Simple Summary
Insecticide resistance is a global issue that mosquito control and other programs are facing that must be addressed to protect public health. Targeted control using the most efficacious insecticide products will help reduce insecticide resistance. A novel compact wind tunnel was developed to quickly assess product efficacy against mosquitoes in a laboratory. Oil- and water-based products were tested against laboratory and field mosquito populations and operational suggestions are provided. The wind tunnel can be used as a screening step to inform field trials and/or when it is not possible to conduct weather-dependent and labor-intensive field trials.
Abstract
Insecticide resistance is increasing globally, and tools must be developed to combat this issue facing mosquito control programs that protect public health and inform operational decisions. Field trials to assess insecticide formulated products (FPs) are logistically demanding and weather-dependent and the Centers for Disease Control and Prevention bottle bioassays are optimized to test technical active ingredient (AI) residue, not aerosolized FP. Here, the methodological gap between AI and FP efficacy testing is addressed. The current study assessed the knockdown/mortality of laboratory and wild populations of Aedes albopictus and Culex pipiens/quinquefasciatus to four FPs (Biomist®, Duet®, AquaDuet®, ReMoa Tri®) in wind tunnel experiments. The number of FP droplets on mosquitoes was analyzed. Regression analyses showed that droplet counts on mosquitoes were significantly (p < 0.05) related to mosquito mortality for some FPs. The wild Culex population was resistant to all FPs in the wind tunnel. Here, when wind tunnel experiments resulted in a relatively low mortality rate (<90%), this indicates mosquito resistance to the FP. In these situations, a field trial would likely not achieve good results and may not be necessary. Alternatively, when wind tunnel experiments resulted in a nearly perfect mortality rate, a confirmatory field trial could be conducted, if needed.
1. Introduction
Billions of people are at risk and approximately 700,000 deaths occur each year worldwide from mosquito-borne diseases [1]. West Nile encephalitis (WNE) is the most prevalent locally acquired mosquito-borne disease in the United States (US) and is caused by mosquito-borne transmission of West Nile virus (WNV) [2]. The US experienced 59,141 cases of WNV infections, resulting in >27,000 hospitalizations and ≈2958 deaths during 1999–2023 [2]. In 2024 alone, 1466 cases were reported across 49 states, reaffirming WNV as the most prevalent locally transmitted pathogen by mosquitoes [2]. Furthermore, despite being primarily nuisance biters, floodwater and other mosquitoes can impede emergency response after natural disasters such as hurricanes [3]. Therefore, mosquito control strategies must be optimized to address a variety of mosquito species and populations.
Insecticide resistance (IR) is increasing globally; between 2010 and 2020, 78 of 88 malaria-endemic countries reported resistance to at least one insecticide in malaria vectors [4]. Even in some countries where resistance to pyrethroids has been documented, this class of insecticide continues to be used by mosquito control programs (MCPs) [5]. The lack of alternatives in available tools, operational manpower, and strategic bandwidth has led to the continued use of insecticide classes such as pyrethroids, despite their declining efficacy.
Current methods for assessing insecticide formulated product (FP) efficacy for mosquito control can be cumbersome (e.g., large-scale wind tunnels) and/or require logistically challenging, time-consuming, expensive, and weather-dependent field trials with multiple personnel working at dusk/dawn periods [1,6,7,8]. One study constructed a large-scale (2.4 m long) wind tunnel (i.e., dispersion tunnel) to test how wind speed affected droplet deposition on cages [9]. The same study concluded that higher wind speeds would be needed to move smaller droplets through mesh cages and larger droplets are most likely to be filtered out through the mesh of mosquito cages. Another large-scale (≈3.6 m long) wind tunnel device was used to expose flies and cockroaches to FP for 5 s [10]. The same study did not transfer insects from wind tunnel cages to clean cages after treatment, as they observed negligible differences in mortality, which reduced the handling of insects. In another study, a 1.2 m wind tunnel was used to expose mosquitoes to FP (Duet® contains prallethrin which has excitation properties) or components of Duet® (i.e., synergized prallethrin, sumethrin) using an airbrush [11]. The same study recorded videos of mosquitoes during treatments to evaluate activity levels and counted/measured the size of the droplets landing on different body parts of the mosquitoes. Droplets were measured on dissected mosquito body parts mounted on slides using a compound microscope (no fluorescent dye was used) [11]. Mosquitoes exposed to Duet® or prallethrin alone showed more movement than control mosquitoes and increased movement may resulted in a greater number of droplets landing on the mosquitoes [11]. Most droplets were found on the legs and wings and were 2.5–5 µm in diameter [11]. The same study showed no correlation between the volume of droplets (i.e., droplet size or dose) and the number of droplets, indicating that several small droplets might have the same effect as fewer large droplets. Others have shown that droplets with a diameter from 2 to 16 µm dispersed in an ultra-low volume (ULV) FP cloud are the most likely to contact flying mosquitoes, even if larger (e.g., 32 µm) droplets were found on slides in the field [12].
