Long-Term Phenological Shifts in Butterfly Species from Transylvania, Romania—A Case Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Period
2.2. Data Acquisition
2.2.1. Butterfly Data
2.2.2. Climate Data
2.3. Statistical Analyses
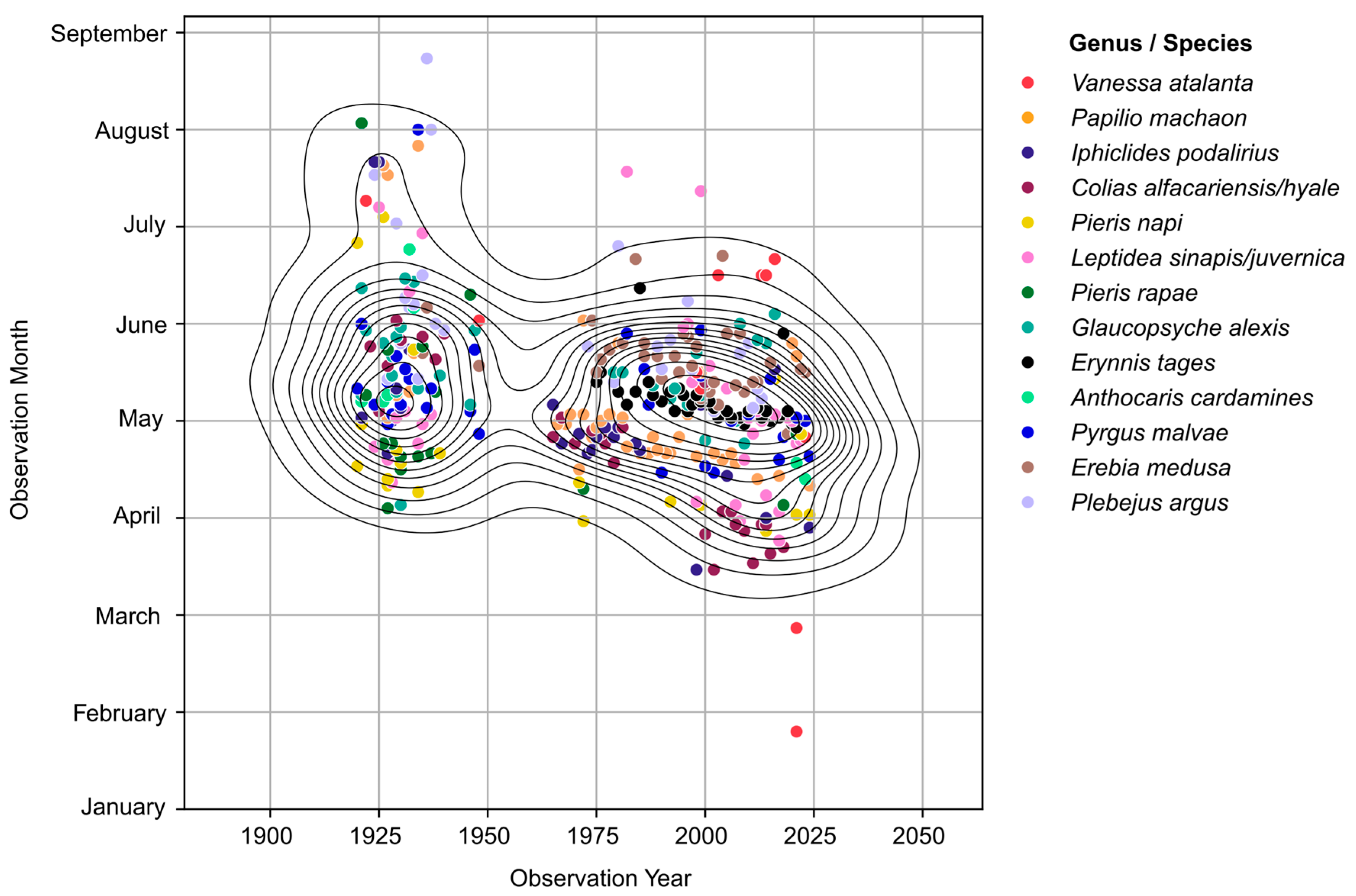
3. Results
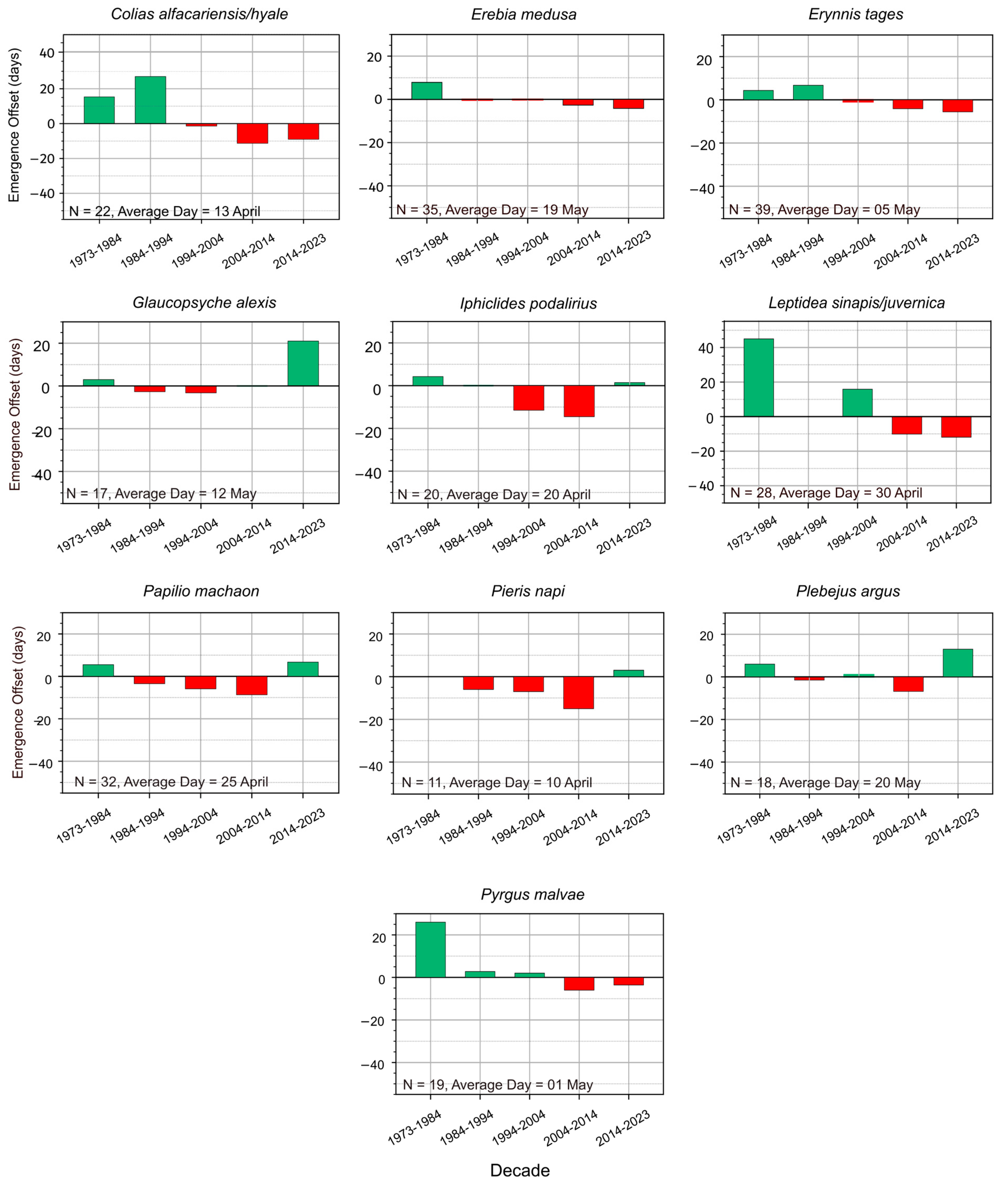
3.1. Species with Emergence in Spring
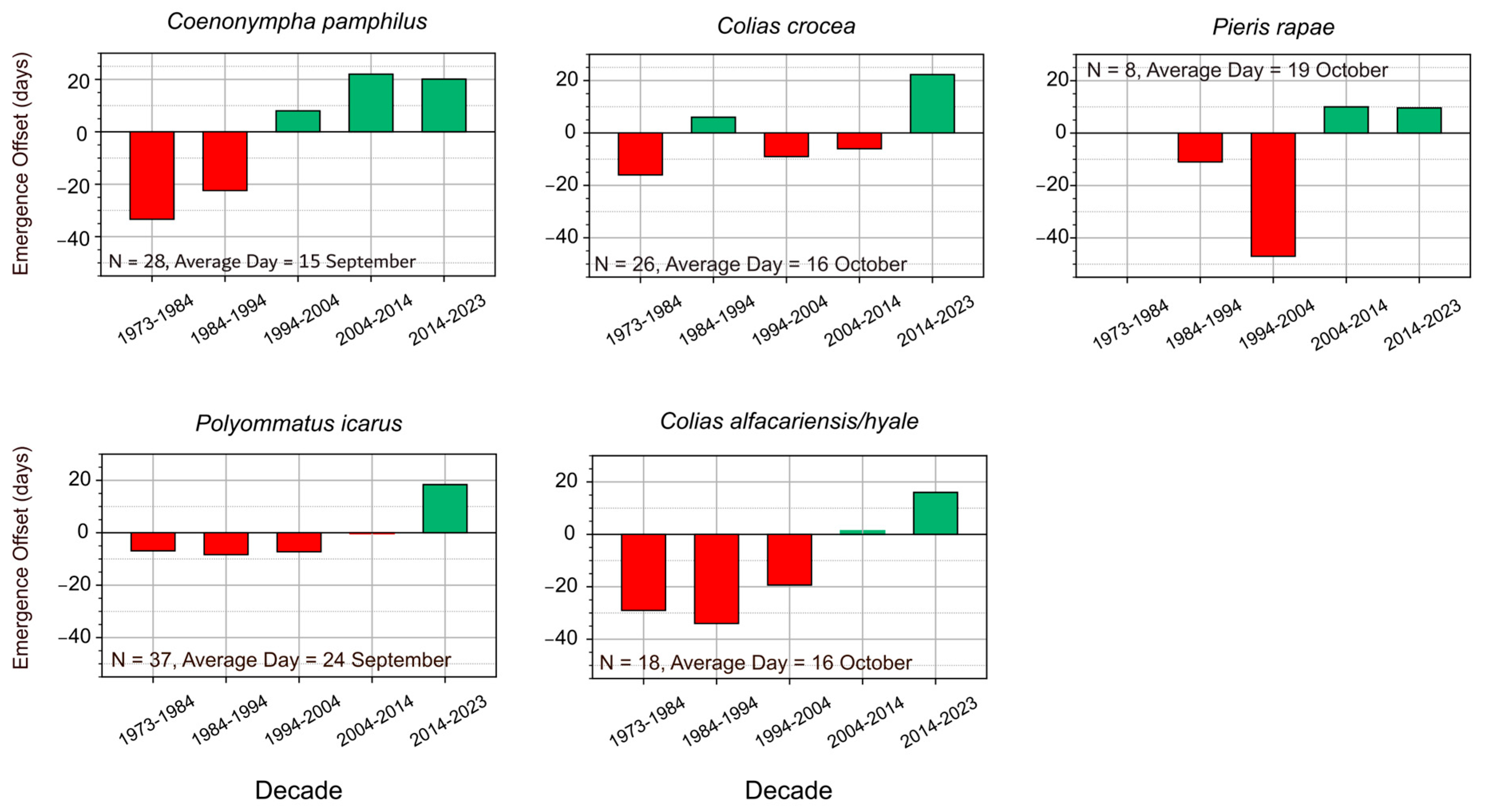
3.2. Species with Multiple Generations and Long Flight Periods
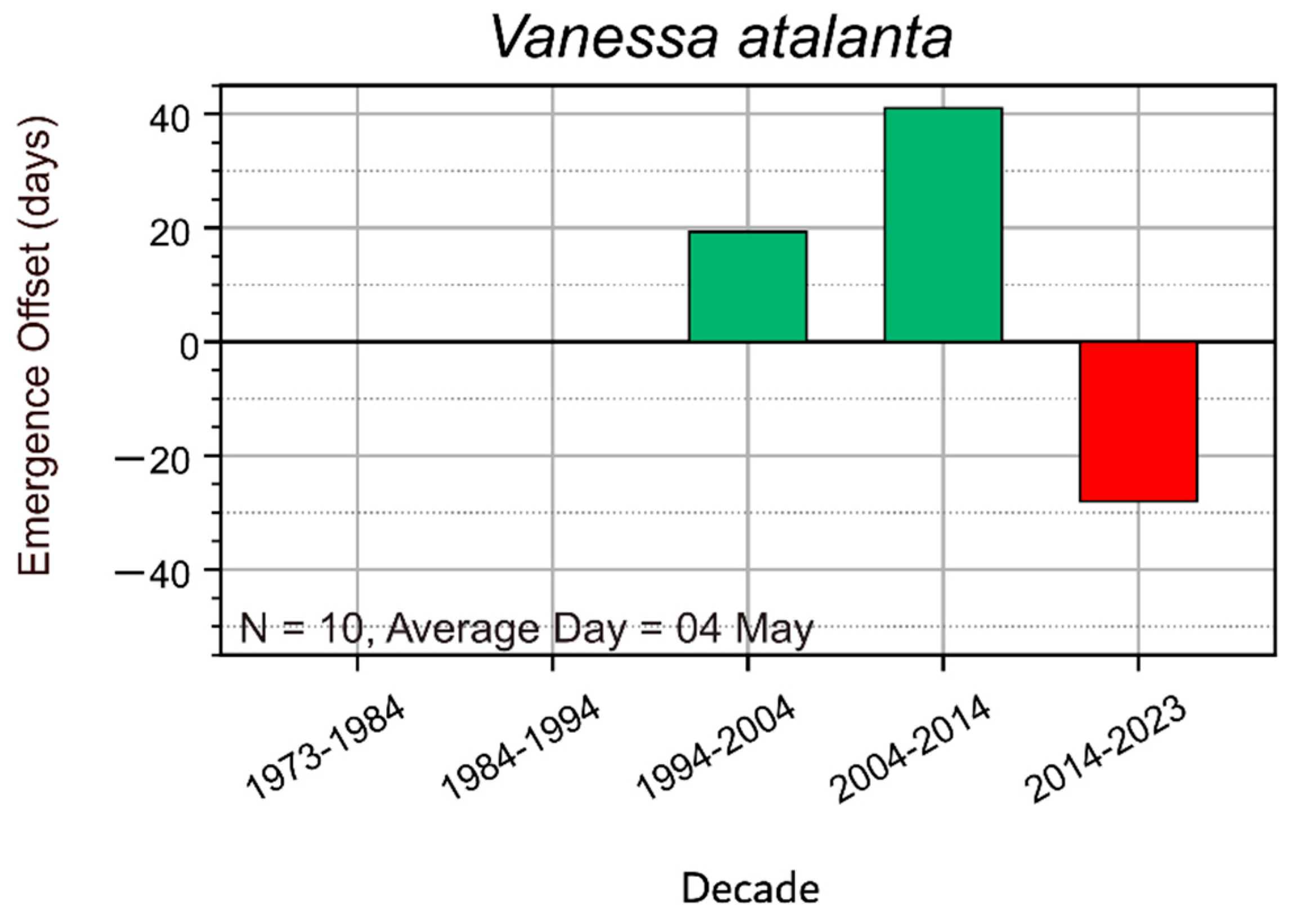
3.3. Migratory Species
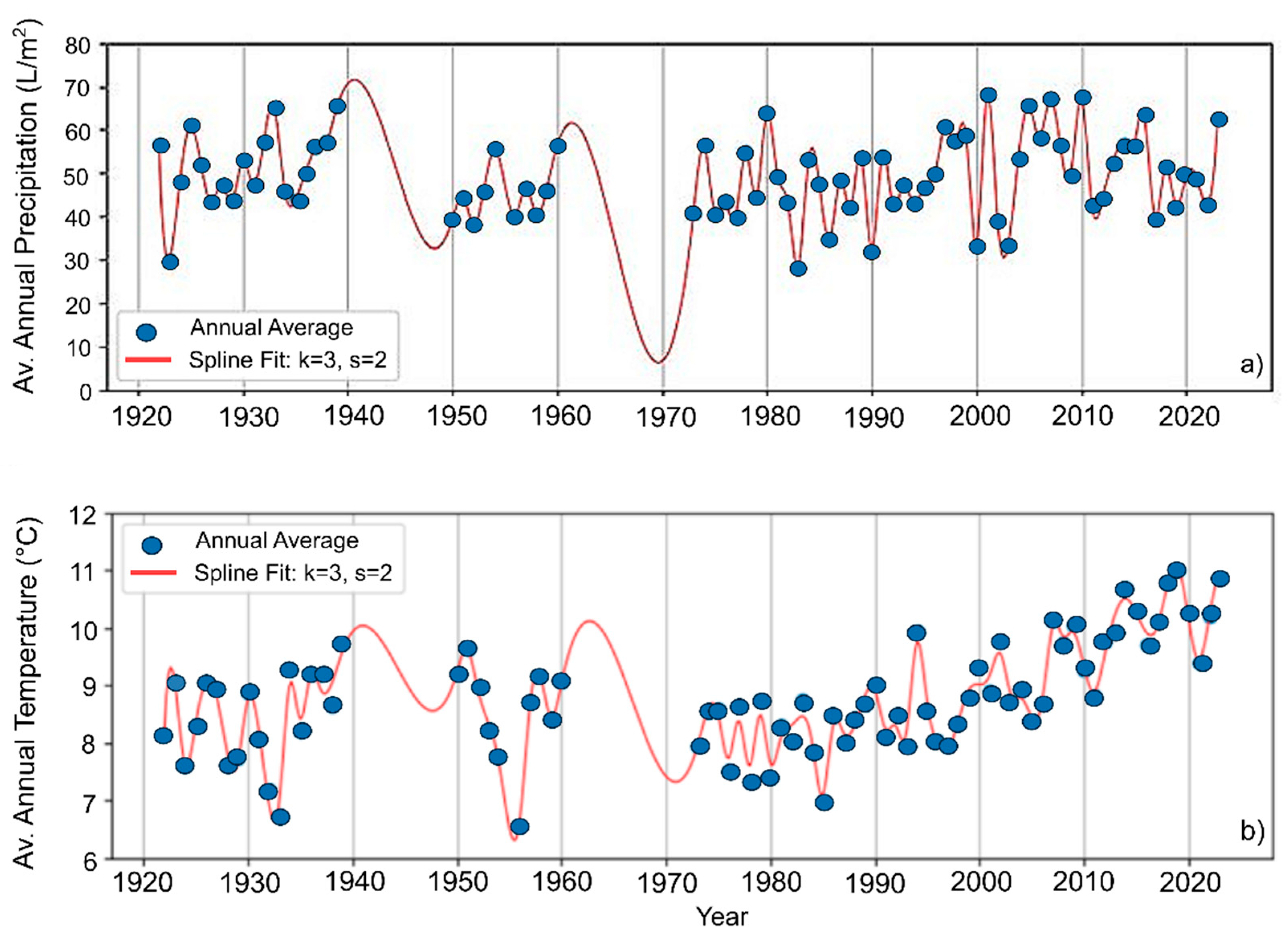
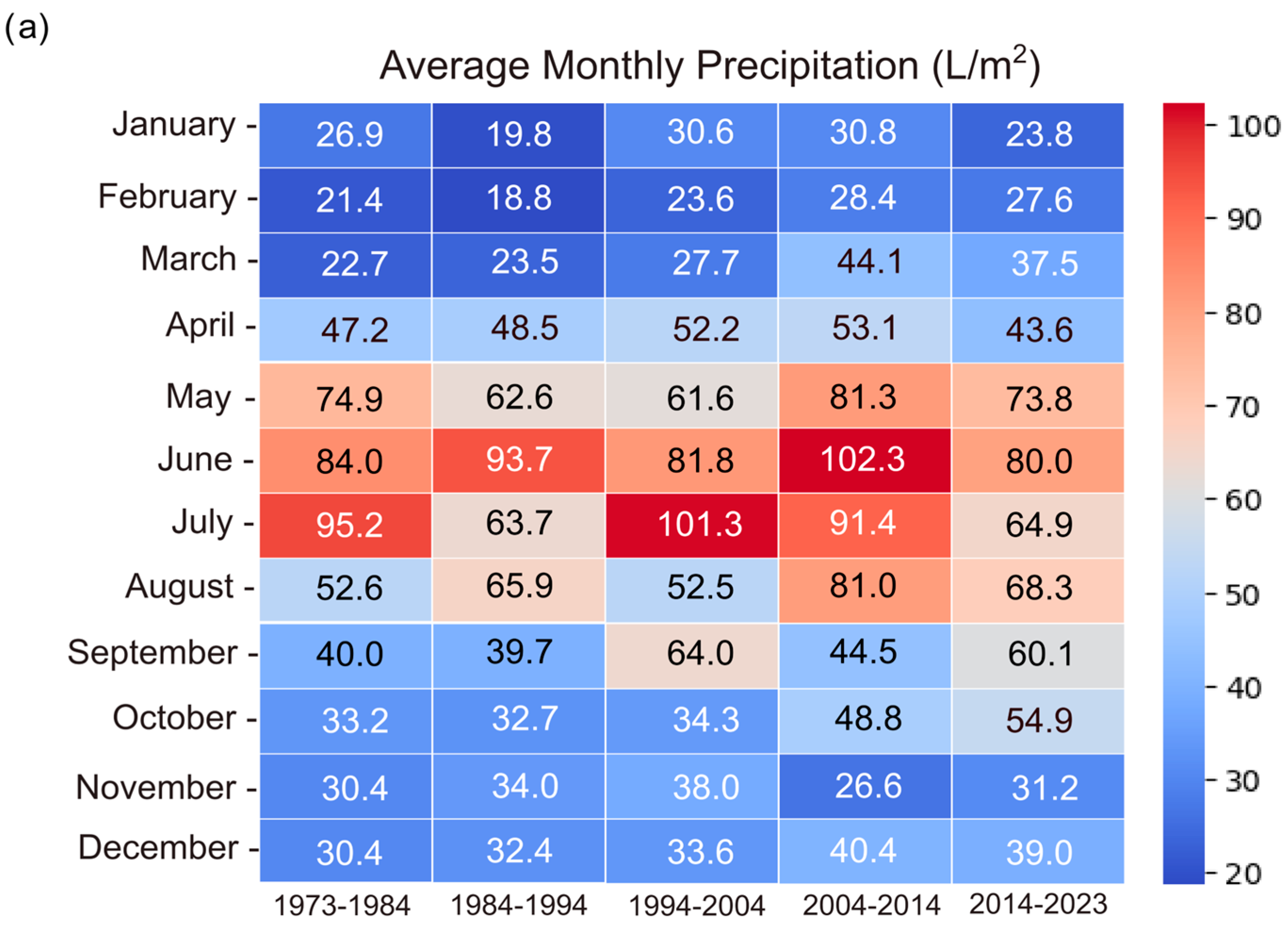
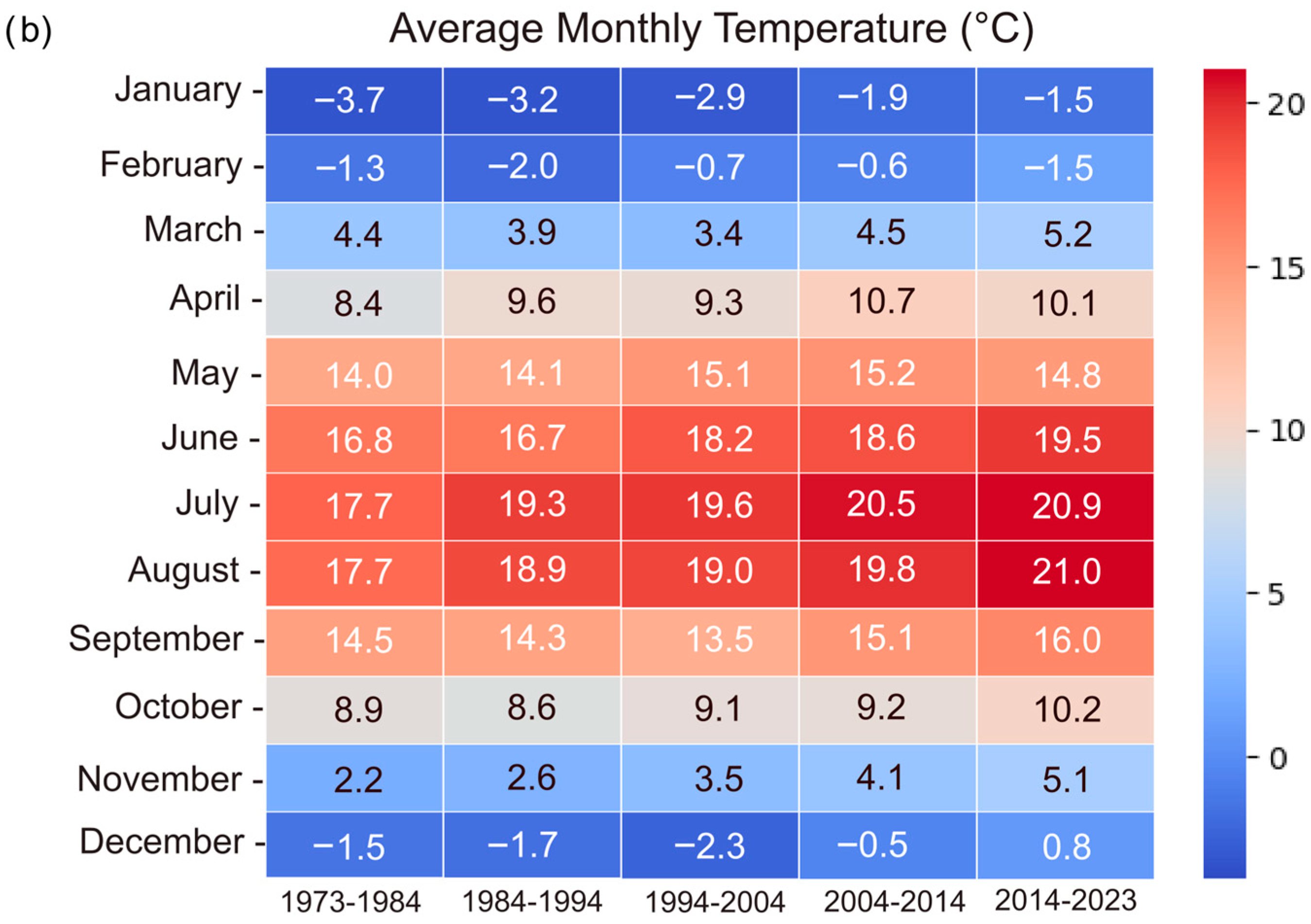
3.4. Climate
4. Discussions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vitali, F.; Habel, J.C.; Ulrich, W.; Schmitt, T. Global Change Drives Phenological and Spatial Shifts in Central European Longhorn Beetles (Coleoptera, Cerambycidae) during the Past 150 Years. Oecologia 2023, 202, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Forrest, J.R. Complex Responses of Insect Phenology to Climate Change. Curr. Opin. Insect Sci. 2016, 17, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Bartomeus, I.; Ascher, J.S.; Wagner, D.; Danforth, B.N.; Colla, S.; Kornbluth, S.; Winfree, R. Climate-Associated Phenological Advances in Bee Pollinators and Bee-Pollinated Plants. Proc. Natl. Acad. Sci. USA 2011, 108, 20645–20649. [Google Scholar] [CrossRef] [PubMed]
- Hassall, C.; Owen, J.; Gilbert, F. Phenological Shifts in Hoverflies (Diptera: Syrphidae): Linking Measurement and Mechanism. Ecography 2017, 40, 853–863. [Google Scholar] [CrossRef]
- Johansson, F.; Orizaola, G.; Nilsson-Örtman, V. Temperate Insects with Narrow Seasonal Activity Periods Can Be as Vulnerable to Climate Change as Tropical Insect Species. Sci. Rep. 2020, 10, 8822. [Google Scholar] [CrossRef]
- Müller, J.; Hothorn, T.; Yuan, Y.; Seibold, S.; Mitesser, O.; Rothacher, J.; Freund, J.; Wild, C.; Wolz, M.; Menzel, A. Weather Explains the Decline and Rise of Insect Biomass over 34 Years. Nature 2024, 628, 349–354. [Google Scholar] [CrossRef]
- Rumohr, Q.; Baden, C.U.; Bergtold, M.; Marx, M.T.; Oellers, J.; Schade, M.; Toschki, A.; Maus, C. Drivers and Pressures behind Insect Decline in Central and Western Europe Based on Long-Term Monitoring Data. PLoS ONE 2023, 18, e0289565. [Google Scholar] [CrossRef]
- Hufnagel, L.; Kocsis, M. Impacts of Climate Change on Lepidoptera Species and Communities. Appl. Ecol. Environ. Res. 2011, 9, 43–72. [Google Scholar] [CrossRef]
