RNAi of vATPasea Affects Survival and Larval-Pupal Development in Plutella xylostella
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing
2.2. Molecular Cloning
2.3. Synthesis of dsRNA Molecules
2.4. Introduction of dsRNA
2.5. Real-Time Quantitative PCR (qRT-PCR)
2.6. Data Analysis
3. Results
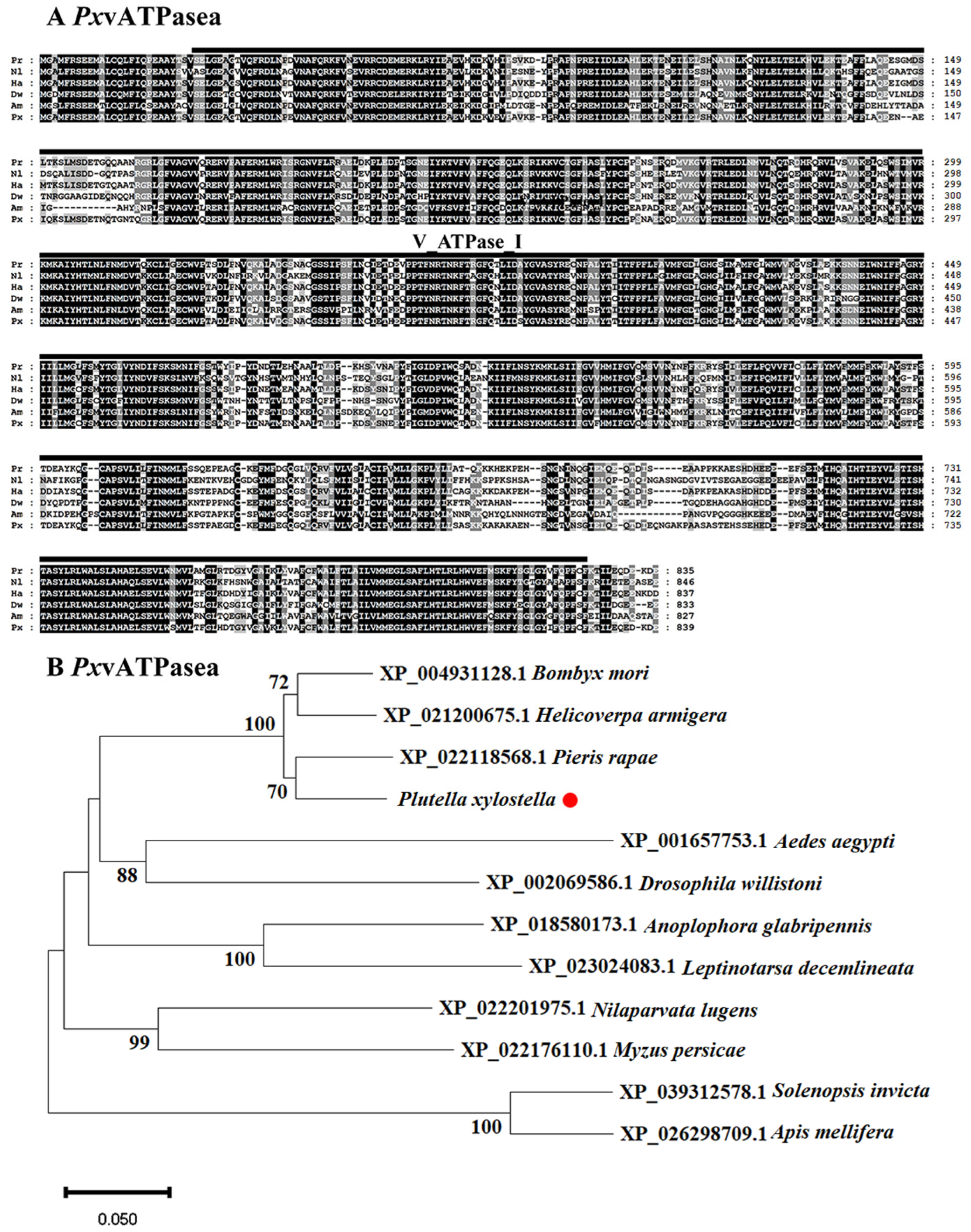
3.1. Identification of PxvATPasea
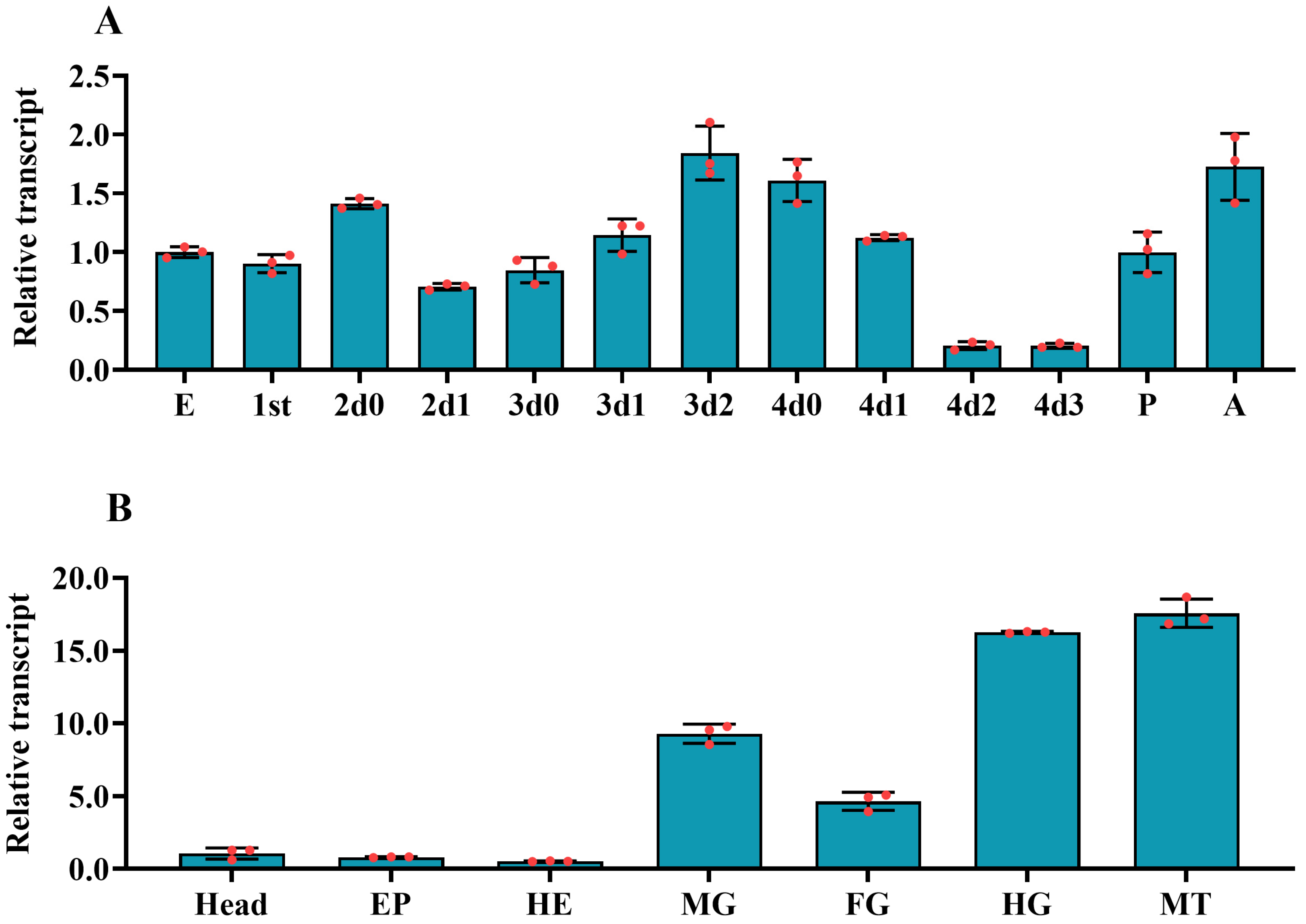
3.2. The Expression Profiles of PxvATPasea
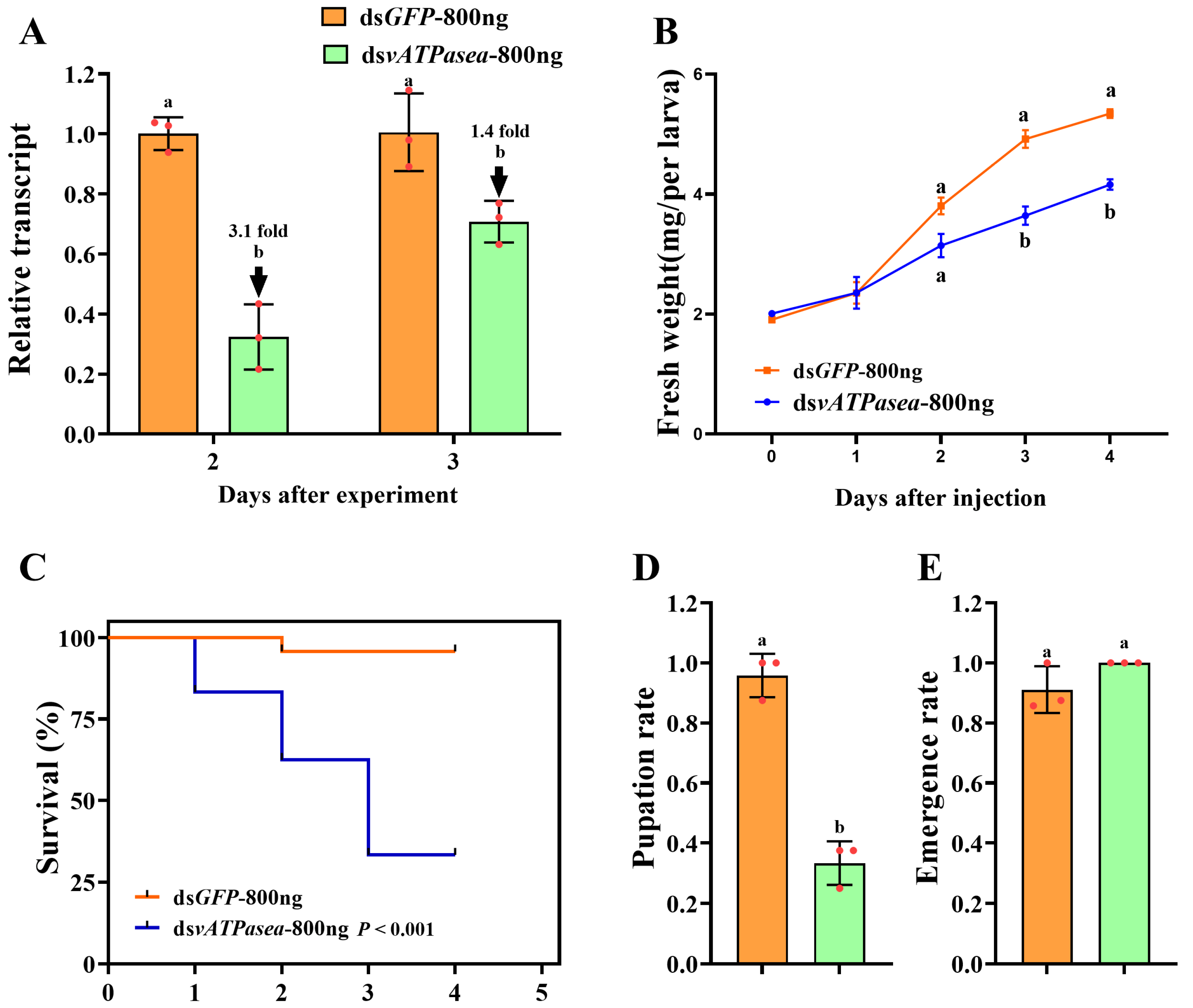
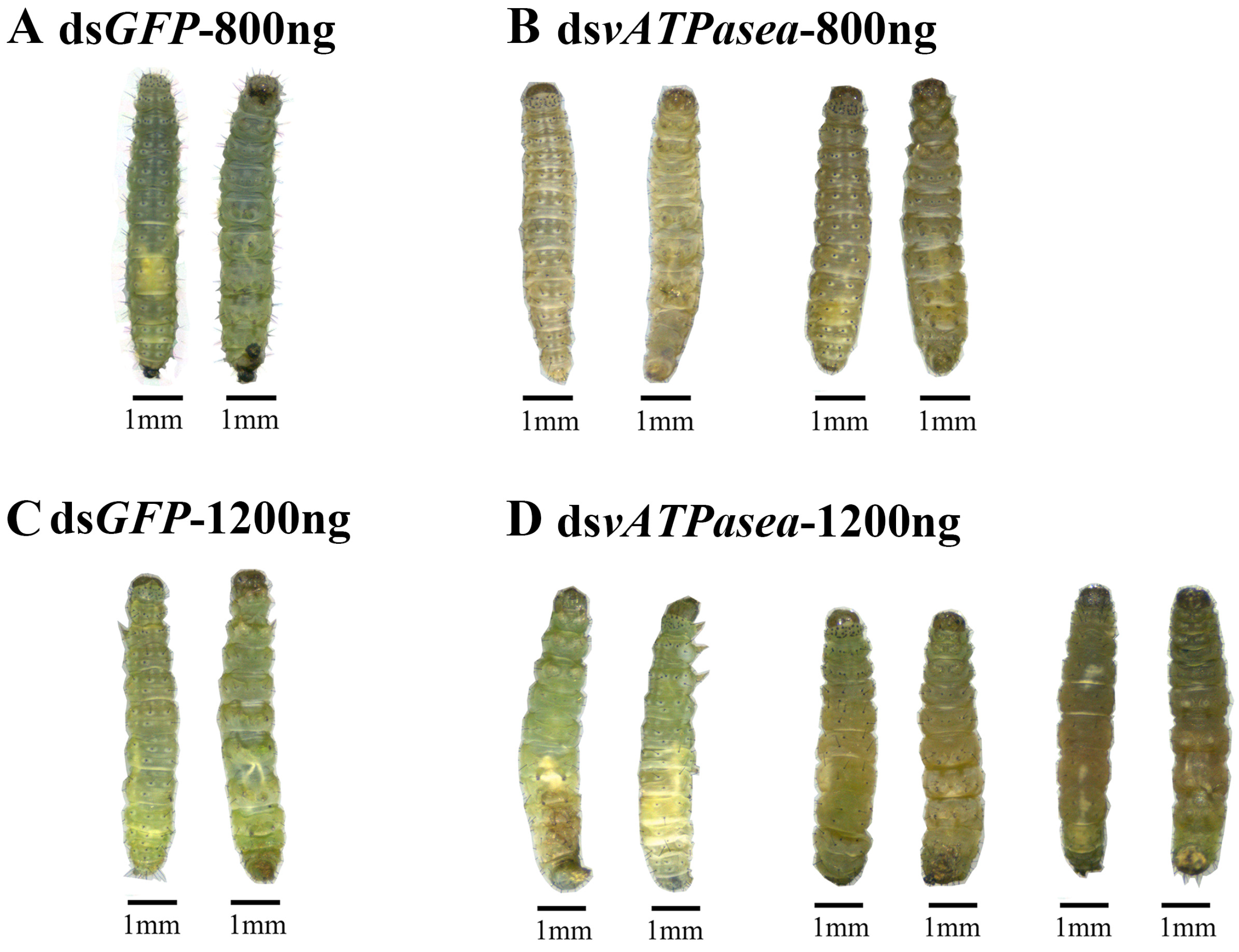
3.3. Effects of RNAi for 800 ng of dsPxvATPasea at the Fourth-Instar Larvae
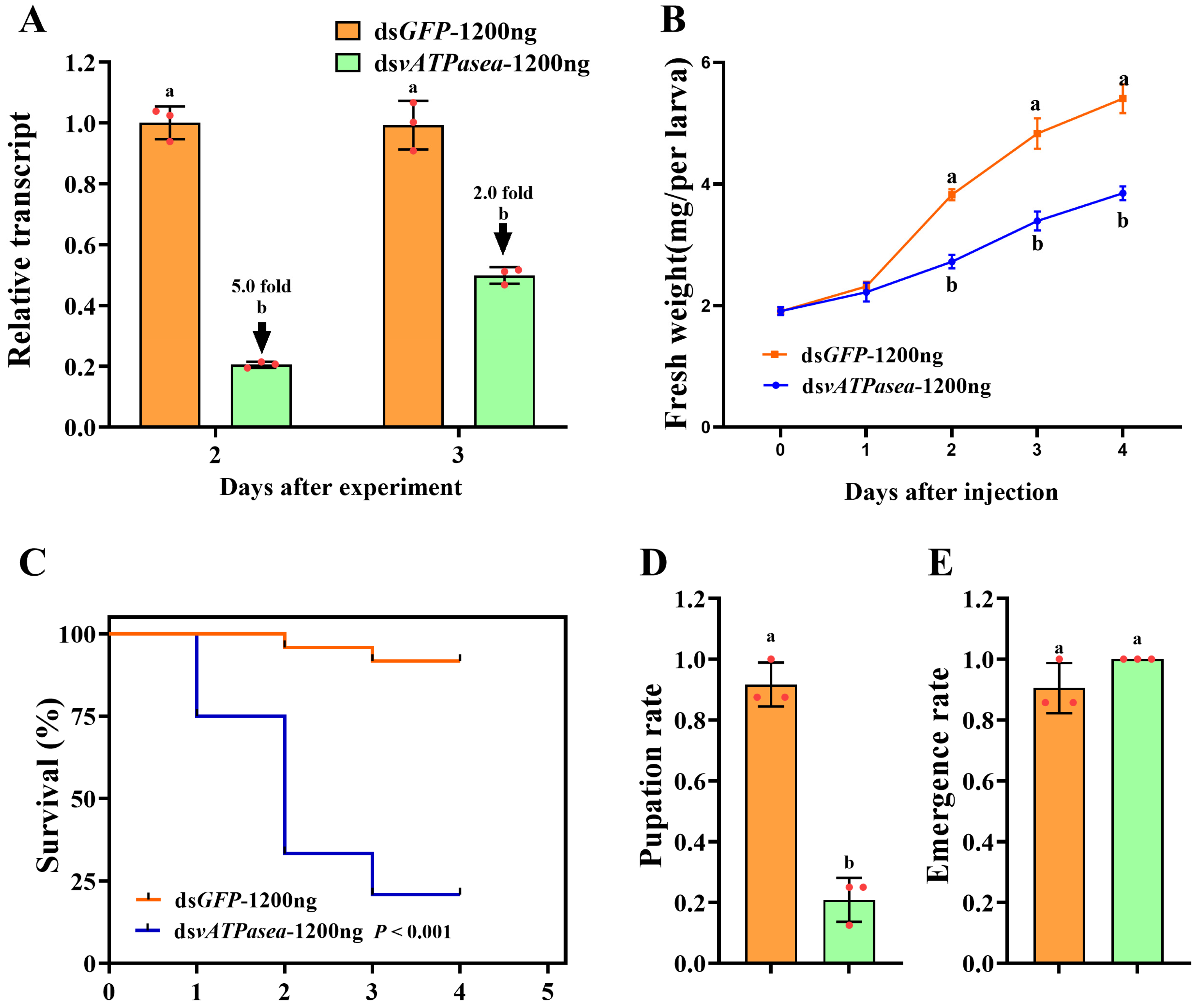
3.4. Impacts on Introduction of 1200 ng dsPxvATPasea at the Fourth-Instar Larvae
4. Discussion
4.1. RNAi Is a Powerful Tool to Manage P. xylostella
4.2. PxvATPasea Is a Potential Candidate Gene to Control the Larvae of P. xylostella
4.3. Enhancing RNAi Efficiency by Stabilizing dsRNA in Insects
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Deng, P.; Peng, Y.; Sheng, Z.; Li, W.; Liu, Y. RNAi silencing CHS1 gene shortens the mortality time of Plutella xylostella feeding Bt-transgenic Brassica napus. Pest Manag. Sci. 2024, 80, 2610–2618. [Google Scholar] [CrossRef] [PubMed]
- Shehzad, M.; Bodlah, I.; Siddiqui, J.A.; Bodlah, M.A.; Fareen, A.G.E.; Islam, W. Recent insights into pesticide resistance mechanisms in Plutella xylostella and possible management strategies. Environ. Sci. Pollut. Res. Int. 2023, 30, 95296–95311. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhu, B.; Zhang, L.; Xiao, Y.; Liang, P.; Wu, K. Influence of seasonal migration on evolution of insecticide resistance in Plutella xylostella. Insect Sci. 2022, 29, 496–504. [Google Scholar] [CrossRef]
- Christiaens, O.; Whyard, S.; Vélez, A.M.; Smagghe, G. Double-stranded RNA technology to control insect pests: Current status and challenges. Front. Plant Sci. 2020, 11, 451. [Google Scholar] [CrossRef]
- Liu, S.; Jaouannet, M.; Dempsey, D.A.; Imani, J.; Coustau, C.; Kogel, K.H. RNA-based technologies for insect control in plant production. Biotechnol. Adv. 2020, 39, 107463. [Google Scholar] [CrossRef]
