The Impact of Systemic Insecticides: Cyantraniliprole and Flupyradifurone on the Mortality of Athalia rosae (Hymenoptera: Tenthredinidae) Based on the Biophoton-Emission of Oilseed Rape
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Parameters and Settings
- untreated;
- cyantraniliprole seed treatment;
- cyantraniliprole in-crop treatment;
- flupyradifurone seed treatment;
- flupyradifurone in-crop treatment.
2.2. Non-Invasive Imaging Methodology
2.3. Evaluation and Statistical Analysis
3. Results
3.1. Effect of Active Ingredients on Insect Mortality
3.2. The Extent of Chewing the Oilseed Rape Leaves
3.3. Results of Delayed Fluorescence Response to the Different Application
3.4. Results of Ultraweak Bioluminescence
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#data/QCL/visualize (accessed on 29 November 2024).
- Williams, I.H. The Major Insect Pests of Oilseed Rape in Europe and Their Management: An Overview. In Biocontrol-Based Integrated Management of Oilseed Rape Pests; Springer: Dordrecht, The Netherlands, 2010; pp. 1–43. [Google Scholar] [CrossRef]
- Alford, D.V.; Nilsson, C.; Ulber, B. Insect Pests of Oilseed Rape Crops. In Biocontrol of Oilseed Rape Pests; Wiley: Hoboken, NJ, USA, 2003; pp. 9–42. [Google Scholar] [CrossRef]
- Oishi, K.; Sawa, M.; Hatakeyama, M.; Kageyama, Y. Genetics and Biology of the Sawfly, Athalia Rosae (Hymenoptera)—Review. Genetica 1993, 88, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Insecticide Resistance Action Committee. Available online: https://irac-online.org/?s=MoA%3A28 (accessed on 29 November 2024).
- Nauen, R.; Jeschke, P.; Velten, R.; Beck, M.E.; Ebbinghaus-Kintscher, U.; Thielert, W.; Wölfel, K.; Haas, M.; Kunz, K.; Raupach, G. Flupyradifurone: A Brief Profile of a New Butenolide Insecticide. Pest Manag. Sci. 2015, 71, 850–862. [Google Scholar] [CrossRef]