- Sánchez-Bayo, F.; Wyckhuys, K.A.G. Worldwide Decline of the Entomofauna: A Review of Its Drivers. Biol. Conserv. 2019, 232, 8–27. [Google Scholar] [CrossRef]
- Cayton, H.L.; Haddad, N.M.; Gross, K.; Diamond, S.E.; Ries, L. Do Growing Degree Days Predict Phenology across Butterfly Species? Ecology 2015, 96, 1473–1479. [Google Scholar] [CrossRef]
- Birch, R.; Nebel, L.; Chittaro, Y.; Hermann, G.; Trusch, R.; Gelbrecht, J.; Markl, G. The Diverse Reactions of Butterflies and Zygaenids (Lepidoptera) to Climate Change—A Large Scale, Multi-Species Study. Glob. Ecol. Biogeogr. 2025, 34, e70112. [Google Scholar] [CrossRef]
- Barve, N. Climate-Change and Mass Mortality Events in Overwintering Monarch Butterflies. Rev. Mex. Biodivers. 2012, 83, 817–824. [Google Scholar] [CrossRef]
- Habel, J.C.; Trusch, R.; Schmitt, T.; Ochse, M.; Ulrich, W. Long-Term Large-Scale Decline in Relative Abundances of Butterfly and Burnet Moth Species across South-Western Germany. Sci. Rep. 2019, 9, 14921. [Google Scholar] [CrossRef] [PubMed]
- Hallmann, C.A.; Sorg, M.; Jongejans, E.; Siepel, H.; Hofland, N.; Schwan, H.; Stenmans, W.; Müller, A.; Sumser, H.; Hörren, T.; et al. More than 75 Percent Decline over 27 Years in Total Flying Insect Biomass in Protected Areas. PLoS ONE 2017, 12, e0185809. [Google Scholar] [CrossRef] [PubMed]
- Warren, M.S.; Maes, D.; Van Swaay, C.A.M.; Goffart, P.; Van Dyck, H.; Bourn, N.A.D.; Wynhoff, I.; Hoare, D.; Ellis, S. The Decline of Butterflies in Europe: Problems, Significance, and Possible Solutions. Proc. Natl. Acad. Sci. USA 2021, 118, e2002551117. [Google Scholar] [CrossRef]
- Buckley, L.B. Temperature-Sensitive Development Shapes Insect Phenological Responses to Climate Change. Curr. Opin. Insect Sci. 2022, 52, 100897. [Google Scholar] [CrossRef]
- Costache, C.; Crişan, A.; Rákosy, L. The Decline of Butterfly Populations Due to Climate and Land Use Change in Romania. In Climate and Land Use Impacts on Natural and Artificial Systems; Elsevier: Amsterdam, The Netherlands, 2021; pp. 271–285. [Google Scholar] [CrossRef]
- Habel, J.C.; Schmitt, T.; Gros, P.; Ulrich, W. Active around the Year: Butterflies and Moths Adapt Their Life Cycles to a Warming World. Glob. Change Biol. 2024, 30, e17103. [Google Scholar] [CrossRef]
- Calvin, K.; Dasgupta, D.; Krinner, G.; Mukherji, A.; Thorne, P.W.; Trisos, C.; Romero, J.; Aldunce, P.; Barrett, K.; Blanco, G.; et al. IPCC, 2023: Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, 1st ed.; Core Writing Team, Lee, H., Romero, J., Eds.; Intergovernmental Panel on Climate Change (IPCC): Geneva, Switzerland, 2023. [Google Scholar] [CrossRef]