- Zhu, K.Y.; Palli, S.R. Mechanisms, applications, and challenges of insect RNA interference. Annu. Rev. Entomol. 2020, 65, 293–311. [Google Scholar] [CrossRef]
- Shen, C.H.; Jin, L.; Fu, K.Y.; Guo, W.C.; Li, G.Q. RNA interference targeting Ras GTPase gene Ran causes larval and adult lethality in Leptinotarsa decemlineata. Pest Manag. Sci. 2022, 78, 3849–3858. [Google Scholar] [CrossRef]
- Zhang, J.; Khan, S.A.; Hasse, C.; Ruf, S.; Heckel, D.G.; Bock, R. Full crop protection from an insect pest by expression of long double-stranded RNAs in plastids. Science 2015, 347, 991–994. [Google Scholar] [CrossRef]
- Wu, M.; Dong, Y.; Zhang, Q.; Li, S.; Chang, L.; Loiacono, F.V.; Ruf, S.; Zhang, J.; Bock, R. Efficient control of western flower thrips by plastid-mediated RNA interference. Proc. Natl. Acad. Sci. USA 2022, 119, e2120081119. [Google Scholar] [CrossRef]
- Liu, F.; Yang, B.; Zhang, A.; Ding, D.; Wang, G. Plant-mediated RNAi for controlling Apolygus lucorum. Front. Plant. Sci. 2019, 10, 64. [Google Scholar] [CrossRef] [PubMed]
- Guan, R.B.; Li, H.C.; Miao, X.X. RNAi pest control and enhanced BT insecticidal efficiency achieved by dsRNA of chymotrypsin-like genes in Ostrinia furnacalis. J. Pest Sci. 2017, 90, 745–757. [Google Scholar] [CrossRef]
- Vatanparast, M.; Kazzazi, M.; Sajjadian, S.M.; Park, Y. Knockdown of Helicoverpa armigera protease genes affects its growth and mortality via RNA interference. Arch. Insect Biochem. Physiol. 2021, 108, e21840. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.H.; Mu, L.L.; Jin, L.; Anjum, A.A.; Li, G.Q. Silencing uridine diphosphate N-acetylglucosamine pyrophosphorylase gene impairs larval development in Henosepilachna vigintioctopunctata. Pest Manag. Sci. 2021, 78, 3894–3902. [Google Scholar]
- Liu, D.; Asad, M.; Liao, J.; Chen, J.; Li, J.; Chu, X.; Pang, S.; Tariq, M.; Abbas, A.N.; Yang, G. The potential role of the Piwi gene in the development and reproduction of Plutella xylostella. Int. J. Mol. Sci. 2023, 24, 12321. [Google Scholar] [CrossRef]