- Pes, M.P.; Melo, A.A.; Stacke, R.S.; Zanella, R.; Perini, C.R.; Silva, F.M.A.; Carús Guedes, J.V. Translocation of Chlorantraniliprole and Cyantraniliprole Applied to Corn as Seed Treatment and Foliar Spraying to Control Spodoptera Frugiperda (Lepidoptera: Noctuidae). PLoS ONE 2020, 15, e0229151. [Google Scholar] [CrossRef] [PubMed]
- Fang, N.; Zhao, X.; Li, Y.; Luo, Y.; Wang, X.; He, H.; Zhang, C.; Jiang, J. Uptake, Translocation and Subcellular Distribution of Broflanilide, Afidopyropen, and Flupyradifurone in Mustard (Brassica Juncea). J. Hazard. Mater. 2023, 452, 131381. [Google Scholar] [CrossRef] [PubMed]
- Barry, J.D.; Portillo, H.E.; Annan, I.B.; Cameron, R.A.; Clagg, D.G.; Dietrich, R.F.; Watson, L.J.; Leighty, R.M.; Ryan, D.L.; Mcmillan, J.A.; et al. Movement of Cyantraniliprole in Plants after Foliar Applications and Its Impact on the Control of Sucking and Chewing Insects. Pest Manag. Sci. 2015, 71, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Lahm, G.P.; Selby, T.P.; Freudenberger, J.H.; Stevenson, T.M.; Myers, B.J.; Seburyamo, G.; Smith, B.K.; Flexner, L.; Clark, C.E.; Cordova, D. Insecticidal Anthranilic Diamides: A New Class of Potent Ryanodine Receptor Activators. Bioorg. Med. Chem. Lett. 2005, 15, 4898–4906. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, A.K.; Qi, S.; Sarpong, R.; Casida, J.E. Insect Ryanodine Receptor: Distinct but Coupled Insecticide Binding Sites for [N-C3H3]Chlorantraniliprole, Flubendiamide, and [3H]Ryanodine. Chem. Res. Toxicol. 2012, 25, 1571–1573. [Google Scholar] [CrossRef]
- Boaventura, D.; Bolzan, A.; Padovez, F.E.O.; Okuma, D.M.; Omoto, C.; Nauen, R. Detection of a Ryanodine Receptor Target-Site Mutation in Diamide Insecticide Resistant Fall Armyworm, Spodoptera Frugiperda. Pest Manag. Sci. 2020, 76, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Nauen, R. Insecticide Mode of Action: Return of the Ryanodine Receptor. Pest Manag. Sci. 2006, 62, 690–692. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.D.; Seo, M.; Stelinski, L.L. Behavioral and Hormetic Effects of the Butenolide Insecticide, Flupyradifurone, on Asian Citrus Psyllid, Diaphorina Citri. Crop Prot. 2017, 98, 102–107. [Google Scholar] [CrossRef]
- Issa, K.A.; Wosula, E.N.; Stephano, F.; Legg, J.P. Evaluation of the Efficacy of Flupyradifurone against Bemisia Tabaci on Cassava in Tanzania. Insects 2022, 13, 920. [Google Scholar] [CrossRef]
- Gerbovits, B.; Keszthelyi, S.; Jócsák, I. Biophoton Emission-Based Approach of the Effects of Systemic Insecticides on the Survival of Eurydema Ventralis Kolenati, 1846 (Hemiptera: Pentatomidae) and on the Photosynthetic Activity of Oilseed Rape. J. Environ. Sci. Health Part B 2024, 59, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Howald, R.; Zwahlen, C.; Nentwig, W. Evaluation of Bt Oilseed Rape on the Non-Target Herbivore Athalia Rosae. Entomol. Exp. Appl. 2003, 106, 87–93. [Google Scholar] [CrossRef]
- Ștef, R.; Grozea, I.; Cotuna, O.; Sărățeanu, V.; Iamandei, M.; Duma Copcea, A.; Iuliana EPURE, L.; Manea, D.; Carabet, A. Use of Acetamiprid in the Management of Athalia Rosae Population from Oilseed Rape Agroecosystem. Sci. Pap. Ser. A Agron. 2023, 66, 409–418. [Google Scholar]
- Hideg, È. On the Spontaneous Ultraweak Light Emission of Plants. J. Photochem. Photobiol. B 1993, 18, 239–244. [Google Scholar] [CrossRef]
- Rastogi, A.; Pospíšil, P. Ultra-Weak Photon Emission as a Non-Invasive Tool for the Measurement of Oxidative Stress Induced by UVA Radiation in Arabidopsis Thaliana. J. Photochem. Photobiol. B 2013, 123, 59–64. [Google Scholar] [CrossRef]
- Wilson, T.; Woodland Hastings, J. Bioluminescence. Annu. Rev. Cell Dev. Biol. 1998, 14, 197–230. [Google Scholar] [CrossRef] [PubMed]