- Hickinbotham, E.J.; Pattison, Z.; Fox, R.; Rushton, S.P. Drivers of Moth Phenology in England and Wales. J. Insect Conserv. 2024, 28, 969–979. [Google Scholar] [CrossRef]
- Bonelli, S.; Cerrato, C.; Barbero, F.; Boiani, M.V.; Buffa, G.; Casacci, L.P.; Fracastoro, L.; Provenzale, A.; Rivella, E.; Zaccagno, M.; et al. Changes in Alpine Butterfly Communities during the Last 40 Years. Insects 2021, 13, 43. [Google Scholar] [CrossRef]
- Hill, G.M.; Kawahara, A.Y.; Daniels, J.C.; Bateman, C.C.; Scheffers, B.R. Climate Change Effects on Animal Ecology: Butterflies and Moths as a Case Study. Biol. Rev. 2021, 96, 2113–2126. [Google Scholar] [CrossRef]
- McDermott Long, O.; Warren, R.; Price, J.; Brereton, T.M.; Botham, M.S.; Franco, A.M.A. Sensitivity of UK Butterflies to Local Climatic Extremes: Which Life Stages Are Most at Risk? J. Anim. Ecol. 2017, 86, 108–116. [Google Scholar] [CrossRef]
- Miao, B.; Peng, Y.; Yang, D.; Kubota, Y.; Economo, E.P.; Liu, C. Climate and Land-use Interactively Shape Butterfly Diversity in Tropical Rainforest and Savanna Ecosystems of Southwestern China. Insect Sci. 2021, 28, 1109–1120. [Google Scholar] [CrossRef]
- Ren, J.; Li, S.; He, M.; Zhang, Y. Butterfly Community Diversity in the Qinling Mountains. Diversity 2022, 14, 27. [Google Scholar] [CrossRef]
- Santorufo, L.; Ienco, A.; Scalercio, S. Climate Warming Drives Divergence of Montane Butterfly Communities in Southern Italy. Reg. Environ. Change 2021, 21, 56. [Google Scholar] [CrossRef]
- Knyazev, S.A.; Saikina, S.M.; Teploukhov, V.Y.; Sitnikov, P.S.; Galich, D.E.; Kosterin, O.E. First Records of Apatura ilia ([Denis & Schiffermüller], 1775) and Limenitis camilla (Linnaeus, 1764) in West Siberia. Acta Biol. Sib. 2024, 10, 107–116. [Google Scholar] [CrossRef]
- Yakovlev, R.V.; Kostyunin, A.E. Range Expansion of Apatura iris (Linnaeus, 1758) in Siberia (Lepidoptera: Nymphalidae). SHILAP Rev. Lepidopterol. 2015, 43, 305–308. [Google Scholar]
- Gordeev, S.Y.; Gordeeva, T.V.; Korsun, O.V. On the Reasons of Limenitis sydyi (Lepidoptera, Nymphalidae) expansion in Transbaikalia. Russ. J. Biol. Invasions 2023, 16, 40–53. [Google Scholar] [CrossRef]
- Gordeev, S.Y.; Gordeeva, T.V. The Causes of Penetration of Apatura Fabricius, 1807 (Lepidoptera, Nymphalidae) Species into Western Transbaikalia. Russ. J. Biol. Invasions 2020, 11, 1–8. [Google Scholar] [CrossRef]
- Shahin, H.; Correia, A.H.; Orazio, C.; Branco, M.; Almeida, M.H. Monitoring Two REINFFORCE Network Arboreta: First Result on Site, Climate and Genetic Interaction Showing Impact on Phenology and Biotic Damages. Sci. For. 2019, 47, 552–570. [Google Scholar] [CrossRef]