- Hou, Q.L.; Zhang, H.Q.; Zhu, J.N.; Chen, E.H. Tyrosine hydroxylase is required for the larval-pupal transformation and immunity of Plutella xylostella: Potential for pest management. J. Agric. Food Chem. 2024, 72, 27818–27829. [Google Scholar]
- Zeng, J.; Kang, W.N.; Jin, L.; Anjum, A.A.; Li, G.Q. Knockdown of Vacuolar ATPase Subunit G Gene affects larval survival and impaired pupation and adult emergence in Henosepilachna vigintioctopunctata. Insects 2021, 12, 935. [Google Scholar] [CrossRef]
- Zeng, J.; Mu, L.L.; Jin, L.; Ali Anjum, A.; Li, G.Q. RNAi of vacuolar-type H (+)-ATPase genes causes growth delay and molting defect in Henosepilachna vigintioctopunctata. Bull. Entomol. Res. 2021, 111, 705–714. [Google Scholar] [CrossRef]
- Zeng, J.; Kang, W.N.; Jin, L.; Anjum, A.A.; Li, G.Q. Vacuolar ATPase subunit F is critical for larval survival in Henosepilachna vigintioctopunctata. Insect Mol. Biol. 2022, 31, 177–189. [Google Scholar]
- Fu, K.Y.; Guo, W.C.; Lü, F.G.; Liu, X.P.; Li, G.Q. Response of the vacuolar ATPase subunit E to RNA interference and four chemical pesticides in Leptinotarsa decemlineata (Say). Pestic. Biochem. Physiol. 2014, 114, 16–23. [Google Scholar] [CrossRef]
- McGuire, C.; Stransky, L.; Cotter, K.; Forgac, M. Regulation of V-ATPase activity. Front. Biosci. Landmark Ed. 2017, 22, 609–622. [Google Scholar] [PubMed]
- Song, Q.; Meng, B.; Xu, H.; Mao, Z. The emerging roles of vacuolar-type ATPase-dependent Lysosomal acidification in neurodegenerative diseases. Transl. Neurodegener. 2020, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Abbas, Y.M.; Wu, D.; Bueler, S.A.; Robinson, C.V.; Rubinstein, J.L. Structure of V-ATPase from the mammalian brain. Science 2020, 367, 1240–1246. [Google Scholar] [CrossRef]
- Kitagawa, N.; Mazon, H.; Heck, A.J.R.; Wilkens, S. Stoichiometry of the peripheral stalk subunits E and G of yeast V1-ATPase determined by mass spectrometry. J. Biol. Chem. 2008, 283, 3329–3337. [Google Scholar] [CrossRef]
- Muench, S.P.; Huss, M.; Song, C.F.; Phillips, C.; Wieczorek, H.; Trinick, J.; Harrison, M.A. Cryo-electron microscopy of the vacuolar ATPase motor reveals its mechanical and regulatory complexity. J. Mol. Biol. 2009, 386, 989–999. [Google Scholar] [CrossRef]