- Jócsák, I.; Malgwi, I.; Rabnecz, G.; Szegő, A.; Varga-Visi, É.; Végvári, G.; Pónya, Z. Effect of Cadmium Stress on Certain Physiological Parameters, Antioxidative Enzyme Activities and Biophoton Emission of Leaves in Barley (Hordeum vulgare L.) Seedlings. PLoS ONE 2020, 15, e0240470. [Google Scholar] [CrossRef] [PubMed]
- Lukács, H.; Jócsák, I.; Somfalvi-Tóth, K.; Keszthelyi, S. Physiological Responses Manifested by Some Conventional Stress Parameters and Biophoton Emission in Winter Wheat as a Consequence of Cereal Leaf Beetle Infestation. Front. Plant Sci. 2022, 13, 839855. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Moreiras, A.M.; Graña, E.; Reigosa, M.J.; Araniti, F. Imaging of Chlorophyll a Fluorescence in Natural Compound-Induced Stress Detection. Front. Plant Sci. 2020, 11, 583590. [Google Scholar] [CrossRef]
- Jócsák, I.; Gyalog, H.; Hoffmann, R.; Somfalvi-Tóth, K. In-Vivo Biophoton Emission, Physiological and Oxidative Responses of Biostimulant-Treated Winter Wheat (Triticum Eastivum L.) as Seed Priming Possibility, for Heat Stress Alleviation. Plants 2022, 11, 640. [Google Scholar] [CrossRef] [PubMed]
- Jócsák, I.; Csima, F.; Somfalvi-Tóth, K. Alterations of Photosynthetic and Oxidative Processes Influenced by the Presence of Different Zinc and Cadmium Concentrations in Maize Seedlings: Transition from Essential to Toxic Functions. Plants 2024, 13, 1150. [Google Scholar] [CrossRef] [PubMed]
- King, A.P.; Eckersley, R.J. Inferential Statistics IV: Choosing a Hypothesis Test. Stat. Biomed. Eng. Sci. 2019, 16, 147–171. [Google Scholar] [CrossRef]
- Ghasemi, A.; Zahediasl, S. Normality Tests for Statistical Analysis: A Guide for Non-Statisticians. Int. J. Endocrinol. Metab. 2012, 10, 486–489. [Google Scholar] [CrossRef]
- Abbott, W.S. A Method of Computing the Effectiveness of an Insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Teixeira, L.A.; Andaloro, J.T. Diamide Insecticides: Global Efforts to Address Insect Resistance Stewardship Challenges. Pestic Biochem. Physiol. 2013, 106, 76–78. [Google Scholar] [CrossRef]