Many small- to medium-scale MCPs do not routinely conduct field trials or use other methods to evaluate FP, partially due to a lack of resources. There is currently a methodological gap between technical active ingredient (AI) testing with the Centers for Disease Control and Prevention (CDC) bottle bioassays and the operational knowledge of FP efficacy [8,13,14,15], which this study seeks to address. More work is needed to link IR data to operational outcomes [14,16]. Resistance to an AI shown in a laboratory bioassay does not necessarily indicate the failure of the FP containing that AI, since FPs may have additional ingredients (e.g., synergists) that enhance effectiveness. Ideally, efficacy testing of FPs should be routinely carried out on target mosquito populations to ensure efficacy and reduce unnecessary spraying of ineffective insecticides [17,18]. Furthermore, since there is widespread resistance to pyrethroids and AIs from other insecticide classes globally, increased testing of FPs is needed to inform operational response to protect public health from mosquito-borne diseases (e.g., dengue, malaria, West Nile encephalitis) and aid in the response after natural disasters such as hurricanes. If a local mosquito population is resistant to an FP, it might be possible to rotate to another class or type of FP; hence, knowledge of FP resistance/susceptibility enables MCPs to make evidence-based operational decisions [14]. Both susceptible and resistant mosquito populations should be evaluated in lab and/or field trials to approximate real-world conditions since IR is increasing globally [19]. Many MCPs do not have the resources for FP efficacy testing; therefore, there is a need for a practical, low-cost solution to rapidly test and identify effective FPs for local mosquito populations before large-scale implementation.
A novel compact wind tunnel prototype was developed that fits inside a small fume hood and proof-of-concept results have been reported [7]. Briefly, the previous method development study exposed Aedes albopictus (Skuse) and Culex pipiens (Linnaeus)/quinquefasciatus (Say) to Biomist® 3 + 15 (hereafter, Biomist®) (FP; permethrin AI) or air (control) in field trial cages within the wind tunnel. Mosquito populations were successfully exposed to Biomist® in the wind tunnel and showed 100% mortality in all tested populations, although only a 2 h time point was monitored in this pilot study [7]. Consequently, the objectives in the current work were to (1) develop a new and improved research-grade wind tunnel prototype and (2) assess the proof-of-concept that the laboratory wind tunnel data can be used as an FP screening tool to inform operational decisions for MCPs.
2. Materials and Methods
2.1. Compact Wind Tunnel Construction
A research-grade compact wind tunnel was constructed, incorporating improvements based on data from a previous prototype (Figure 1) [7]. Improvements include a commercial nozzle designed to optimize droplet sizes, dual valves to independently control air flow and liquid feed, dual filters to remove aerosol and possible volatile organic carbon, and dual gauges to independently control background air and nozzle air pressure. The main difference from the original wind tunnel model is the elimination of a Blaustein Atomizing Module (BLAM), a costly aerosol generator. Rather, the nozzle is equipped with a lower-cost commercial-based air atomizing body, fluid cap, and air cap (Spraying Systems, Co., Glendale Heights, IL, USA). Images of the wind tunnel model used here are shown (Figure 1). Lab-supplied air is filtered through a five-stage desiccant air dryer (Speedaire-2YNL6, Philadelphia, PA, USA) inside a small box that includes a High-Efficiency Particulate Air (HEPA) filter (Global Life Sciences Solutions 0.2 μm PTFE, Buckinghamshire, UK) open to the atmosphere to remove excess air and relieve pressure before entering the chamber. Air enters the inlet of the chamber at 100 L min−1 (LPM) using a mass flow controller (Alicat Scientific, Tucson, AZ, USA). A vacuum pump is used at the outlet (Welch-Ilmvac2585B-50, Niles, IL, USA), which is regulated by a control valve (SMC vacuum regulator, Yorba Linda, CA, USA). Wind tunnel assessments were carried out at ambient room temperature (22.5 °C) and humidity (61.6%).
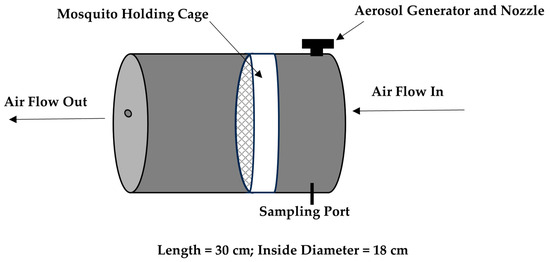
Figure 1.