- Macgregor, C.J.; Thomas, C.D.; Roy, D.B.; Beaumont, M.A.; Bell, J.R.; Brereton, T.; Bridle, J.R.; Dytham, C.; Fox, R.; Gotthard, K.; et al. Climate-Induced Phenology Shifts Linked to Range Expansions in Species with Multiple Reproductive Cycles per Year. Nat. Commun. 2019, 10, 4455. [Google Scholar] [CrossRef]
- Walther, G.-R.; Post, E.; Convey, P.; Menzel, A.; Parmesan, C.; Beebee, T.J.C.; Fromentin, J.-M.; Hoegh-Guldberg, O.; Bairlein, F. Ecological Responses to Recent Climate Change. Nature 2002, 416, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Diamond, S.E.; Cayton, H.; Wepprich, T.; Jenkins, C.N.; Dunn, R.R.; Haddad, N.M.; Ries, L. Unexpected Phenological Responses of Butterflies to the Interaction of Urbanization and Geographic Temperature. Ecology 2014, 95, 2613–2621. [Google Scholar] [CrossRef]
- Parmesan, C. Chapter 24. Butterflies as Bioindicators for Climate Change Effects. In Butterflies: Ecology and Evolution Taking Flight; University of Chicago Press: Chicago, IL, USA, 2003; pp. 541–560. [Google Scholar]
- Roy, D.B.; Sparks, T.H. Phenology of British Butterflies and Climate Change. Glob. Change Biol. 2000, 6, 407–416. [Google Scholar] [CrossRef]
- Betzholtz, P.-E.; Forsman, A.; Franzén, M. Associations of 16-Year Population Dynamics in Range-Expanding Moths with Temperature and Years since Establishment. Insects 2023, 14, 55. [Google Scholar] [CrossRef]
- Hodgson, J.A.; Thomas, C.D.; Oliver, T.H.; Anderson, B.J.; Brereton, T.M.; Crone, E.E. Predicting Insect Phenology across Space and Time: PREDICTING INSECT PHENOLOGY. Glob. Change Biol. 2011, 17, 1289–1300. [Google Scholar] [CrossRef]
- Scriber, J.M.; Maher, E.; Aardema, M.L. Differential Effects of Short Term Winter Thermal Stress on Diapausing Tiger Swallowtail Butterflies (Papilio spp.). Insect Sci. 2012, 19, 277–285. [Google Scholar] [CrossRef]
- Singer, M.C.; Parmesan, C. Phenological Asynchrony between Herbivorous Insects and Their Hosts: Signal of Climate Change or Pre-Existing Adaptive Strategy? Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 3161–3176. [Google Scholar] [CrossRef]
- Bale, J.S.; Hayward, S.A.L. Insect Overwintering in a Changing Climate. J. Exp. Biol. 2010, 213, 980–994. [Google Scholar] [CrossRef]
- Diamond, S.E.; Frame, A.M.; Martin, R.A.; Buckley, L.B. Species’ Traits Predict Phenological Responses to Climate Change in Butterflies. Ecology 2011, 92, 1005–1012. [Google Scholar] [CrossRef] [PubMed]