- Mazhab-Jafari, M.T.; Rohou, A.; Schmidt, C.; Bueler, S.A.; Benlekbir, S.; Robinson, C.V.; Rubinstein, J.L. Atomic model for the membrane-embedded V(O) motor of a eukaryotic V-ATPase. Nature 2016, 539, 118–122. [Google Scholar] [CrossRef]
- Roh, S.H.; Stam, N.J.; Hryc, C.F.; Couoh-Cardel, S.; Pintilie, G.; Chiu, W.; Wilkens, S. The 3.5-Å CryoEM structure of nanodisc-reconstituted yeast vacuolar ATPase V(o) proton channel. Mol. Cell 2018, 69, 993–1004.e3. [Google Scholar] [CrossRef] [PubMed]
- Bonin, C.P.; Mann, R.S. A piggyBac transposon gene trap for the analysis of gene expression and function in drosophila. Genetics 2004, 167, 1801–1811. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Mu, L.L.; Jin, L.; Anjum, A.A.; Li, G.Q. Evaluation of three vacuolar ATPase genes as potential RNAi target in Henosepilachna vigintioctopunctata. J. Asia Pac. Entomol. 2021, 24, 55–63. [Google Scholar] [CrossRef]
- Xie, C.; Xiong, L.; Ye, M.; Shen, L.; Li, J.; Zhang, Z.; You, M.; You, S. Genome-wide analysis of V-ATPase genes in Plutella xylostella (L.) and the potential role of PxVHA-G1 in resistance to Bacillus thuringiensis Cry1Ac toxin. Int. J. Biol. Macromol. 2022, 194, 74–83. [Google Scholar] [CrossRef]
- Niu, R.C.; Meng, F.X.; Zeng, Q.H.; Wang, Y.J.; Liu, T.X.; Chu, D.; Zhang, S.Z. Comprehensive transcriptomic analyses of silk-associated genes and functional characterization of key silk fibroins in Plutella xylostella. Int. J. Mol. Sci. 2025, 26, 2842. [Google Scholar] [CrossRef]
- Ze, L.J.; Xu, P.; Kang, W.N.; Wu, J.J.; Jin, L.; Anjum, A.A.; Li, G.Q. Disruption of kynurenine pathway reveals physiological importance of tryptophan catabolism in Henosepilachna vigintioctopunctata. Amino Acids 2021, 53, 1091–1104. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Ze, L.J.; Kang, W.N.; Wu, J.J.; Jin, L.; Anjum, A.A.; Li, G.Q. Functional divergence of white genes in Henosepilachna vigintioctopunctata revealed by RNA interference. Insect Mol. Biol. 2020, 29, 466–476. [Google Scholar] [CrossRef]
