Ecological Balance in Unmanaged Beech Reserves: Scolytids or Their Natural Saproxylic Beetle Enemies?
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Studied Beetle Groups
2.3. Beetle Sampling
2.4. Analysis
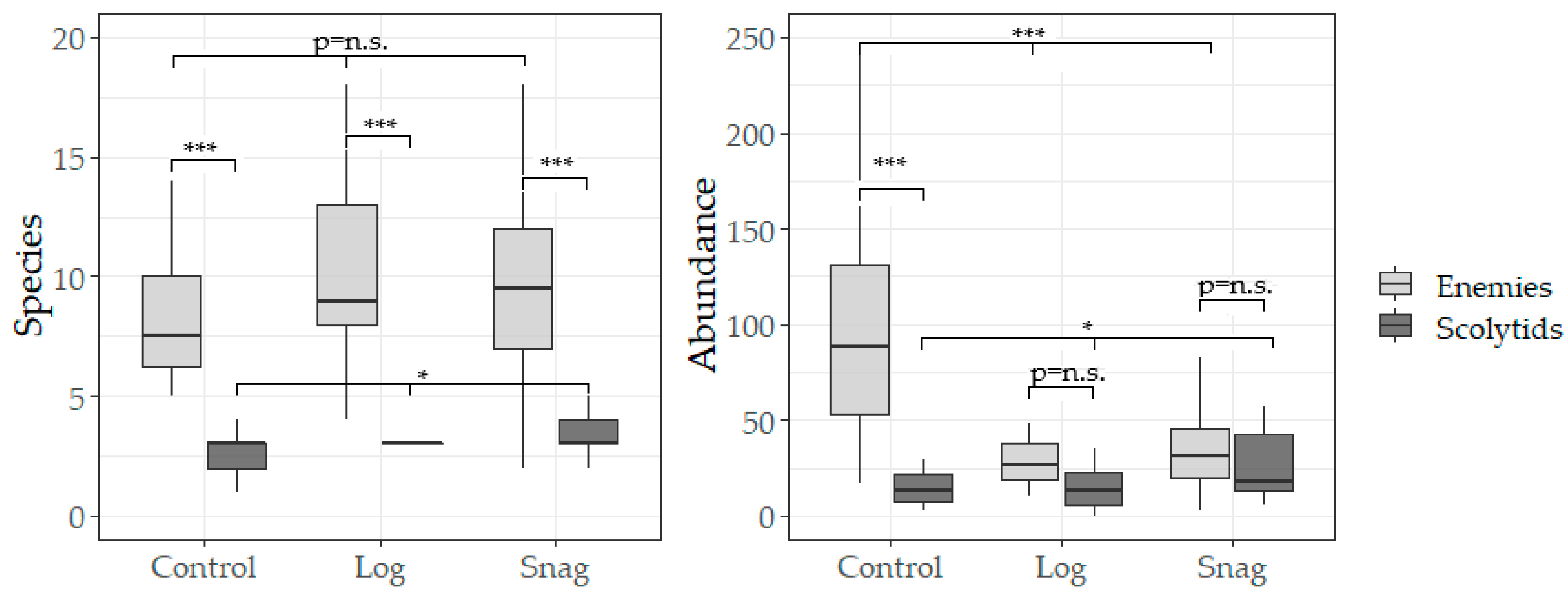
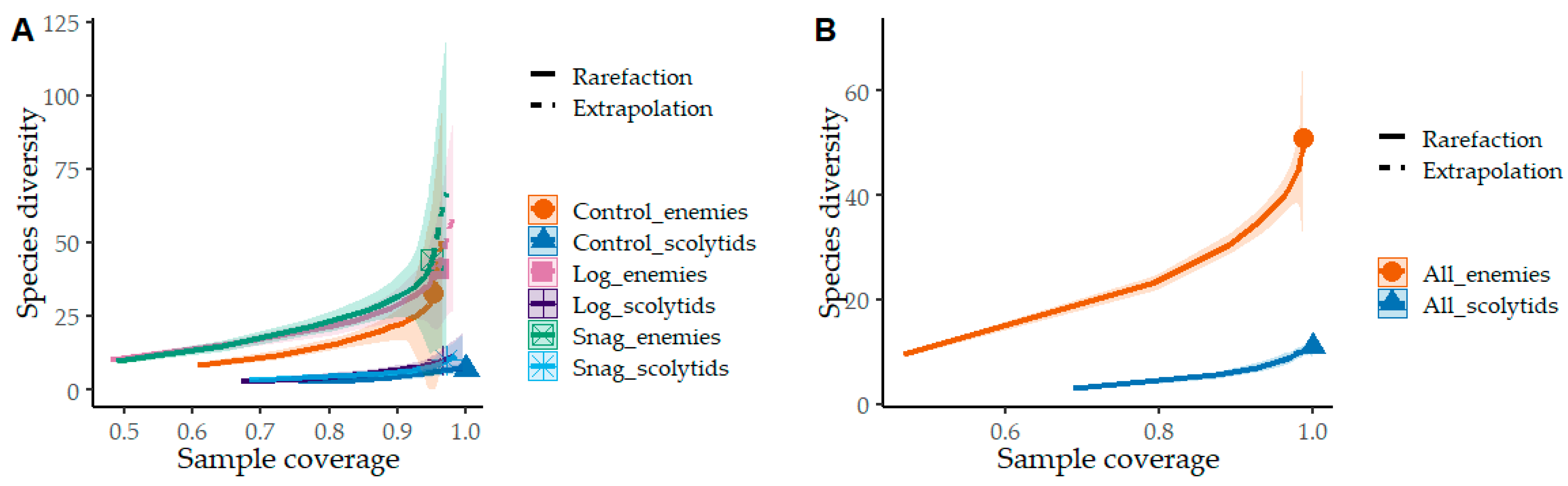
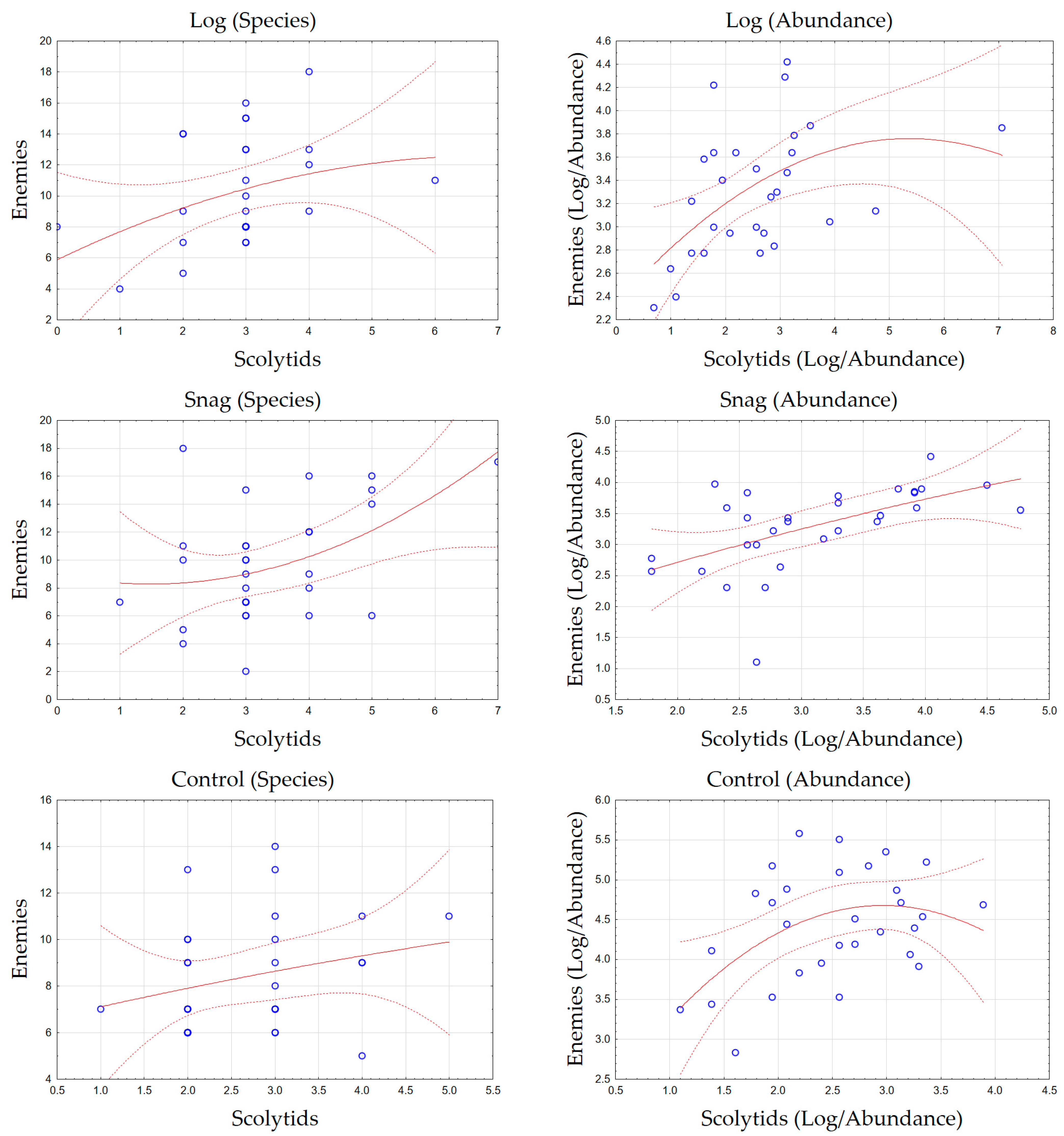
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kunin, W.E. Robust Evidence of Declines in Insect Abundance and Biodiversity. Nature 2019, 574, 641–642. [Google Scholar] [CrossRef]
- Duflot, R.; Fahrig, L.; Mönkkönen, M. Management Diversity Begets Biodiversity in Production Forest Landscapes. Biol. Conserv. 2022, 268, 109514. [Google Scholar] [CrossRef]
- Zumr, V.; Remeš, J.; Pulkrab, K. How to Increase Biodiversity of Saproxylic Beetles in Commercial Stands Through Integrated Forest Management in Central Europe. Forests 2021, 12, 814. [Google Scholar] [CrossRef]
- Hilmers, T.; Friess, N.; Bässler, C.; Heurich, M.; Brandl, R.; Pretzsch, H.; Seidl, R.; Müller, J.; Butt, N. Biodiversity Along Temperate Forest Succession. J. Appl. Ecol. 2018, 55, 2756–2766. [Google Scholar] [CrossRef]
- Sabatini, F.M.; Burrascano, S.; Keeton, W.S.; Levers, C.; Lindner, M.; Pötzschner, F.; Verkerk, P.J.; Bauhus, J.; Buchwald, E.; Chaskovsky, O.; et al. Where Are Europe’s Last Primary Forests? Divers. Distrib. 2018, 24, 1426–1439. [Google Scholar] [CrossRef]
- Brunet, J.; Fritz, O.; Richnau, G. Biodiversity in European beech forests—A review with recommendations for sustainable forest management. Ecol. Bull. 2010, 53, 77–94. [Google Scholar]
- Müller, J.; Bußler, H.; Kneib, T. Saproxylic Beetle Assemblages Related to Silvicultural Management Intensity and Stand Structures in a Beech Forest in Southern Germany. J. Insect Conserv. 2008, 12, 107–124. [Google Scholar] [CrossRef]
- Jabin, M.; Mohr, D.; Kappes, H.; Topp, W. Influence of Deadwood on Density of Soil Macro-Arthropods in a Managed Oak–Beech Forest. For. Ecol. Manag. 2004, 194, 61–69. [Google Scholar] [CrossRef]