Wind tunnel prototype general diagram. The mosquito holding cage (white object) is placed inside the wind tunnel prior to insecticide aerosolization. Background air enters the inlet and exits the outlet of the chamber at 100 LPM.
2.2. Mosquito Populations and Mortality
Mosquito populations used here are listed in Table 1. North Carolina (NC) is in a hybrid zone for Cx. pipiens and Cx. quinquefasciatus, and hence these populations are referred to as Cx. pipiens/quinquefasciatus. The F0 generation mosquitoes were collected from the field as eggs, reared, and the adults were used in experiments. Aedes albopictus eggs were collected from the field using seed germination paper placed within a 0.5 L black plastic cup with tap water and retrieved after 5–7 d. For the Curry Court (Pitt County, NC, USA) Culex pipiens/quinquefasciatus population, egg rafts were collected from the field in black plastic water pans (52 × 38 × 13 cm) with hay infusion as an attractant. The F2 generation Ae. albopictus from March 9, 1764 Dr. NE (hereafter, March Drive) (Brunswick County, NC, USA) were collected from the field and propagated for two generations in the laboratory to increase sample size using established methods [20,21,22].

Table 1.
Mosquito populations used in wind tunnel assessments. All wild populations were collected in North Carolina.
Female mosquitoes 4–6 d old were aspirated from colony cages and transferred to 15.2 cm diameter cardboard with mesh screen (Clarke Mosquito, St. Charles, IL, USA) (11–18 mosquitoes/cage; 3–4 replicate cages/group) for use in wind tunnel experiments. After FP exposure in the wind tunnel, mosquitoes from each group were transferred to separate clean 0.5 L cardboard cages, provided 20% sucrose, and placed in a 28 °C incubator with 14 h light:10 h dark. Knockdown was recorded at 2 h post-exposure and mortality was recorded at 24, 36, and/or 48 h post-exposure. The 36-h time point was used as the final mortality assessment time for mosquitoes exposed to Biomist®, Duet®, and AquaDuet® (Clarke Mosquito Control, St. Charles, IL, USA), while the 48-h time point was used for ReMoa Tri® (Valent BioSciences, Libertyville, IL, USA). Monitoring mortality at the later (48 h) time point is recommended for ReMoa Tri® [18].
2.3. Aerosol Characterization (Droplet Size)
Prior to mosquito exposure, aerosols generated in wind tunnel assays were characterized and optimized following the manufacturer instructions using an Aerodynamic Particle Sizer (spectrometer) (APS 3321, TSI Incorporated, Shoreview, MN, USA) with probe inserted through a sample port located near the nozzle. Each FP was aerosolized for at least five minutes to determine droplet size (mass median diameter [MMD]).
In this study, the wind tunnel was operated with a liquid insecticide flow rate of 0.4 mL/min and a nozzle air pressure of 2 PSIG to achieve the largest droplet size under the conditions of this test and to minimize FP waste. To achieve particle-free air, lab air flowed through a five-stage desiccant air dryer (Speedaire-2YNL6, Philadelphia, PA, USA) prior to entering the nozzle [7]. Three replicate mosquito cages (11–18 mosquitoes/cage) per population and FP were each exposed to aerosolized FP droplets for 10 s in the wind tunnel using established methods [7]. Additional replicate mosquito cages were exposed to air in the wind tunnel for 10 s as negative controls or with FPs for analysis of droplet distribution and spread.
2.4. Formulated Products
Aerosol testing and mosquito exposures were conducted with the following FPs: (1) Biomist® 3 + 15 (3% permethrin, 15% piperonyl butoxide [PBO], 82% other ingredients, including petroleum distillate [oil-based]), (2) Duet® (1% prallethrin, 5% sumethrin, 5% PBO, 89% other ingredients, including petroleum distillate [oil-based]), (3) AquaDuet® (1% prallethrin, 5% sumethrin, 5% PBO, 89% other ingredients [water-based]), and (4) ReMoa Tri® (4% fenpropathrin, 1.5% abamectin, 0.33% octanoic acid, 0.33% nonanoic acid, 0.33% decanoic acid, 93.51% other ingredients [oil-based]).
2.5. Droplet Spread and Number
To determine droplet spread on mosquitoes, riboflavin (fluorescent dye) was mixed with each FP (1 g/L; dye:product ratio) (Biomist®, Duet®, AquaDuet®, ReMoa Tri®) before being applied to two replicate mosquito cages (11–18 mosquitoes/cage) per population and FP. Fluorescent dyes are a commonly used method to visualize FP droplets [23]. An additional three cages were used for mortality assessments. A VisiLED ultraviolet (UV)/bright field ring light S80-55 with VisiLED MC 1100 controller to adjust light settings (Schott, Duryea, PA, USA) was used and attached to an SZ61 dissecting microscope (Olympus, Center Valley, PA, USA). The ring light emits UVA light wavelengths of 340–420 nm. Here, the controller was set to four-segment mode to improve contrast and the rotary knob allowed for seamless switching between bright field and UV modes, following manufacturer specifications. The brightness level was set to 10 during mosquito droplet assessment for optimum visualization. The number of droplets was counted for mosquitoes in two replicate cages per population and FP. Total droplets were recorded for the following body parts for each mosquito population and treatment group: proboscis, antennae, head, thorax, wings, legs, and abdomen. Total droplets were also tabulated for each individual mosquito.
