Impacts of Sublethal Doses of Spinetoram on the Biological Traits and Detoxifying Enzymes of the Tomato Leaf Miner, Tuta absoluta (Lepidoptera: Gelechiidae)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of T. absoluta
2.2. Assessment of Acute Toxicity
2.3. Treatment with Sublethal Spinetoram Concentrations
2.4. Assay of Detoxification Enzymes
2.5. Statistical Analysis
3. Results
3.1. Assessment of the Toxicity Test of Spinetoram
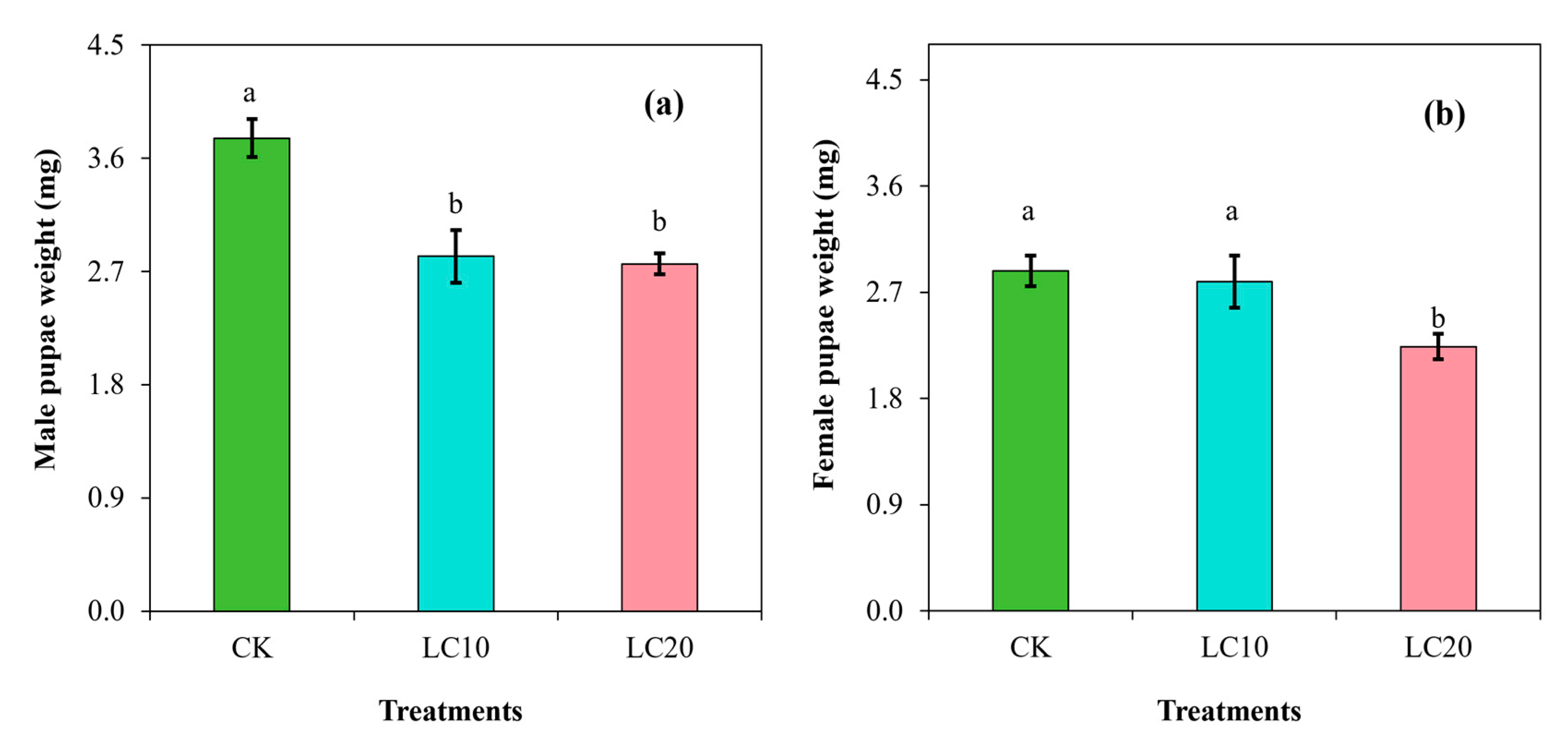
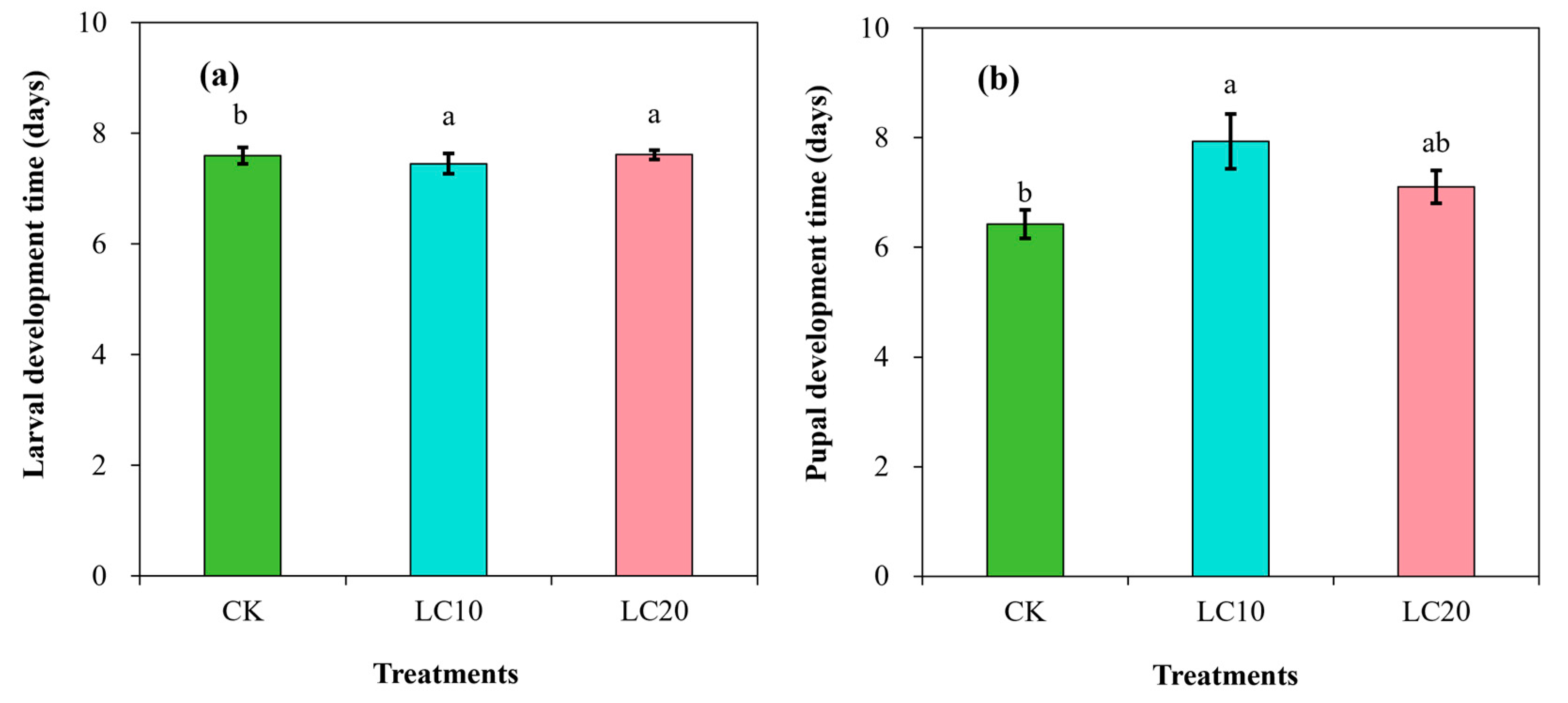
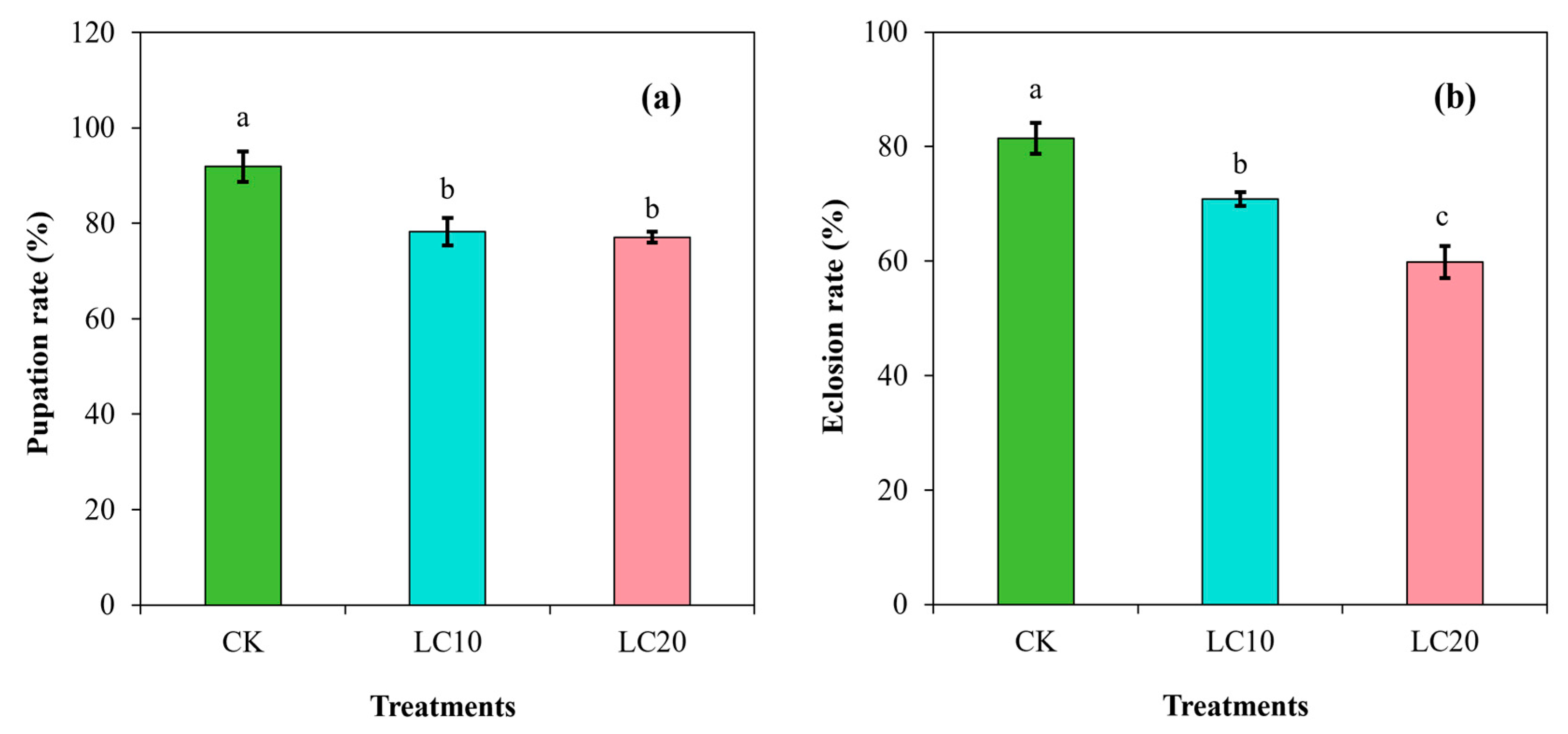