- Jonsell, M.; Widenfalk, L.A.; Hellqvist, S. Substrate Specificity Among Diptera in Decaying Bioenergy Wood: Can They Be Conserved by the Same Measures as Are Currently Applied to Beetles? Biodivers. Conserv. 2020, 29, 2623–2662. [Google Scholar] [CrossRef]
- Graf, M.; Seibold, S.; Gossner, M.M.; Hagge, J.; Weiß, I.; Bässler, C.; Müller, J. Coverage Based Diversity Estimates of Facultative Saproxylic Species Highlight the Importance of Deadwood for Biodiversity. For. Ecol. Manag. 2022, 517, 120275. [Google Scholar] [CrossRef]
- Zuo, J.; Muys, B.; Berg, M.P.; Hefting, M.M.; van Logtestijn, R.S.P.; van Hal, J.; Cornelissen, J.H.C. Earthworms Are Not Just “Earth” Worms: Multiple Drivers to Large Diversity in Deadwood. For. Ecol. Manag. 2023, 530, 120746. [Google Scholar] [CrossRef]
- Atrena, A.; Banelytė, G.G.; Læssøe, T.; Riis-Hansen, R.; Bruun, H.H.; Rahbek, C.; Heilmann-Clausen, J. Quality of Substrate and Forest Structure Determine Macrofungal Richness Along a Gradient of Management Intensity in Beech Forests. For. Ecol. Manag. 2020, 478, 118512. [Google Scholar] [CrossRef]
- Tillon, L.; Bouget, C.; Paillet, Y.; Aulagnier, S. How Does Deadwood Structure Temperate Forest Bat Assemblages? Eur. J. For. Res. 2016, 135, 433–449. [Google Scholar] [CrossRef]
- Basile, M.; Krištín, A.; Mikusiński, G.; Thorn, S.; Żmihorski, M.; Pasinelli, G.; Brockerhoff, E.G. Salvage Logging Strongly Affects Woodpecker Abundance and Reproduction: A Meta-Analysis. Curr. For. Rep. 2023, 9, 1–14. [Google Scholar] [CrossRef]
- Zumr, V.; Remeš, J. Saproxylic beetles as an indicator of forest biodiversity and the influence of forest management on their crucial life attributes: Review. Rep. For. Res. 2020, 65, 242–257. [Google Scholar]
- Zahradník, P. Seznam Brouk (Coleoptera) České Republiky a Slovenska. Lesnická Práce, s.r.o., Kostelec nad Černými Lesy; Lesnická Práce: Kostelec nad Černými lesy, Czechia, 2017; ISBN 978-80-7458-092-5. 544p. [Google Scholar]
- Pfeffer, A. Fauna ČSR, Volume 6, Kůrovci—Scolytoidea; Czechoslovak Academy of Sciences: Prague, Czech Republic, 1955; pp. 324 pp + 18 pp appendices. [Google Scholar]