2.6. Statistical Analyses
Chi-square tests (p < 0.05) were used to determine differences in mortality rates between FPs and mosquito populations (SAS Institute, version 9.4, Cary, NC, USA). Mosquito cages were used as the unit of replication using per-cage mortality proportions (i.e., number dead/total mosquitoes per cage). Analysis of variance (p < 0.05) was used to determine differences in means of droplet counts between mosquitoes in different treatment groups. Normality was assessed using Kolmogorov–Smirnoff tests and data were transformed prior to statistical analyses, if necessary. If significant differences were observed in the mean, Duncan means comparison tests were used to determine which means were significantly different (p < 0.05). Regression analysis (p < 0.05) was used to predict if total droplets on mosquito bodies were related to mosquito mortality rates for different FPs.
3. Results
Aerosol characterization and mosquito exposures were conducted with the following FPs: (1) Biomist®, (2) Duet®, (3) AquaDuet®, and (4) ReMoa Tri®. Droplet sizes (MMD) of FPs were determined by APS prior to wind tunnel mosquito assessments (Biomist® = 4.70 µm, Duet® = 4.40 µm, AquaDuet® = 4.37 µm, ReMoa Tri® = 4.60 µm).
Fluorescent droplets were visualized on mosquitoes exposed in the wind tunnel using UV filter and microscopy and tabulated for the four different FPs and mosquito populations (Figure 2, Table 2). The total number of droplets on an individual mosquito body ranged from 0 to 20. No significant differences (p > 0.05) were observed in log-transformed total droplet counts between FPs and mosquito populations.
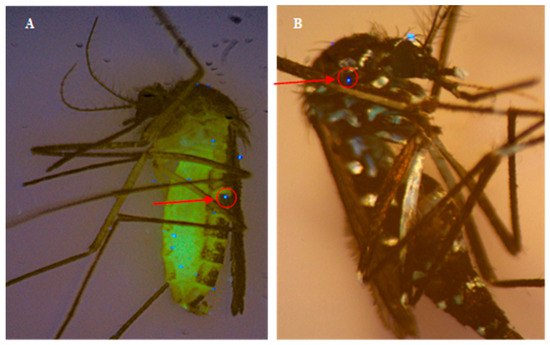
Figure 2.
Examples of microscope images of FP with fluorescent dye (riboflavin) on (A) Culex and (B) Aedes mosquitoes under UV light filter after wind tunnel exposure. Red arrow/circle indicates example of fluorescent droplet on mosquito body.

Table 2.
Droplet counts for FPs on mosquito bodies in wind tunnel. Mean of total droplets per mosquito ± standard error (range of droplets/mosquito).
Significant differences were observed in droplet counts between body parts and this varied by FP and mosquito population. Abdomen and legs consistently showed the highest number of droplets in this experiment. Body parts with significantly highest droplet counts for each FP (Duncan statistical comparison) included the following: Biomist® (p = 0.001): Abdomen and legs; Duet® (p < 0.0001): Abdomen, legs, thorax, and wings; AquaDuet® (p = 0.005): Abdomen; and ReMoa Tri® (p = 0.001): Abdomen, wings, and legs.
Mosquito Mortality
Figure 3 shows mortality (36 or 48 h) after exposure to FPs in the wind tunnel to Biomist®, Duet®, AquaDuet®, and ReMoa Tri®. No mortality was observed in the control groups and 16–100% mortality was observed for mosquitoes exposed to FP. The lowest mortality rates were observed in the Culex wild population: Biomist® (43% mortality), Duet® (16% mortality), AquaDuet® (6% mortality), and ReMoa Tri® (86% mortality). For the other three mosquito populations (Aedes lab, Aedes wild, Culex lab), most mortality rates were > 97%, showing susceptibility. The exception to this was the Culex lab colony showing 79% mortality when exposed to AquaDuet®.
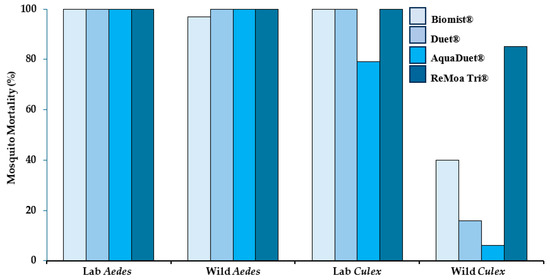
Figure 3.