- Wu, J.J.; Cheng, M.D.; Ze, L.J.; Shen, C.H.; Jin, L.; Li, G.Q. Dissecting the isoform-specific roles of FTZ-F1 in the larval-larval and larval-pupal ecdyses in Henosepilachna vigintioctopunctata. Insects 2022, 13, 228. [Google Scholar] [CrossRef]
- Hameed, M.S.; Wang, Z.; Vasseur, L.; Yang, G. Molecular Characterization and the Function of Argonaute3 in RNAi Pathway of Plutella xylostella. Int. J. Mol. Sci. 2018, 19, 1249. [Google Scholar] [CrossRef] [PubMed]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef]
- Huvenne, H.; Smagghe, G. Mechanisms of dsRNA uptake in insects and potential of RNAi for pest control: A review. J. Insect Physiol. 2010, 56, 227–235. [Google Scholar] [CrossRef]
- Baum, J.A.; Bogaert, T.; Clinton, W.; Heck, G.R.; Feldmann, P.; Ilagan, O.; Johnson, S.; Plaetinck, G.; Munyikwa, T.; Pleau, M.; et al. Control of coleopteran insect pests through RNA interference. Nat. Biotechnol. 2007, 25, 1322–1326. [Google Scholar] [CrossRef]
- Mao, Y.B.; Cai, W.J.; Wang, J.W.; Hong, G.J.; Tao, X.Y.; Wang, L.J.; Huang, Y.P.; Chen, X.Y. Silencing a cotton bollworm P450 monooxygenase gene by plant-mediated RNAi impairs larval tolerance of gossypol. Nat. Biotechnol. 2007, 25, 1307–1313. [Google Scholar] [CrossRef]
- Wytinck, N.; Manchur, C.L.; Li, V.H.; Whyard, S.; Belmonte, M.F. dsRNA uptake in plant pests and pathogens: Insights into RNAi-based insect and fungal control technology. Plants 2020, 9, 1780. [Google Scholar] [CrossRef]
- Yan, S.; Ren, B.; Zeng, B.; Shen, J. Improving RNAi efficiency for pest control in crop species. Biotechniques 2020, 68, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Zotti, M.; Dos Santos, E.A.; Cagliari, D.; Christiaens, O.; Taning, C.N.T.; Smagghe, G. RNA interference technology in crop protection against arthropod pests, pathogens and nematodes. Pest Manag. Sci. 2018, 74, 1239–1250. [Google Scholar] [CrossRef]
- Spit, J.; Philips, A.; Wynant, N.; Santos, D.; Plaetinck, G.; Vanden Broeck, J. Knockdown of nuclease activity in the gut enhances RNAi efficiency in the Colorado potato beetle, Leptinotarsa decemlineata, but not in the desert locust, Schistocerca gregaria. Insect Biochem. Mol. Biol. 2017, 81, 103–116. [Google Scholar] [CrossRef]