- Müller, J.; Noss, R.F.; Bussler, H.; Brandl, R. Learning from a “Benign Neglect Strategy” in a National Park: Response of Saproxylic Beetles to Dead Wood Accumulation. Biol. Conserv. 2010, 143, 2559–2569. [Google Scholar] [CrossRef]
- Plath, E.; Trauth, C.; Gerhards, J.; Griebel, L.; Fischer, K. Dieback of Managed Spruce Stands in Western Germany Promotes Beetle Diversity. J. For. Res. 2024, 35, 48. [Google Scholar] [CrossRef]
- Hlásny, T.; König, L.; Krokene, P.; Lindner, M.; Montagné-Huck, C.; Müller, J.; Qin, H.; Raffa, K.F.; Schelhaas, M.-J.; Svoboda, M.; et al. Bark Beetle Outbreaks in Europe: State of Knowledge and Ways Forward for Management. Curr. For. Rep. 2021, 7, 138–165. [Google Scholar] [CrossRef]
- Resco de Dios, V.; Schütze, S.J.; Cunill Camprubí, À.; Balaguer-Romano, R.; Boer, M.M.; Fernandes, P.M. Protected Areas as Hotspots of Wildfire Activity in Fire-Prone Temperate and Mediterranean Biomes. J. Environ. Manag. 2025, 385, 125669. [Google Scholar] [CrossRef]
- Beetz, K.; Marrs, C.; Busse, A.; Poděbradská, M.; Kinalczyk, D.; Kranz, J.; Forkel, M. Effects of Bark Beetle Disturbance and Fuel Types on Fire Radiative Power and Burn Severity in the Bohemian-Saxon Switzerland. For. Int. J. For. Res. 2024, 98, 59–70. [Google Scholar] [CrossRef]
- Křístek, J. Ochrana Lesů a Přírodního Prostředí; Učebnice; Matice lesnická: Písek, Czech Republic, 2002. [Google Scholar]
- Marchioro, M.; Faccoli, M.; Dal Cortivo, M.; Branco, M.; Roques, A.; Garcia, A.; Ruzzier, E. New species and new records of exotic Scolytinae (Coleoptera, Curculionidae) in Europe. Biodivers. Data J. 2022, 10, e93995. [Google Scholar] [CrossRef]
- Basile, M.; Lachat, T.; Balducci, L.; Chianucci, F.; Chojnacki, L.; Archaux, F.; Avtzis, D.; Bouget, C.; De Smedt, P.; Doerfler, I.; et al. Managed Forests Are a Stronghold of Non-Native Beetles in Europe. J. Appl. Ecol. 2025, 62, 1243–1257. [Google Scholar] [CrossRef]
- Fiala, T.; Holuša, J.; Procházka, J.; Čížek, L.; Dzurenko, M.; Foit, J.; Galko, J.; Kašák, J.; Kulfan, J.; Lakatos, F.; et al. Xylosandrus Germanus in Central Europe: Spread into and Within the Czech Republic. J. Appl. Entomol. 2020, 144, 423–433. [Google Scholar] [CrossRef]