- Keszthelyi, S.; Gerbovits, B.; Jócsák, I. Impact Analysis of Different Applications of Cyantraniliprole on Control of Horse Chestnut Leaf Miner (Cameraria ohridella) Larvae Supported by Biophoton Emission. Biol. Futur. 2023, 74, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Roditakis, E.; Stavrakaki, M.; Grispou, M.; Achimastou, A.; Van Waetermeulen, X.; Nauen, R.; Tsagkarakou, A. Flupyradifurone Effectively Manages Whitefly Bemisia Tabaci MED (Hemiptera: Aleyrodidae) and Tomato Yellow Leaf Curl Virus in Tomato. Pest Manag. Sci. 2017, 73, 1574–1584. [Google Scholar] [CrossRef] [PubMed]
- Molnár, I.; Somlyay, I.; Tóth, E.; Farkas, I. Cyazypyr® (Ciantraniliprol) Hatóanyagú Magcsávázó Szer a Káposztalégy Ellen (Delia radicum L.) Őszi Káposztarepcében. In A Multidisciplinary Journal in Agricultural Sciences; Georgikon for Agriculture: Keszthely, Hungary, 2016; Volume 20, pp. 88–92. [Google Scholar]
- Stamm, M.D.; Heng-Moss, T.M.; Baxendale, F.P.; Siegfried, B.D.; Blankenship, E.E.; Nauen, R. Uptake and Translocation of Imidacloprid, Clothianidin and Flupyradifurone in Seed-Treated Soybeans. Pest Manag. Sci. 2016, 72, 1099–1109. [Google Scholar] [CrossRef]
- Campbell, J.W.; Cabrera, A.R.; Stanley-Stahr, C.; Ellis, J.D. An Evaluation of the Honey Bee (Hymenoptera: Apidae) Safety Profile of a New Systemic Insecticide, Flupyradifurone, Under Field Conditions in Florida. J. Econ. Entomol. 2016, 109, 1967–1972. [Google Scholar] [CrossRef]
- Hesselbach, H.; Scheiner, R. Effects of the Novel Pesticide Flupyradifurone (Sivanto) on Honeybee Taste and Cognition. Sci. Rep. 2018, 8, 4954. [Google Scholar] [CrossRef]