- Wepprich, T.; Henry, E.; Haddad, N.M. Voltinism Shifts in Response to Climate Warming Generally Benefit Populations of Multivoltine Butterflies. Ecol. Lett. 2025, 28, e70018. [Google Scholar] [CrossRef] [PubMed]
- Enriquez, T.; Visser, B. The Importance of Fat Accumulation and Reserves for Insect Overwintering. Curr. Opin. Insect Sci. 2023, 60, 101118. [Google Scholar] [CrossRef]
- Stefanescu, C.; Herrando, S.; Páramo, F. Butterfly Species Richness in the North-west Mediterranean Basin: The Role of Natural and Human-induced Factors. J. Biogeogr. 2004, 31, 905–915. [Google Scholar] [CrossRef]
- Lafranchis, T.; Jutzeler, D.; Guillosson, Y.J.; Kan, P.; Kan, B. La Vie des Papillons. Ecologie, Biologie et Comportement des Rhopalocéres de France; Diatheo: Paris, France, 2015. [Google Scholar]
- Gallou, A.; Baillet, Y.; Ficetola, G.F.; Després, L. Elevational Gradient and Human Effects on Butterfly Species Richness in the French Alps. Ecol. Evol. 2017, 7, 3672–3681. [Google Scholar] [CrossRef]
- Altermatt, F. Temperature-related Shifts in Butterfly Phenology Depend on the Habitat. Glob. Change Biol. 2012, 18, 2429–2438. [Google Scholar] [CrossRef]
- Kharouba, H.M.; Lewthwaite, J.M.M.; Guralnick, R.; Kerr, J.T.; Vellend, M. Using Insect Natural History Collections to Study Global Change Impacts: Challenges and Opportunities. Philos. Trans. R. Soc. B Biol. Sci. 2019, 374, 20170405. [Google Scholar] [CrossRef]
- Meineke, E.K.; Davies, T.J.; Daru, B.H.; Davis, C.C. Biological Collections for Understanding Biodiversity in the Anthropocene. Philos. Trans. R. Soc. B Biol. Sci. 2019, 374, 20170386. [Google Scholar] [CrossRef]
- Rákosy, L. A Field Guide to the Butterflies of Romania, 1st ed.; Pelagic Identification Guides; Pelagic Publishing Ltd.: Exeter, UK, 2024. [Google Scholar]
- Devictor, V.; Van Swaay, C.; Brereton, T.; Brotons, L.; Chamberlain, D.; Heliölä, J.; Herrando, S.; Julliard, R.; Kuussaari, M.; Lindström, Å.; et al. Differences in the Climatic Debts of Birds and Butterflies at a Continental Scale. Nat. Clim. Change 2012, 2, 121–124. [Google Scholar] [CrossRef]
- Popescu-Gorj, A. Catalogue de La Collection de Lépidoptères “Prof. A. Ostrogovich” Du Muséum d’histoire Naturelle “Grigore Antipa” Bucarest; Musée d’Histoire Naturelle “Grigore Antipa”: Bucarest, Romania, 1964. [Google Scholar]
- Rákosy, L. A Valuable Collection of Lepidoptera in the Zoological Museum of the University in Cluj-Napoca. Stud. Univ. Babeş-Bolyai Biol. 1988, 32, 55–58. [Google Scholar]
- Rákosy, L.; Lászlóffy, Z. Fauna de Macrolepidoptere de La Fânatele Clujului (Lepidoptera) (Cluj, Romania). Bul. Inf. Soc. Lepid. Rom. 1997, 8, 165–186. [Google Scholar]