- Fiala, T.; Holuša, J. A Monitoring Network for the Detection of Invasive Ambrosia and Bark Beetles in the Czech Republic: Principles and Proposed Design. Front. For. Glob. Change 2023, 6, 1239748. [Google Scholar] [CrossRef]
- Langellotto, G.A.; Denno, R.F. Responses of Invertebrate Natural Enemies to Complex-Structured Habitats: A Meta-Analytical Synthesis. Oecologia 2004, 139, 1–10. [Google Scholar] [CrossRef]
- Zumr, V.; Nakládal, O.; Gallo, J.; Remeš, J. Deadwood Position Matters: Diversity and Biomass of Saproxylic Beetles in a Temperate Beech Forest. For. Ecosyst. 2024, 11, 100174. [Google Scholar] [CrossRef]
- Müller, J.; Brunet, J.; Brin, A.; Bouget, C.; Brustel, H.; Bussler, H.; Forster, B.; Isacsson, G.; Kohler, F.; Lachat, T.; et al. Implications from large-scale spatial diversity patterns of saproxylic beetles for the conservation of European Beech forests. Insect Conserv. Divers. 2013, 6, 162–169. [Google Scholar] [CrossRef]
- Holuša, J.; Resnerová, K.; Dvořáková, B.; Hradecký, J.; Šipoš, J.; Fiala, T. The Relationship Between Nemozoma Elongatum (Coleoptera: Trogossitidae) And Its Primary Bark-Beetle Prey-Species. Ann. For. Sci. 2025, 82, 11. [Google Scholar] [CrossRef]
- Horák, J.; Nakládal, O. Beetles associated with trees and predation between them: Part III—Annotated checklist of beetles with predation potential. Lesn. Čas.—For. J. 2009, 55, 181–193. [Google Scholar]
- Šrámek, O. Základní Informace o Státní Přírodní Rezervaci “Voděradské bučiny”,—Ms., dep. in Rezervační Kniha AOPK ČR; RP Střední Čechy: Praha 6, Czech Republic, 1976; 8p. [Google Scholar]
- Care Plan Voděradské Bučiny. Available online: https://portal.nature.cz/w/uzemi-3274#/ (accessed on 3 September 2025).
- Seibold, S.; Brandl, R.; Buse, J.; Hothorn, T.; Schmidl, J.; Thorn, S.; Müller, J. Association of Extinction Risk of Saproxylic Beetles with Ecological Degradation of Forests in Europe. Conserv. Biol. 2014, 29, 382–390. [Google Scholar] [CrossRef]