- Luo, J.; Liang, S.; Li, J.; Xu, Z.; Li, L.; Zhu, B.; Li, Z.; Lei, C.; Lindsey, K.; Chen, L.; et al. A transgenic strategy for controlling plant bugs (Adelphocoris suturalis) through expression of double-stranded RNA homologous to fatty acyl-coenzyme A reductase in cotton. New Phytol. 2017, 215, 1173–1185. [Google Scholar] [CrossRef]
- Xu, L.; Duan, X.; Lv, Y.; Zhang, X.; Nie, Z.; Xie, C.; Ni, Z.; Liang, R. Silencing of an aphid carboxylesterase gene by use of plant-mediated RNAi impairs Sitobion avenae tolerance of Phoxim insecticides. Transgenic Res. 2014, 23, 389–396. [Google Scholar] [CrossRef]
- Mao, J.; Zeng, F. Plant-mediated RNAi of a gap gene-enhanced tobacco tolerance against the Myzus persicae. Transgenic Res. 2014, 23, 145–152. [Google Scholar] [CrossRef]
- Gunning, P.W.; Ghoshdastider, U.; Whitaker, S.; Popp, D.; Robinson, R.C. The evolution of compositionally and functionally distinct actin filaments. J. Cell Sci. 2015, 128, 2009–2019. [Google Scholar] [CrossRef] [PubMed]
- San Miguel, K.; Scott, J.G. The next generation of insecticides: dsRNA is stable as a foliar-applied insecticide. Pest Manag. Sci. 2016, 72, 801–809. [Google Scholar] [CrossRef]
- Park, M.H. The post-translational synthesis of a polyamine-derived amino acid, hypusine, in the eukaryotic translation initiation factor 5A (eIF5A). J. Biochem. 2006, 139, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Christiaens, O.; Iga, M.; Velarde, R.A.; Rougé, P.; Smagghe, G. Halloween genes and nuclear receptors in ecdysteroid biosynthesis and signalling in the pea aphid. Insect Mol. Biol. 2010, 19, 187–200. [Google Scholar] [CrossRef]
- Wu, J.J.; Mu, L.L.; Kang, W.N.; Ze, L.J.; Shen, C.H.; Jin, L.; Anjum, A.A.; Li, G.Q. RNA interference targeting ecdysone receptor blocks the larval-pupal transition in Henosepilachna vigintioctopunctata. Insect Sci. 2021, 28, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.Y.; Deng, P.; Zhang, Q.; Li, A.; Fu, K.Y.; Guo, W.C.; Li, G.Q. Ecdysone receptor isoforms play distinct roles in larval-pupal-adult transition in Leptinotarsa decemlineata. Insect Sci. 2020, 27, 487–499. [Google Scholar] [CrossRef]
