Cabbage, Spring 2023. Arthropod Manag. Tests 2023, 48, tsad100. [Google Scholar] [CrossRef]
- Shelton, A.M.; Kroening, M.K.; Eigenbrode, S.D.; Petzold, C.; Hoffmann, M.P.; Wyman, J.A.; Wilsey, W.T.; Cooley, R.J.; Pedersen, L.H. Diamondback moth (Lepidoptera: Plutellidae) contamination of cabbage transplants and the potential for insecticide resistance problems. J. Entomol. Sci. 1996, 31, 347–354. [Google Scholar] [CrossRef]

| Colony | Insecticide | LC50 mg ai/L a | 95% Fiducial Limits | N | Slope | Intercept | SE | x2 | p | RR (FRT) b | RR (CSP) c | RR (NP) d | RR (MAN) e | RR (T-S) f |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SMN | Chlorantraniliprole | 411.99 | 252.72–683.99 | 287 | 1.26 | −3.31 | 0.22 | 32.1 | <0.0001 | 2942.7 | 27.0 | 0.7 | 1.0 | 211.3 |
| SMN | Cyantraniliprole | 269.22 | 59.57–867.58 | 255 | 0.54 | −1.31 | 0.12 | 17.4 | <0.0001 | 1582.4 | 31.8 | 23.6 | 14.7 | 1416.9 |
| TIF | Chlorantraniliprole | 71.55 | 13.17–223.50 | 204 | 0.58 | −1.07 | 0.15 | 13.9 | 0.0002 | 511.0 | 4.7 | 0.1 | 0.2 | 36.7 |
| TIF | Cyantraniliprole | 65.01 | 1.35–209.33 | 175 | 0.61 | −1.10 | 0.22 | 7.2 | 0.0072 | 382.4 | 7.7 | 5.7 | 3.6 | 342.2 |
| Population | Insecticide | Year | Percent Mortality | Z-Score | p | p-Adjusted | Resistance Level |
|---|---|---|---|---|---|---|---|
| FRT | Chlorantraniliprole | - | 100.0 | - | - | - | S |
| CSP | Chlorantraniliprole | 2018 | 61.4 | 0.51 | 0.307 | 0.460 | S |
| MAN | Chlorantraniliprole | 2018 | 20.0 | 2.36 | 0.009 | 0.051 † | R |
| NP | Chlorantraniliprole | 2018 | 26.0 | 2.00 | 0.022 | 0.078 † | R |
| OMG | Chlorantraniliprole | 2021 | 20.7 | 2.43 | 0.007 | 0.056 † | R |
| WRT | Chlorantraniliprole | 2021 | 13.7 | 2.79 | 0.002 | 0.059 † | R |
| LNG | Chlorantraniliprole | 2022 | 20.0 | 2.41 | 0.008 | 0.051 † | R |
| GRD | Chlorantraniliprole | 2022 | 17.5 | 2.56 | 0.005 | 0.057 † | R |
| BRK | Chlorantraniliprole | 2022 | 11.7 | 3.03 | 0.001 | 0.053 † | R |
| OKB | Chlorantraniliprole | 2023 | 34.5 | 1.30 | 0.095 | 0.253 | S |
| FRT | Cyantraniliprole | - | 100.0 | - | - | - | S |
| CSP | Cyantraniliprole | 2018 | 90.0 | 0.53 | 0.297 | 0.372 | S |
| MAN | Cyantraniliprole | 2018 | 87.5 | 0.68 | 0.248 | 0.338 | S |
| NP | Cyantraniliprole | 2018 | 72.0 | 1.08 | 0.139 | 0.241 | S |
| OMG | Cyantraniliprole | 2021 | 9.4 | 2.89 | 0.001 | 0.021 * | R |
| WRT | Cyantraniliprole | 2021 | 3.3 | 3.22 | 0.001 | 0.028 * | R |
| LNG | Cyantraniliprole | 2022 | 10.0 | 2.91 | 0.001 | 0.026 * | R |
| GRD | Cyantraniliprole | 2022 | 10.0 | 2.83 | 0.002 | 0.020 * | R |
| BRK | Cyantraniliprole | 2022 | 25.0 | 2.17 | 0.014 | 0.044 * | R |
| OKB | Cyantraniliprole | 2023 | 33.3 | 1.82 | 0.034 | 0.085 † | R |
| Comparison | Z-Score | p | p-Adjusted |
|---|---|---|---|
| FRT Chlorantraniliprole vs. 2018 Chlorantraniliprole | 2.09 | 0.035 | 0.053 † |
| FRT Cyantraniliprole vs. 2018 Cyantraniliprole | 0.63 | 0.523 | 0.6042 |
| FRT Chlorantraniliprole vs. 2021–2023 Chlorantraniliprole | 3.18 | 0.001 | 0.003 * |
| FRT Cyantraniliprole vs. 2021–2023 Cyantraniliprole | 3.57 | <0.001 | 0.001 * |
| 2018 Chlorantraniliprole vs. 2021–2023 Chlorantraniliprole | 1.52 | 0.126 | 0.171 |
| 2018 Cyantraniliprole vs. 2021–2023 Cyantraniliprole | 4.99 | <0.001 | <0.001 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dunn, T.P.; Al Baki, M.A.; Cremonez, P.S.G.; Riley, D.G.; Sparks, A.N., Jr.; Smith, H.; Champagne, D.E. Temporal Variation in Target Site Mutations Is Associated with Diamide Cross-Resistance in Diamondback Moth Populations (Lepidoptera: Plutellidae) from Florida and Georgia, USA. Insects 2025, 16, 1179. https://doi.org/10.3390/insects16111179
Dunn TP, Al Baki MA, Cremonez PSG, Riley DG, Sparks AN Jr., Smith H, Champagne DE. Temporal Variation in Target Site Mutations Is Associated with Diamide Cross-Resistance in Diamondback Moth Populations (Lepidoptera: Plutellidae) from Florida and Georgia, USA. Insects. 2025; 16(11):1179. https://doi.org/10.3390/insects16111179
Chicago/Turabian StyleDunn, Thomas P., Md. Abdullah Al Baki, Paulo S. G. Cremonez, David G. Riley, Alton N. Sparks, Jr., Hugh Smith, and Donald E. Champagne. 2025. "Temporal Variation in Target Site Mutations Is Associated with Diamide Cross-Resistance in Diamondback Moth Populations (Lepidoptera: Plutellidae) from Florida and Georgia, USA" Insects 16, no. 11: 1179. https://doi.org/10.3390/insects16111179
APA StyleDunn, T. P., Al Baki, M. A., Cremonez, P. S. G., Riley, D. G., Sparks, A. N., Jr., Smith, H., & Champagne, D. E. (2025). Temporal Variation in Target Site Mutations Is Associated with Diamide Cross-Resistance in Diamondback Moth Populations (Lepidoptera: Plutellidae) from Florida and Georgia, USA. Insects, 16(11), 1179. https://doi.org/10.3390/insects16111179