- Ulyshen, M.D. Saproxylic Insects, Zoological Monographs; Springer International Publishing: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Bracalini, M.; Martini, A.; Tagliaferri, L.; Panzavolta, T. Impact of Trapping Programs for Ips typographus (Linnaeus) (Curculionidae: Scolytinae) on Predators, Parasitoids, and Other Non-Target Insects. Forests 2025, 16, 1510. [Google Scholar] [CrossRef]
- Montgomery, G.A.; Belitz, M.W.; Guralnick, R.P.; Tingley, M.W. Standards and Best Practices for Monitoring and Benchmarking Insects. Front. Ecol. Evol. 2021, 8, 579193. [Google Scholar] [CrossRef]
- Parmain, G.; Bouget, C.; Müller, J.; Horak, J.; Gossner, M.M.; Lachat, T.; Isacsson, G. Can Rove Beetles (Staphylinidae) Be Excluded in Studies Focusing on Saproxylic Beetles in Central European Beech Forests? Bull. Entomol. Res. 2015, 105, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Zumr, V.; Nakládal, O.; Bílek, L.; Remeš, J. The Diameter of Beech Snags Is an Important Factor for Saproxylic Beetle Richness: Implications for Forest Management and Conservation. For. Ecosyst. 2023, 10, 100143. [Google Scholar] [CrossRef]
- Alinvi, O.; Ball, J.P.; Danell, K.; Hjältén, J.; Pettersson, R.B. Sampling Saproxylic Beetle Assemblages in Dead Wood Logs: Comparing Window and Eclector Traps to Traditional Bark Sieving and a Refinement. J. Insect Conserv. 2007, 11, 99–112. [Google Scholar] [CrossRef]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019; Available online: https://www.john-fox.ca/Companion/ (accessed on 10 September 2025).
- Brooks, M.E.; Kristensen, K.; van Benthem, K.J.; Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Mächler, M.; Bolker, B.M. Glmmtmb Balances Speed and Flexibility Among Packages for Zero-Inflated Generalized Linear Mixed Modeling. R J. 2017, 9, 378–400. [Google Scholar] [CrossRef]
- Huang, A. Mean-parametrized Conway–Maxwell–Poisson regression models for dispersed counts. Stat. Model. 2017, 17, 1–22. [Google Scholar] [CrossRef]
- Hartig, F. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models, R package version 0.4.7. 2024. Available online: https://github.com/florianhartig/dharma (accessed on 10 September 2025).