- Abe, M.; Saito, M.; Tsukahara, A.; Shiokawa, S.; Ueno, K.; Shimamura, H.; Nagano, M.; Toshima, J.Y.; Toshima, J. Functional complementation reveals that 9 of the 13 human V-ATPase subunits can functionally substitute for their yeast orthologs. J. Biol. Chem. 2019, 294, 8273–8285. [Google Scholar] [CrossRef]
- Wieczorek, H.; Beyenbach, K.W.; Huss, M.; Vitavska, O. Vacuolar-type proton pumps in insect epithelia. J. Exp. Biol. 2009, 212, 1611–1619. [Google Scholar] [CrossRef] [PubMed]
- Muench, S.P.; Scheres, S.H.; Huss, M.; Phillips, C.; Vitavska, O.; Wieczorek, H.; Trinick, J.; Harrison, M.A. Subunit positioning and stator filament stiffness in regulation and power transmission in the V1 motor of the Manduca sexta V-ATPase. J. Mol. Biol. 2014, 426, 286–300. [Google Scholar] [CrossRef]
- Liu, X.J.; Liang, X.Y.; Guo, J.; Shi, X.K.; Merzendorfer, H.; Zhu, K.Y.; Zhang, J.Z. V-ATPase subunit a is required for survival and midgut development of Locusta migratoria. Insect Mol. Biol. 2022, 31, 60–72. [Google Scholar] [CrossRef]
- Ma, Y.F.; Zhao, Y.Q.; Zhou, Y.Y.; Feng, H.Y.; Gong, L.L.; Zhang, M.Q.; Hull, J.J.; Dewer, Y.; Roy, A.; Smagghe, G.; et al. Nanoparticle-delivered RNAi-based pesticide target screening for the rice pest white-backed planthopper and risk assessment for a natural predator. Sci. Total Environ. 2024, 926, 171286. [Google Scholar] [CrossRef]
- Guan, R.B.; Li, H.C.; Fan, Y.J.; Hu, S.R.; Christiaens, O.; Smagghe, G.; Miao, X.X. A nuclease specific to lepidopteran insects suppresses RNAi. J. Biol. Chem. 2018, 293, 6011–6021. [Google Scholar] [CrossRef] [PubMed]
- Shukla, J.N.; Kalsi, M.; Sethi, A.; Narva, K.E.; Fishilevich, E.; Singh, S.; Mogilicherla, K.; Palli, S.R. Reduced stability and intracellular transport of dsRNA contribute to poor RNAi response in lepidopteran insects. RNA Biol. 2016, 13, 656–669. [Google Scholar] [CrossRef]