Mosquito mortality 36 h post-exposure in the wind tunnel to insecticide formulated products Biomist®, AquaDuet®, and Duet® and 48 h post-exposure for ReMoa Tri®. Four different mosquito populations were exposed to each product in the wind tunnel for 10 s before being transferred to clean cages for mortality assessment.
Regression analyses showed that log-transformed values for total droplet count on mosquitoes (subset of cages characterized) were significantly related to mosquito mortality for Biomist® (df = 1,49, F = 11.54, p = 0.001, R2 = 0.191) and ReMoa Tri® (df = 1,57, F = 10.66, p = 0.002, R2 = 0.158), but not for AquaDuet® (df = 1,50, F = 2.56, p = 0.116, R2 = 0.049) or Duet® (df = 1,52, F = 0.88, p = 0.352, R2 = 0.017).
4. Discussion
No significant differences were observed in the droplet counts on mosquitoes exposed to different FPs in the wind tunnel. Wind tunnel experiments are designed to show whether the FP causes mosquito mortality [10]. We know how much FP was delivered to each cage of mosquitoes and how many droplets hit each mosquito in the cage in the wind tunnel under the conditions of this test. Further studies will compare mortality rates of caged mosquito populations between wind tunnel and field trial assessments. Since droplets are screened when passing through the mesh mosquito cage, mortality rates of uncaged mosquitoes would likely be different [9,24]. For example, in a field setting, droplets are carried from the ULV machine nozzle source and become dispersed throughout the environment, depending on distance from the spray line, wind, and other factors. In some cases, free flying mosquitoes may experience higher mortality than caged mosquitoes since the droplets are not screened [24]; however, in other cases, wind may disperse droplets to such an extent that not many droplets impinge on mosquitoes. Another study showing no correlation between volume (i.e., droplet size, dose) and the number of FP droplets indicates that several small droplets might have the same effect as fewer large droplets [11]. This can be considered when evaluating technical differences in the application of FPs via topical, wind tunnel, field trial, or other methods. The wind tunnel model used here is a closed system where mosquito cages are positioned 18 cm from the aerosolized FP and droplets are ensured to impinge on mosquito bodies. During field applications of FPs using a ULV machine, droplets are more dispersed, spread by wind throughout the atmosphere across a large area, and are expected to impinge on mosquitoes that are actively flying through the spray.
In wind tunnel experiments, the mean of the total number of droplets per mosquito is related to mosquito mortality in the Biomist® and ReMoa Tri® group, but not the Duet® or AquaDuet® group. Regression models show low explanatory power; hence, under the conditions of this test, droplet counts on mosquito bodies alone do not explain mortality and the interaction of droplet counts with other factors should also be considered. Another study is underway to compare droplet counts on mosquitoes in a field trial to the wind tunnel. An IR study using the topical insecticide application technique showed that FPs (Aqualuer 20–20, Fyfanon) consistently applied to mosquitoes on body parts such as the head, thorax, and abdomen (but not the wings, legs, or proboscis) showed no differences in mortality [25]. The same study diluted each FP 1000-fold with BVA-13 oil to deliver (via syringe to chilled mosquito) a 267 µm droplet that was expected to contain the same amount of insecticide as in a 27 µm droplet of undiluted FP that would be expected to hit a mosquito during a ULV field treatment. Mortality was assessed after 24 h [25]. However, since the 267 µm droplet size in the aforementioned study exceeds the usual size of ULV droplets and our wind tunnel or field trial droplets, no direct comparisons can be made. Two different topical insecticide method development studies described applying (via syringe to chilled mosquitoes) one 0.5 µL drop of each AI/acetone mixture to the ventral or dorsal thorax of each mosquito and assessing mortality after 24 h [26,27]. It should be noted that mosquitoes in the field absorb residual insecticides through their legs while contacting surfaces or via any part of their bodies when flying through an aerosolized ULV treatment [11]. Hence, the topical application of a droplet of AI to the ventral thorax is a starting point for measuring IR in a technical sense rather than being comparable to field applications of FPs [26]. This is a similar limitation to the CDC bottle bioassay that evaluates IR to residual AI, but not aerosolized FP [8,14,26].