- Askeyev, O.V.; Sparks, T.H.; Askeyev, I.V.; Tishin, D.V.; Tryjanowski, P. East versus West: Contrasts in Phenological Patterns? Glob. Ecol. Biogeogr. 2010, 19, 783–793. [Google Scholar] [CrossRef]
- Lancaster, L.T. Widespread Range Expansions Shape Latitudinal Variation in Insect Thermal Limits. Nat. Clim. Change 2016, 6, 618–621. [Google Scholar] [CrossRef]
- Menzel, A.; Yuan, Y.; Matiu, M.; Sparks, T.; Scheifinger, H.; Gehrig, R.; Estrella, N. Climate Change Fingerprints in Recent European Plant Phenology. Glob. Change Biol. 2020, 26, 2599–2612. [Google Scholar] [CrossRef]
- Toro-Delgado, E.; Vila, R.; Talavera, G.; Turner, E.C.; Hayes, M.P.; Horrocks, N.P.C.; Bladon, A.J. Regional Differences in Thermoregulation between Two European Butterfly Communities. J. Anim. Ecol. 2024, 93, 183–195. [Google Scholar] [CrossRef]
- Altermatt, F. Climatic Warming Increases Voltinism in European Butterflies and Moths. Proc. R. Soc. B Biol. Sci. 2010, 277, 1281–1287. [Google Scholar] [CrossRef]
- Donoso, I.; Stefanescu, C.; Martínez-Abraín, A.; Traveset, A. Phenological Asynchrony in Plant–Butterfly Interactions Associated with Climate: A Community-wide Perspective. Oikos 2016, 125, 1434–1444. [Google Scholar] [CrossRef]
- Zografou, K.; Swartz, M.T.; Adamidis, G.C.; Tilden, V.P.; McKinney, E.N.; Sewall, B.J. Species Traits Affect Phenological Responses to Climate Change in a Butterfly Community. Sci. Rep. 2021, 11, 3283. [Google Scholar] [CrossRef]
- Végvári, Z.; Juhász, E.; Tóth, J.P.; Barta, Z.; Boldogh, S.; Szabó, S.; Varga, Z. Life-history Traits and Climatic Responsiveness in Noctuid Moths. Oikos 2015, 124, 235–242. [Google Scholar] [CrossRef]
- Wepprich, T.; Adrion, J.R.; Ries, L.; Wiedmann, J.; Haddad, N.M. Butterfly Abundance Declines over 20 Years of Systematic Monitoring in Ohio, USA. PLoS ONE 2019, 14, e0216270. [Google Scholar] [CrossRef]
- Stocker, T.; Qin, D.; Plattner, G.; Tignor, M.; Allen, S.; Boschung, J.; Nauels, A.; Xia, Y.; Bex, B.; Midgley, B. (Eds.) IPCC, 2013: Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- Freimuth, J.; Bossdorf, O.; Scheepens, J.F.; Willems, F.M. Climate Warming Changes Synchrony of Plants and Pollinators. Proc. R. Soc. B Biol. Sci. 2022, 289, 20212142. [Google Scholar] [CrossRef]
- Tamura, Y.; Osawa, T.; Tabuchi, K.; Yamasaki, K.; Niiyama, T.; Sudo, S.; Ishigooka, Y.; Yoshioka, A.; Takada, M.B. Estimating Plant–Insect Interactions under Climate Change with Limited Data. Sci. Rep. 2022, 12, 10554. [Google Scholar] [CrossRef]