- Wang, K.; Peng, Y.; Pu, J.; Fu, W.; Wang, J.; Han, Z. Variation in RNAi efficacy among insect species is attributable to dsRNA degradation in vivo. Insect Biochem. Mol. Biol. 2016, 77, 1–9. [Google Scholar] [CrossRef]
- Liu, J.; He, Q.; Lin, X.; Smagghe, G. Recent progress in nanoparticle-mediated RNA interference in insects: Unveiling new frontiers in pest control. J. Insect Physiol. 2025, 104884. [Google Scholar] [CrossRef] [PubMed]
- Sandal, S.; Singh, S.; Bansal, G.; Kaur, R.; Mogilicherla, K.; Pandher, S.; Roy, A.; Kaur, G.; Rathore, P.; Kalia, A. Nanoparticle-Shielded dsRNA Delivery for Enhancing RNAi Efficiency in Cotton Spotted Bollworm Earias vittella (Lepidoptera: Nolidae). Int. J. Mol. Sci. 2023, 24, 9161. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Wang, Q.; Kang, Z.H.; Wen, J.X.; Yang, Y.B.; Lu, X.J.; Guo, W.; Zhao, D. Novel Environmentally Friendly RNAi Biopesticides: Targeting V-ATPase in Holotrichia parallela Larvae Using Layered Double Hydroxide Nanocomplexes. J. Agric. Food Chem. 2024, 72, 11381–11391. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, X.; Luo, J.; Lu, L.; Zhu, L.; Wang, S.; Yang, K.; Wan, X.; Wu, Y.; Akmal, B.; Wu, G.; et al. RNAi of vATPasea Affects Survival and Larval-Pupal Development in Plutella xylostella. Insects 2025, 16, 1054. https://doi.org/10.3390/insects16101054
Yu X, Luo J, Lu L, Zhu L, Wang S, Yang K, Wan X, Wu Y, Akmal B, Wu G, et al. RNAi of vATPasea Affects Survival and Larval-Pupal Development in Plutella xylostella. Insects. 2025; 16(10):1054. https://doi.org/10.3390/insects16101054
Chicago/Turabian StyleYu, Xuetao, Jinhua Luo, Lin Lu, Li Zhu, Siyuan Wang, Kang Yang, Xia Wan, Yuhua Wu, Boboev Akmal, Gang Wu, and et al. 2025. "RNAi of vATPasea Affects Survival and Larval-Pupal Development in Plutella xylostella" Insects 16, no. 10: 1054. https://doi.org/10.3390/insects16101054
APA StyleYu, X., Luo, J., Lu, L., Zhu, L., Wang, S., Yang, K., Wan, X., Wu, Y., Akmal, B., Wu, G., Yan, X., & Shen, C. (2025). RNAi of vATPasea Affects Survival and Larval-Pupal Development in Plutella xylostella. Insects, 16(10), 1054. https://doi.org/10.3390/insects16101054