Another study showed 85% mortality (resistant) for wild Cx. quinquefasciatus after field trial exposure to ReMoa Tri® [18]. Aedes albopictus exposed to Duet® in another field trial showed higher mortality (80%) in front yards compared to back yards (56%), although no differences were observed in droplet density between front and back yards [28]. Here, wild Ae. albopictus showed 100% mortality to Duet® after exposure in the wind tunnel. Resistant mosquitoes should be used in IR assessments to approximate the real world as resistance is increasing globally [19]. Others have also shown lower droplet density with distance from the field trial spray line that can be impacted by high or gusty wind conditions [29]. Under the conditions of this test, when the wind tunnel experiment resulted in a relatively low mosquito mortality rate (<90%), this indicated FP resistance, following CDC guidelines. In these situations, a field trial would likely not achieve good results and may not even be necessary. On the other hand, when the wind tunnel experiment gives a nearly perfect mortality rate, a field trial can be used to confirm the result.
Limitations and Future Work
The current study utilizes an APS to measure MMD rather than a DC-IV device, which is used in the field to adjust ULV atomizer settings and achieve targeted aerosol characteristics. The air flow rate and droplet counts (drops/mm2) used in wind tunnel experiments here are lower than wind speeds and droplet counts observed in field trials; hence, higher air flow rates and liquid FP feed rates are being used in ongoing experiments to approximate field conditions. Here, fluorescent droplet counts and spread on mosquito bodies exposed to FPs in the wind tunnel were analyzed and this work will be expanded in future work to include a comparison of droplets analyzed on mosquito bodies exposed during field trials. Mortality was recorded at 36 h or 48 h, and this depended on the mode of action of the product being tested. In the future, a uniform end point will be used for consistency. In future studies, randomized run order and/or blinding during mortality assessments will be considered as this is a common practice in toxicology assays. A new wind tunnel design is being developed with further improvements (e.g., HEPA-filtered air, wind speed approximating field conditions) to decrease cost, simplify the design, and increase ease of use. Testing of additional FPs (e.g., ground-based ULV treatments, aerial treatments, oil- and water-based) and mosquito populations from NC and other MCPs is underway.
5. Conclusions
The wind tunnel described here has the potential to directly benefit MCPs and/or other agencies for the rapid assessment of FP efficacy. Insecticide testing could be provided as a service for small- to medium-scale programs unable to conduct field trials or for those interested in evaluating certain FPs to inform purchasing decisions. The wind tunnel can be used as a screening step before field trials or as a proxy if/when field trials are not possible. For example, if target mosquitoes are found to be resistant to a chosen FP in the wind tunnel where droplets are guaranteed to impinge on mosquitoes, there is likely no need to conduct a field trial on that FP for that population. Alternatively, if mosquitoes are susceptible to the FP in the wind tunnel, recommendations would be made to MCPs to use that FP for mosquito control for that mosquito population and/or conduct further testing via field trials. This seasonally collected information could empower MCPs to analyze long term IR trends and target mosquitoes with the most effective FP each year.
Additional studies are needed to improve the operational use of IR data in the field [8,13,30]. Surveys of US MCPs showed that 84% (in 2017) and 74% (in 2023) lack the capacity to conduct insecticide testing themselves [31,32]. Hence, it would be useful for MCPs to have a tool for routinely assessing FPs to inform operational decisions [15]. Topical applications can also assess IR to AIs and FPs but are labor-intensive. A wind tunnel could help MCPs bridge the gap between the CDC bottle bioassay that tests AIs and the operational decisions for using FPs.
Author Contributions
Conceptualization, S.R. and S.S.; Methodology, S.R., S.S., W.M., E.R., R.S., A.W. and N.B.; Analysis, S.R., S.S., Q.W., W.M., E.R., R.S. and P.J.; Writing—Original draft preparation, S.R. and S.S.; Project administration, S.R. and S.S.; Funding acquisition, S.R. and S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded, in part, by the NC Biotechnology Center (Award #2024-FLG-0062). Part of S. Sousan’s efforts is supported by the National Institute of Environmental Health Sciences of the National Institutes of Health (Award #P30ES025128). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
We thank A. Young and A. Canady for providing the Ae. albopictus field mosquitoes, G. Oakley for help with constructing the wind tunnel, and C. Rogers and M. Foley for feedback on the wind tunnel invention. US Patent (19/306,274) application was filed in September 2025 for the wind tunnel design by the East Carolina University (ECU) Office of Licensing & Commercialization. Claims are directed towards a compact wind tunnel consisting of different mechanical and electrical elements for applying aerosolized solutions to determine arthropod resistance. We thank the helpful comments of the two anonymous reviewers who improved the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| WNE | West Nile encephalitis |
| US | United States |
| WNV | West Nile virus |
| IR | Insecticide resistance |
| MCP | Mosquito control programs |
| FP | Formulated product |
| ULV | Ultra-low volume |
| CDC | Centers for Disease Control and Prevention |
| AI | Active ingredient |
| BLAM | Blaustein Atomizing Module |
| HEPA | High-efficiency particulate air |
| NC | North Carolina |
| MMD | Mass median diameter |
| APS | Aerodynamic Particle Sizer |
| PBO | Piperonyl butoxide |
| UV | Ultraviolet |
References
- WHO. Guidelines for Efficacy Testing of Insecticides for Indoor and Outdoor Ground-Applied Space Spray Applications. 2009. Available online: https://www.who.int/publications/i/item/9789241503235 (accessed on 25 September 2025).