- Kharouba, H.M.; Vellend, M. Flowering Time of Butterfly Nectar Food Plants Is More Sensitive to Temperature than the Timing of Butterfly Adult Flight. J. Anim. Ecol. 2015, 84, 1311–1321. [Google Scholar] [CrossRef]
- Baur, J.; Jagusch, D.; Michalak, P.; Koppik, M.; Berger, D. The Mating System Affects the Temperature Sensitivity of Male and Female Fertility. Funct. Ecol. 2022, 36, 92–106. [Google Scholar] [CrossRef]
- Sales, K.; Vasudeva, R.; Dickinson, M.E.; Godwin, J.L.; Lumley, A.J.; Michalczyk, Ł.; Hebberecht, L.; Thomas, P.; Franco, A.; Gage, M.J.G. Experimental Heatwaves Compromise Sperm Function and Cause Transgenerational Damage in a Model Insect. Nat. Commun. 2018, 9, 4771. [Google Scholar] [CrossRef]
- Sales, K.; Vasudeva, R.; Gage, M.J.G. Fertility and Mortality Impacts of Thermal Stress from Experimental Heatwaves on Different Life Stages and Their Recovery in a Model Insect. R. Soc. Open Sci. 2021, 8, 201717. [Google Scholar] [CrossRef]
- Thackeray, S.J.; Henrys, P.A.; Hemming, D.; Bell, J.R.; Botham, M.S.; Burthe, S.; Helaouet, P.; Johns, D.G.; Jones, I.D.; Leech, D.I.; et al. Phenological Sensitivity to Climate across Taxa and Trophic Levels. Nature 2016, 535, 241–245. [Google Scholar] [CrossRef]
- Xiao, H.; Chen, J.; Chen, L.; Chen, C.; Wu, S. Exposure to Mild Temperatures Decreases Overwintering Larval Survival and Post-Diapause Reproductive Potential in the Rice Stem Borer Chilo Suppressalis. J. Pest Sci. 2017, 90, 117–125. [Google Scholar] [CrossRef]
- Löckinger, M.; Trutschnig, W.; Ulrich, W.; Gros, P.; Schmitt, T.; Habel, J.C. Ecological Performance Determines Phenological Responses of Butterflies in Northern Austria. Glob. Ecol. Conserv. 2024, 54, e03114. [Google Scholar] [CrossRef]
- Zhang, Z. Effects of Climate Change on Butterfly Species. Theor. Nat. Sci. 2023, 4, 235–245. [Google Scholar] [CrossRef]
- Filazzola, A.; Matter, S.F.; Roland, J. Inclusion of Trophic Interactions Increases the Vulnerability of an Alpine Butterfly Species to Climate Change. Glob. Change Biol. 2020, 26, 2867–2877. [Google Scholar] [CrossRef]
- Gergelz, P.; Gór, A.; Hudák, T.; Ilonczai, Z.; Szombathely, E. Nappali Lepkéink. Our Butterflies, in Hungarian; Kitaibel: Budapest, Hungary, 2017. [Google Scholar]
- Höttinger, H.; Pendl, M.; Wiemers, M.; Pospisil, A. Insekten in Wien ß Tagfalter; Österreichische Gesellschaft für Entomofaunistik: Vienna, Austria, 2013. [Google Scholar]
- Kühn, E.; Musche, M.; Harpke, A.; Feldmann, R.; Ulbrich, K.; Wiemers, M.; Settele, J. Tagfalter-Monitoring Deutschland: Jahresauswertung, 2018. Oedippus 2019, 36, 6–38. [Google Scholar]