- Prasad, A.; Sedlářová, M.; Kale, R.S.; Pospíšil, P. Lipoxygenase in Singlet Oxygen Generation as a Response to Wounding: In Vivo Imaging in Arabidopsis Thaliana. Sci. Rep. 2017, 7, 9831. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.; Gouripeddi, P.; Devireddy, H.R.N.; Ovsii, A.; Rachakonda, D.P.; Van Wijk, R.; Pospíšil, P. Spectral Distribution of Ultra-Weak Photon Emission as a Response to Wounding in Plants: An In Vivo Study. Biology 2020, 9, 139. [Google Scholar] [CrossRef] [PubMed]

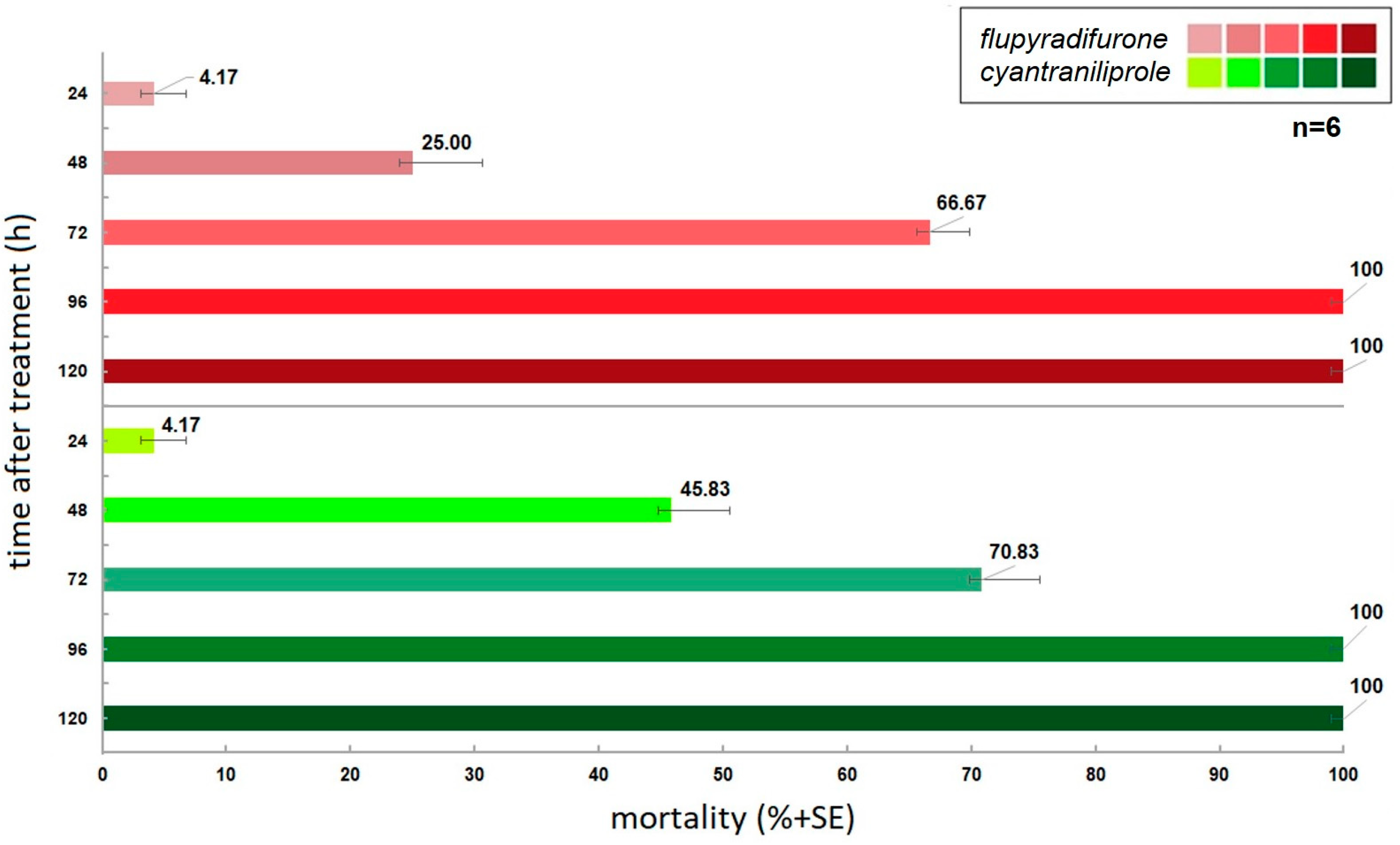



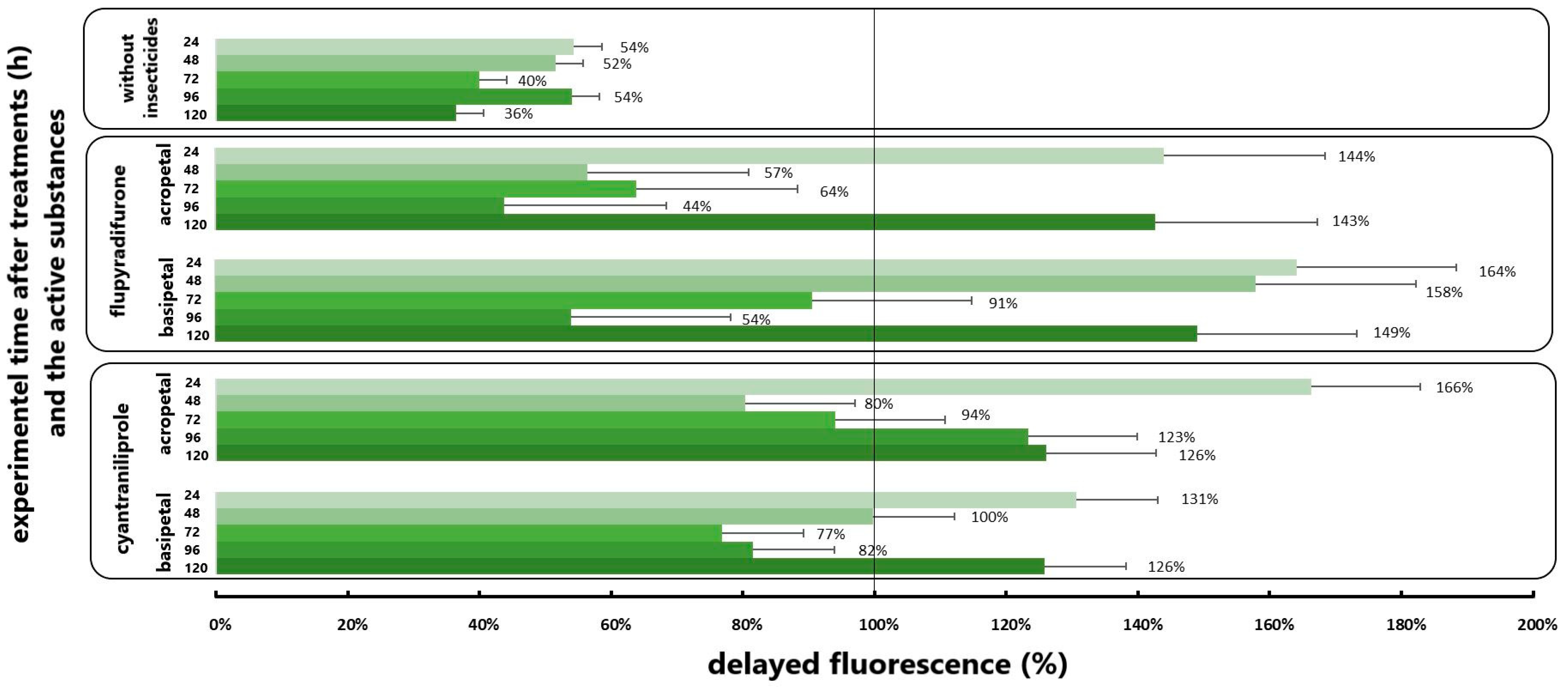
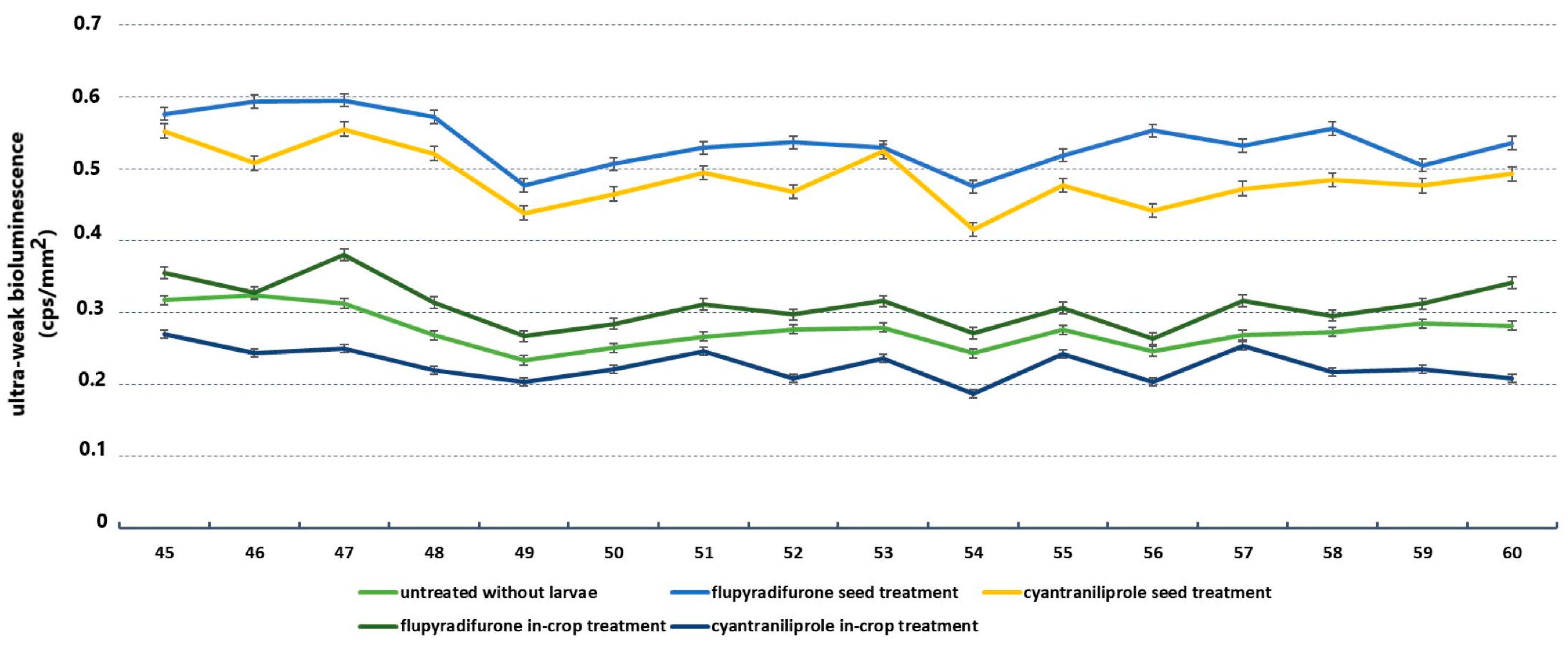

| two-way ANOVA | Treatments | DF | F | p | Mean(Mortality %) |
| active ingredients/time | 1 | 50.061 | <0.0001 * | flupyradifurone basipetal: 59.77% acropetal: 53.33% | |
| basipetal/time | 1 | 35.142 | <0.0001 * | ||
| acropetal/time | 1 | 29.005 | <0.0001 * | cyantraniliprole basipetal: 64.17% acropetal: 54.166% | |
| cyantraniliprole/flupyradifurone | 1 | 0.576 | >0.05 |
| DF | F | p | Mean (cps/mm2) | ||
|---|---|---|---|---|---|
| without larvae | flupyradifurone acropetal/flupyradifurone basipetal | 1 | 5.405 | <0.04 * | 567.42:350.83 |
| cyantraniliprole acropetal/flupyradifurone acropetal | 1 | 2.621 | >0.05 | 392.70:567.42 | |
| cyantraniliprole basipetal/flupyradifurone basipetal | 1 | 1.876 | >0.05 | 422.46:350.83 | |
| with larvae | Untreated/flupyradifurone acropetal | 1 | 5.388 | <0.04 * | 300.53:549.39 |
| Untreated/flupyradifurone basipetal | 1 | 6.721 | <0.02 * | 300.53:539.96 | |
| Untreated/cyantraniliprole acropetal | 1 | 0.972 | >0.05 | 300.53:461.74 | |
| Untreated/cyantraniliprole basipetal | 1 | 6.897 | <0.03 * | 300.53:382.12 | |
| flupyradifurone acropetal/flupyradifurone basipetal | 1 | 0.005 | >0.05 | 549.39:539.96 | |
| cyantraniliprole acropetal/cyantraniliprole basipetal | 1 | 0.936 | >0.05 | 461.74:382.12 | |
| two-way ANOVA | Treatments | DF | F | p | Mean (cps/mm2) |
| Untreated/active ingredients | 3 | 313.53 | <0.0001 * | 0.28:0.40 | |