- Chao, A.; Jost, L. Coverage-Based Rarefaction and Extrapolation: Standardizing Samples by Completeness Rather Than Size. Ecology 2012, 93, 2533–2547. [Google Scholar] [CrossRef] [PubMed]
- Chao, A.; Gotelli, N.J.; Hsieh, T.C.; Sander, E.L.; Ma, K.H.; Colwell, R.K.; Ellison, A.M. Rarefaction and Extrapolation with Hill Numbers: A Framework for Sampling and Estimation in Species Diversity Studies. Ecol. Monogr. 2014, 84, 45–67. [Google Scholar] [CrossRef]
- Hsieh, T.C.; Ma, K.H.; Chao, A. iNEXT: iNterpolation and EXTrapolation for Species Diversity, R Package Version 3.0.0. 2022. Available online: http://chao.stat.nthu.edu.tw/wordpress/software-download/ (accessed on 10 September 2025).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 2 October 2023).
- Šmilauer, P.; Lepš, J. Multivariate Analysis of Ecological Data Using Canoco 5; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar] [CrossRef]
- Dufrene, M.; Legendre, P. Species assemblages and indicator species: The need for a flexible asymmetrical approach. Ecol. Monogr. 1997, 67, 345–366. [Google Scholar] [CrossRef]
- De Cáceres, M.; Legendre, P. Associations between species and groups of sites: Indices and statistical inference. Ecology 2009, 90, 3566–3574. [Google Scholar] [CrossRef] [PubMed]
- Fürjes-Mikó, Á.; Csősz, S.; Paulin, M.J.; Csóka, G. The Role of Red Wood Ants (Formica rufa Species Group) in Central European Forest Ecosystems—A Literature Review. Insects 2025, 16, 518. [Google Scholar] [CrossRef] [PubMed]
- Zahradník, P. Ips typographus (L.), Lesní Ochraná Služba, Lesnická Práce 4/2007. Available online: https://www.silvarium.cz/images/letaky-los/2007/2007_lykozrout_smrkovy.pdf (accessed on 10 September 2025).
- Parisi, F.; Mazziotta, A.; Chirici, G.; D’amico, G.; Vangi, E.; Francini, S.; Travaglini, D. Effects of Forest Management on Beetle (Coleoptera) Communities in Beech Forests (Fagus sylvatica) in the Apennines of Central Italy (Tuscany). Forests 2024, 15, 1085. [Google Scholar] [CrossRef]
- Bouget, C.; Brustel, H.; Brin, A.; Noblecourt, T. Sampling saproxylic beetles with window flight traps: Methodological insights. Rev. D’ecologie Terre Vie 2008, 63, 21–32. [Google Scholar] [CrossRef]
- Seibold, S.; Bässler, C.; Brandl, R.; Büche, B.; Szallies, A.; Thorn, S.; Ulyshen, M.D.; Müller, J. Microclimate and Habitat Heterogeneity as the Major Drivers of Beetle Diversity in Dead Wood. J. Appl. Ecol. 2016, 53, 934–943. [Google Scholar] [CrossRef]
- Lettenmaier, L.; Seibold, S.; Bässler, C.; Brandl, R.; Gruppe, A.; Müller, J.; Hagge, J. Beetle Diversity Is Higher in Sunny Forests Due to Higher Microclimatic Heterogeneity in Deadwood. Oecologia 2022, 198, 825–834. [Google Scholar] [CrossRef]
- Gossner, M.M.; Falck, K.; Weisser, W.W. Effects of Management on Ambrosia Beetles and Their Antagonists in European Beech Forests. For. Ecol. Manag. 2019, 437, 126–133. [Google Scholar] [CrossRef]
- Bilek, L.; Remes, J.; Zahradnik, D. Managed Vs. Unmanaged. Structure Of Beech Forest Stands (Fagus Sylvatica L.) After 50 Years of Development, Central Bohemia. For. Syst. 2011, 20, 122–138. [Google Scholar] [CrossRef]