- Lee, S.; Jeon, H.; Kim, M. Spatial Distribution of Butterflies in Accordance with Climate Change in the Korean Peninsula. Sustainability 2020, 12, 1995. [Google Scholar] [CrossRef]
- Konvicka, M.; Kuras, T.; Liparova, J.; Slezak, V.; Horázná, D.; Klečka, J.; Kleckova, I. Low Winter Precipitation, but Not Warm Autumns and Springs, Threatens Mountain Butterflies in Middle-High Mountains. PeerJ 2021, 9, e12021. [Google Scholar] [CrossRef]
- Schulte, T.; Eller, O.; Niehuis, M.; Rennwald, E. Die Tagfalter Der Pfalz, Bd. 1.—Flora Und Fauna in Rheinland-Pfalz. Beiheft 2007, 36, 592. [Google Scholar]
- Ulrich, W.; Habel, J.C.; Gros, P.; Schmitt, T. Recent Increasing Homogenisation in Austrian Butterfly Communities over the Past Decades. Oikos 2024, 2024, e10179. [Google Scholar] [CrossRef]

| Species | Group | Total |
|---|---|---|
| Anthocaris cardamines | Spring emerging | 14 |
| Colias alfacariensis/hyale | Spring emerging | 35 |
| Erebia medusa | Spring emerging | 41 |
| Erynnis tages | Spring emerging | 39 |
| Glaucopsyche alexis | Spring emerging | 40 |
| Iphiclides podalirius | Spring emerging | 34 |
| Leptidea sinapis | Spring emerging | 44 |
| Papilio machaon | Spring emerging | 46 |
| Pieris napi | Spring emerging | 28 |
| Pieris rapae | Spring emerging | 23 |
| Plebejus argus | Spring emerging | 31 |
| Pyrgus malvae | Spring emerging | 40 |
| Coenonympha pamphilus | Multivolitin, long flight period | 49 |
| Colias croceus | Multivolitin, long flight period | 50 |
| Colias alfacariensis/hyale | Multivolitin, long flight period | 29 |
| Pieris rapae | Multivolitin, long flight period | 16 |
| Polyommatus icaurs | Multivolitin, long flight period | 54 |
| Vanessa atalanta | Migratory | 13 |
| Total | 626 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costache, C.; Rakosy, L.; Rakosy, D. Long-Term Phenological Shifts in Butterfly Species from Transylvania, Romania—A Case Study. Insects 2025, 16, 1071. https://doi.org/10.3390/insects16101071
Costache C, Rakosy L, Rakosy D. Long-Term Phenological Shifts in Butterfly Species from Transylvania, Romania—A Case Study. Insects. 2025; 16(10):1071. https://doi.org/10.3390/insects16101071
Chicago/Turabian StyleCostache, Cristina, László Rakosy, and Demetra Rakosy. 2025. "Long-Term Phenological Shifts in Butterfly Species from Transylvania, Romania—A Case Study" Insects 16, no. 10: 1071. https://doi.org/10.3390/insects16101071
APA StyleCostache, C., Rakosy, L., & Rakosy, D. (2025). Long-Term Phenological Shifts in Butterfly Species from Transylvania, Romania—A Case Study. Insects, 16(10), 1071. https://doi.org/10.3390/insects16101071