- CDC. Data and Maps for West Nile Virus. 2024. Available online: https://www.cdc.gov/west-nile-virus/data-maps/index.html (accessed on 25 September 2025).
- Brown, J.S.; Byrd, B.D.; Connelly, C.R.; Richards, S.L. Operational insights into mosquito control disaster response in coastal North Carolina: Experiences with the Federal Emergency Management Agency after Hurricane Florence. J. Environ. Health 2022, 85, 24–31. [Google Scholar]
- Venkatesan, P. The 2023 WHO world malaria report. Lancet Microbe 2024, 5, e214. [Google Scholar] [CrossRef]
- van den Berg, H.; da Silva Bezerra, H.S.; Al-Eryani, S.; Chanda, M.; Nagpal, B.N.; Knox, T.B.; Velayudhan, R.; Yadav, R.S. Recent trends in global insecticide use for disease vector control and potential implications for resistance management. Sci. Rep. 2021, 11, 23867. [Google Scholar] [CrossRef]
- Fritz, B.K.; Hoffmann, W.C.; Farooq, M.; Walker, T.; Bonds, J. Filtration effects due to bioassay cage design and screen type. J. Am. Mosq. Cont. Assoc. 2010, 26, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.L.; Sousan, S.; White, A.V.; Murray, W.; Peyton, K.; Slade, R. Development of novel compact wind tunnel for testing formulated products in mosquitoes. Pest Manag. Sci. 2024, 80, 3140–3148. [Google Scholar] [CrossRef] [PubMed]
- Lopez, K.; Irwin, P.; Bartlett, D.; Kukla, C.; Paskewitz, S.; Bartholomay, L. A multi-assay assessment of insecticide resistance in Culex pipiens (Diptera: Culicidae) informs a decision-making framework. PLoS ONE 2025, 20, e0324194. [Google Scholar] [CrossRef]
- Hoffman, W.C.; Fritz, B.K.; Farooq, M.; Cooperband, M.F. Effects of wind speed on aerosol spray penetration in adult mosquito bioassay cages. J. Am. Mosq. Cont. Assoc. 2008, 24, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Morgan, N.O.; Retzer, H.J. Aerosol wind tunnel for vector insecticide evaluation. J. Econ. Entomol. 1981, 74, 389–392. [Google Scholar] [CrossRef]
- Cooperband, M.F.; Golden, F.V.; Clark, G.G.; Jany, W.; Allan, S.A. Prallethrin-induced excitation increases contact between sprayed ultralow volume droplets and flying mosquitoes (Diptera: Culicidae) in a wind tunnel. J. Med. Entomol. 2010, 47, 1099–1106. [Google Scholar] [CrossRef]
- Lofgren, C.S.; Anthony, D.W.; Mount, G.A. Size of aerosol droplets impinging on mosquitoes as determined with a scanning electron microscope. J. Econ. Entomol. 1973, 66, 1085–1088. [Google Scholar] [CrossRef]
- CDC. CONUS Manual for Evaluating Insecticide Resistance in Mosquitoes Using the CDC Bottle Bioassay Kit. 2019. Available online: https://www.cdc.gov/mosquitoes/pdfs/conus-508.pdf (accessed on 25 September 2025).