- Christensen, M.; Hahn, K.; Mountford, E.P.; Ódor, P.; Standovár, T.; Rozenbergar, D.; Diaci, J.; Wijdeven, S.; Meyer, P.; Winter, S.; et al. Dead Wood in European Beech (Fagus Sylvatica) Forest Reserves. For. Ecol. Manag. 2005, 210, 267–282. [Google Scholar] [CrossRef]
- Přívětivý, T.; Janík, D.; Unar, P.; Adam, D.; Král, K.; Vrška, T. How Do Environmental Conditions Affect the Deadwood Decomposition of European Beech (Fagus Sylvatica L.)? For. Ecol. Manag. 2016, 381, 177–187. [Google Scholar] [CrossRef]
- Holuša, J.; Henzlová, I.; Dvořáková, B.; Resnerová, K.; Šipoš, J.; Holuša, O.; Bláha, J.; Berčák, R.; Procházka, J.; Trombik, J.; et al. Abundance of Taphrorychus Bicolor in Beech Forests: Influence of Forest Size and Optimal Conditions. For. Ecol. Manag. 2025, 575, 122362. [Google Scholar] [CrossRef]
- Bouget, C.; Duelli, P. The Effects of Windthrow On Forest Insect Communities: A Literature Review. Biol. Conserv. 2004, 118, 281–299. [Google Scholar] [CrossRef]

| Log | Snag | Control | ||
|---|---|---|---|---|
| Scolytids (Sum) | Abbreviations | 1665 | 897 | 455 |
| Anisandrus dispar (Fabricius, 1792) | AniDis(F | 3 | 1 | 2 |
| Ernoporicus fagi (Fabricius, 1798) | ErnFa(F | 16 | 21 | 23 |
| Platypus cylindrus (Fabricius, 1792) | PlaCyl(F | 2 | 3 | |
| Scolytus carpini (Ratzeburg, 1837) | ScoCar(R | 1 | 1 | 2 |
| Scolytus intricatus (Ratzeburg, 1837) | ScoInt(R | 3 | ||
| Taphrorychus bicolor (Herbst, 1793) | TapBic(H | 1466 | 476 | 191 |
| Taphrorychus hirtellus (Eichhoff, 1878) | TapHir(E | 1 | ||
| Trypodendron domesticum(Linnaeus, 1758) | TryDom(L | 4 | 18 | 6 |
| Xyleborinus saxesenii (Ratzeburg, 1837) | XylSax(R | 17 | 111 | |
| Xyleborus monographus (Fabricius, 1792) | XylMon(F | 11 | 4 | 2 |
| Xylosandrus germanus (Blandford, 1894) | XylGer(B | 142 | 261 | 229 |
| Enemies (Sum) | 908 | 958 | 3110 | |
| Aplocnemus impressus (Marsham, 1802) | AplImp(M | 1 | ||
| Aplocnemus nigricornis (Fabricius, 1792) | AplNig(F | 2 | ||
| Bitoma crenata (Fabricius, 1775) | BitCre(F | 10 | 16 | 7 |
| Cerylon deplanatum (Gyllenhal, 1827) | CerDep(G | 2 | ||
| Cerylon fagi C.N.F.Brisout de Barneville, 1867 | CerFag(C | 44 | 5 | 21 |
| Cerylon ferrugineum Stephens, 1830 | CerFer(S | 61 | 58 | 78 |
| Cerylon histeroides (Fabricius, 1792) | CerHis(F | 30 | 17 | 53 |
| Colydium elongatum (Fabricius, 1787) | ColElo(F | 8 | 7 | 3 |
| Corticeus bicolor (Olivier, 1790) | CorBic(O | 1 | 3 | |
| Corticeus fraxini (Kugelann, 1794) | CorFra(K | 1 | ||
| Corticeus unicolor Piller & Mitterpacher, 1783 | CorUni(P | 38 | 64 | 20 |
| Cucujus cinnaberinus (Scopoli, 1763) | CucCin(S | 4 | ||
| Dasytes aeratus Stephens, 1829 | DasAer(S | 4 | 6 | 1 |
| Dasytes niger (Linnaeus, 1761) | DasNig(L | 1 | 6 | 7 |
| Dasytes plumbeus (O. F. Müller, 1776) | DasPlu(O | 206 | 163 | 2420 |
| Epuraea distincta (Grimmer, 1841) | EpuDis(G | 1 | ||
| Epuraea longula Erichson, 1845 | EpuLon(E | 27 | 1 | 1 |
| Epuraea marseuli Reitter, 1872 | EpuMar(R | 4 | 1 | |