| flupyradifurone acropetal/cyantraniliprole acropetal | 1 | 270.959 | <0.0001 * | 0.39:0.56 | |
| flupyradifurone basipetal/cyantraniliprole basipetal | 1 | 757.901 | <0.0001 * | 0.46:0.25 | |
| flupyradifurone acropetal/cyantraniliprole basipetal | 1 | 314.476 | <0.0001 * | 0.39:0.25 | |
| flupyradifurone basipetal/cyantraniliprole acropetal | 1 | 94.435 | <0.0001 * | 0.46:0.56 | |
| cyantraniliprole acropetal/cyantraniliprole basipetal | 1 | 531.282 | <0.0001 * | 0.56:0.25 | |
| flupyradifurone acropetal/flupyradifurone basipetal | 1 | 255.704 | <0.0001 * | 0.39:0.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerbovits, B.; Jócsák, I.; Keszthelyi, S. The Impact of Systemic Insecticides: Cyantraniliprole and Flupyradifurone on the Mortality of Athalia rosae (Hymenoptera: Tenthredinidae) Based on the Biophoton-Emission of Oilseed Rape. Insects 2025, 16, 35. https://doi.org/10.3390/insects16010035
Gerbovits B, Jócsák I, Keszthelyi S. The Impact of Systemic Insecticides: Cyantraniliprole and Flupyradifurone on the Mortality of Athalia rosae (Hymenoptera: Tenthredinidae) Based on the Biophoton-Emission of Oilseed Rape. Insects. 2025; 16(1):35. https://doi.org/10.3390/insects16010035
Chicago/Turabian StyleGerbovits, Bálint, Ildikó Jócsák, and Sándor Keszthelyi. 2025. "The Impact of Systemic Insecticides: Cyantraniliprole and Flupyradifurone on the Mortality of Athalia rosae (Hymenoptera: Tenthredinidae) Based on the Biophoton-Emission of Oilseed Rape" Insects 16, no. 1: 35. https://doi.org/10.3390/insects16010035
APA StyleGerbovits, B., Jócsák, I., & Keszthelyi, S. (2025). The Impact of Systemic Insecticides: Cyantraniliprole and Flupyradifurone on the Mortality of Athalia rosae (Hymenoptera: Tenthredinidae) Based on the Biophoton-Emission of Oilseed Rape. Insects, 16(1), 35. https://doi.org/10.3390/insects16010035