- Richards, S.L.; Byrd, B.D.; Reiskind, M.H.; White, A.V. Assessing insecticide resistance in adult mosquitoes: Perspectives on current methods. Environ. Health Insights 2020, 14, 1178630220952790. [Google Scholar] [CrossRef]
- Dritz, D.A.; Novelo, M.; Wheeler, S.S. Evaluating the metrics of insecticide resistance and efficacy: Comparison of the CDC bottle bioassay with formulated and technical-grade insecticide and a sentinel cage field trial. Trop. Med. Inf. Dis. 2025, 10, 219. [Google Scholar] [CrossRef]
- Lehane, A.; Parker Crockett, C.; Norris, E.; Wheeler, S.S.; Harrington, L.C. Measuring insecticide resistance in a vacuum: Exploring next steps to link resistance data with mosquito control efficacy. J. Med. Entomol. 2024, 61, 584–594. [Google Scholar] [CrossRef] [PubMed]
- Hribar, L.J.; Boehmler, M.B.; Murray, H.L.; Pruszynski, C.A.; Leal, A.L. Mosquito surveillance and insecticide resistance monitoring conducted by the Florida Keys Mosquito Control District, Monroe County, Florida, USA. Insects 2022, 13, 927. [Google Scholar] [CrossRef] [PubMed]
- Unlu, I.; Buckner, E.A.; Medina, J.; Vasquez, C.; Cabrara, A.; Romero-Weaver, A.L.; Ramirez, D.; Kendziorski, N.L.; Kosinski, K.H.; Fedirko, T.J.; et al. Insecticide resistance of Miami-Dade Culex quinquefasciatus populations and initial field efficacy of a new resistance-breaking adulticide formulation. PLoS ONE 2024, 19, e0296046. [Google Scholar] [CrossRef]
- Estep, A.S.; Sanscrainte, N.D.; Farooq, M.; Lucas, K.J.; Heinig, R.L.; Norris, E.J.; Becnel, J.J. Impact of Aedes aegypti V1016I and F1534C knockdown resistance genotypes on operational interventions. Sci. Rep. 2025, 15, 10146. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.L.; Balanay, J.G.; Fields, M.; Vandock, K. Baseline insecticide susceptibility screening against six active ingredients for Aedes and Culex (Diptera: Culicidae) mosquito populations. J. Med. Entomol. 2017, 54, 682–695. [Google Scholar] [CrossRef]
- Richards, S.L.; Balanay, J.G.; White, A.V.; Hope, J.; Vandock, K.; Byrd, B.D.; Reiskind, M.H. Insecticide susceptibility screening against Culex and Aedes (Diptera: Culicidae) mosquitoes from the United States. J. Med. Entomol. 2018, 55, 398–407. [Google Scholar] [CrossRef]
- Richards, S.L.; Byrd, B.D.; Reiskind, M.H.; White, A.V. Evaluation of insecticide resistance in Aedes albopictus in North Carolina, 2017. J. Med. Entomol. 2019, 56, 761–773. [Google Scholar] [CrossRef]
- Farooq, M.; Waits, C. Suitability of mixing fluorescent dye in adulticides and its impact on droplet characteristics and pesticide efficacy. J. Am. Mosq. Cont. Assoc. 2015, 31, 353–359. [Google Scholar] [CrossRef]
- Bonds, J.A.S.; Greer, M.; Coughlin, J.; Patel, V. Caged mosquito bioassay: Studies on cage exposure pathways, effects of mesh on pesticide filtration, and mosquito containment. J. Am. Mosq. Cont. Assoc. 2010, 26, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Aldridge, R.L.; Kaufman, P.E.; Bloomquist, J.R.; Gezan, S.A.; Linthicum, K.J. Impact of topical application site on the efficacy of permethrin and malathion to Culex quinquefasciatus. J. Am. Mosq. Cont. Assoc. 2016, 32, 300–307. [Google Scholar] [CrossRef]
- Jensen, B.M.; Althoff, R.A.; Rydberg, S.E.; Royster, E.N.; Estep, A.; Huijben, S. Topical application bioassay to quantify insecticide toxicity for mosquitoes and fruit flies. J. Vis. Exp. 2022, 179, e63391. [Google Scholar] [CrossRef]
- Li, T.; Wang, Y.; Liu, N. Mosquito adult bioassays. Cold Spring Harb. Protoc. 2023, 5, pdb-prot108041. [Google Scholar] [CrossRef]
- Unlu, I.; Baker, M.A.; Indelicato, N.; Drews, D.; Zeng, D.; Vaidyanathan, R. Nighttime applications of two formulations of pyrethroids are effective against diurnal Aedes albopictus. J. Am. Mosq. Cont. Assoc. 2018, 34, 158–162. [Google Scholar] [CrossRef]
- McDuffie, D.; Kacinskas, S.; Li, S.; Parker-Crockett, C.; Lucas, K.J. Evaluation of ground and aerial ultra-low volume applications using ReMoa Tri against deltamethrin-resistant Aedes aegypti from Collier County, Florida. Trop. Med. Inf. Dis. 2025, 10, 119. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.L.; Byrd, B.D.; Breidenbaugh, M.; Vandock, K. Survey of United States mosquito control programs reveals opportunities to improve the operational value of Centers for Disease Control and Prevention bottle bioassays. J. Med. Entomol. 2022, 59, 1827–1830. [Google Scholar] [CrossRef] [PubMed]
- NACCHO. Mosquito Control in the US Report. 2017. Available online: https://www.naccho.org/uploads/downloadable-resources/Mosquito-control-in-the-U.S.-Report.pdf (accessed on 25 September 2025).
- NACCHO. Vector Surveillance and Control at the Local Level: Findings from the 2023 Vector Control Assessment. 2024. Available online: https://www.naccho.org/uploads/downloadable-resources/Vector-Assessment-2023-Sep.2024.pdf (accessed on 25 September 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).