| Epuraea neglecta (Heer, 1841) | EpuNeg(H | 15 | 1 | |
| Epuraea pallescens (Stephens, 1830) | EpuPal(S | 1 | 1 | 2 |
| Epuraea pygmaea (Gyllenhal, 1808) | EpuPyg(G | 1 | ||
| Epuraea variegata (Herbst, 1793) | EpuVar(H | 50 | 3 | |
| Glischrochilus quadripunctatus (Linnaeus, 1758) | GliQua(L | 3 | 9 | 1 |
| Ipidia binotata Reitter, 1875 | IpiBin(R | 10 | 11 | 3 |
| Litargus connexus (Geoffroy in Fourcroy, 1785) | LitCon(G | 27 | 26 | 11 |
| Malachius bipustulatus (Linnaeus, 1758) | MalBip(L | 1 | ||
| Nemozoma elongatum (Linnaeus, 1761) | NemElo(L | 12 | 22 | 47 |
| Opilo mollis (Linnaeus, 1758) | OpiMol(L | 3 | 7 | |
| Paromalus flavicornis (Herbst, 1792) | ParFla(H | 2 | 48 | 1 |
| Paromalus parallelepipedus (Herbst, 1792) | ParPar(H | 1 | 8 | 1 |
| Pediacus depressus (Herbst, 1797) | PedDep(H | 3 | ||
| Placonotus testaceus (Fabricius, 1787) | PlaTes(F | 5 | 6 | |
| Platysoma compressum (Herbst, 1783) | PlaCom(H | 1 | 1 | |
| Plegaderus caesus (Herbst, 1792) | PleCae(H | 1 | 21 | 1 |
| Pyrochroa coccinea (Linnaeus, 1761) | PyrCoc(L | 2 | 1 | 6 |
| Rhizophagus bipustulatus (Fabricius, 1792) | RhiBip(F | 151 | 195 | 200 |
| Rhizophagus cribratus (Gyllenhal, 1827) | RhiCri(G | 1 | ||
| Rhizophagus dispar (Paykull, 1800) | RhiDis(P | 20 | 13 | 3 |
| Rhizophagus fenestralis (Linnaeus,1758) | RhiFen(L | 2 | 4 | 5 |
| Rhizophagus ferrugineus (Paykull, 1800) | RhiFer(P | 1 | ||
| Rhizophagus nitidulus (Fabricius, 1798) | RhiNit(F | 6 | 2 | 1 |
| Rhizophagus perforatus Erichson, 1845 | RhiPer(E | 5 | 1 | |
| Salpingus planirostris (Fabricius, 1787) | SalPla(F | 1 | 4 | |
| Salpingus ruficollis (Linnaeus, 1761) | SalRuf(L | 4 | 6 | 5 |
| Schizotus pectinicornis (Linnaeus, 1758) | SchPec(L | 32 | 1 | 9 |
| Silvanoprus fagi (Guérin-Ménéville, 1844) | SilFag(G | 1 | ||
| Silvanus unidentatus (Olivier, 1790) | SilUni(O | 1 | ||
| Thanasimus formicarius (Linnaeus, 1758) | ThaFor(L | 1 | 4 | 1 |
| Tillus elongatus (Linnaeus, 1758) | TilElo(L | 9 | 111 | 1 |
| Uleiota planatus (Linnaeus, 1761) | UlePla(L | 21 | 4 | 9 |
| Vincenzellus ruficollis (Panzer, 1794) | VinRuf(P | 84 | 94 | 184 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zumr, V.; Nakládal, O.; Bílek, L.; Remeš, J. Ecological Balance in Unmanaged Beech Reserves: Scolytids or Their Natural Saproxylic Beetle Enemies? Insects 2025, 16, 1087. https://doi.org/10.3390/insects16111087
Zumr V, Nakládal O, Bílek L, Remeš J. Ecological Balance in Unmanaged Beech Reserves: Scolytids or Their Natural Saproxylic Beetle Enemies? Insects. 2025; 16(11):1087. https://doi.org/10.3390/insects16111087
Chicago/Turabian StyleZumr, Václav, Oto Nakládal, Lukáš Bílek, and Jiří Remeš. 2025. "Ecological Balance in Unmanaged Beech Reserves: Scolytids or Their Natural Saproxylic Beetle Enemies?" Insects 16, no. 11: 1087. https://doi.org/10.3390/insects16111087
APA StyleZumr, V., Nakládal, O., Bílek, L., & Remeš, J. (2025). Ecological Balance in Unmanaged Beech Reserves: Scolytids or Their Natural Saproxylic Beetle Enemies? Insects, 16(11), 1087. https://doi.org/10.3390/insects16111087