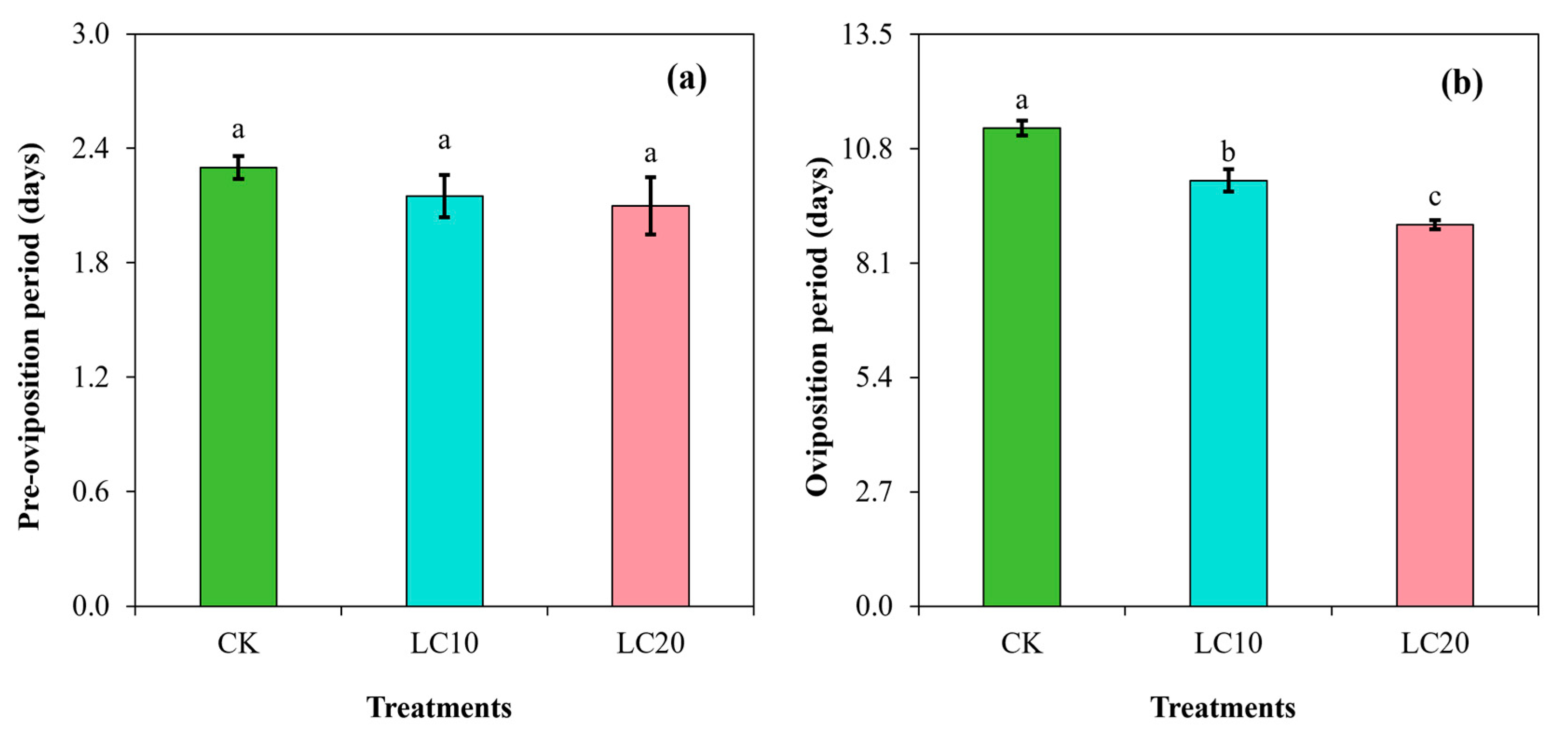
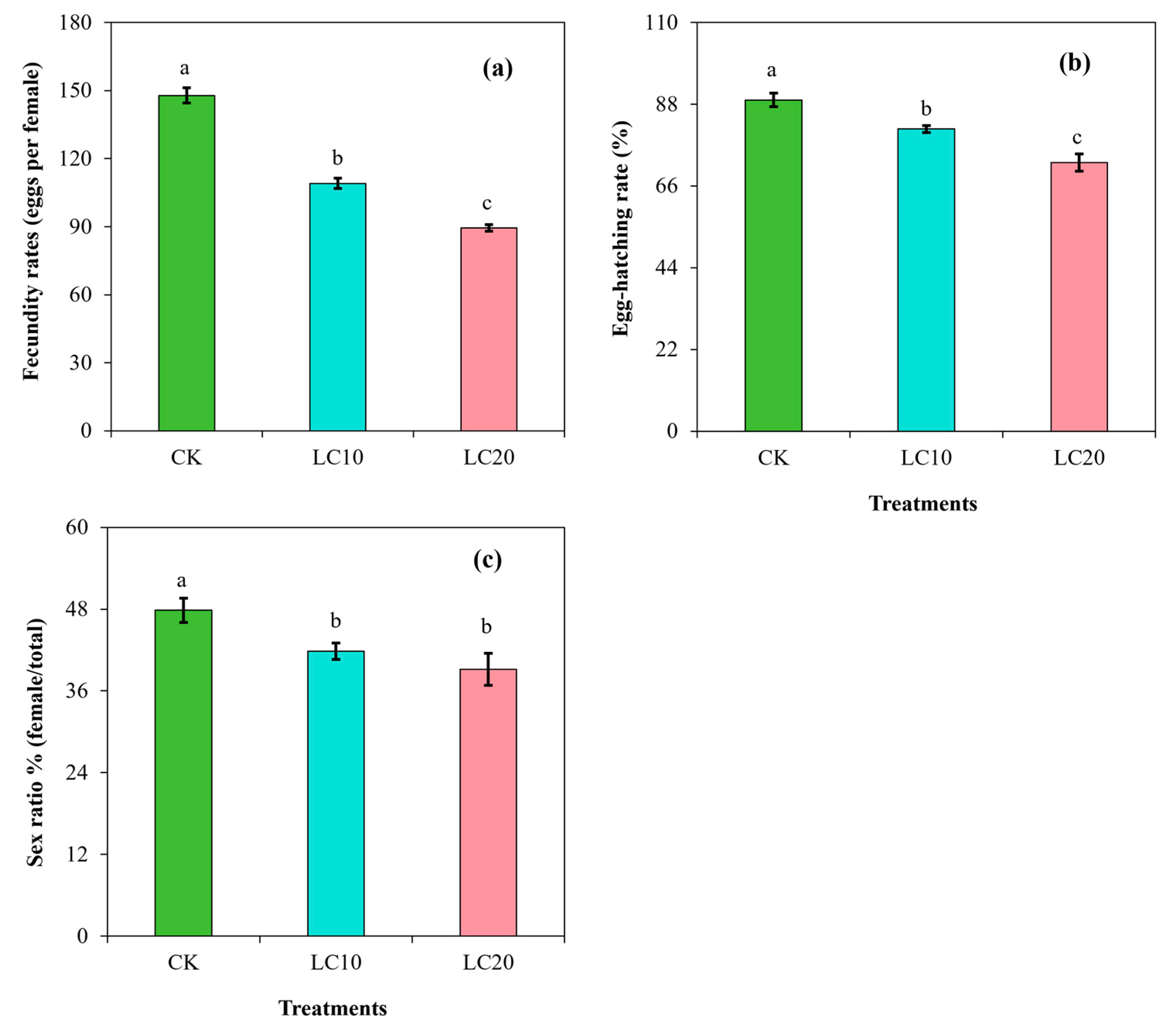
3.2. Spinetoram Sublethal Effect on the Biological Characteristics of T. absoluta
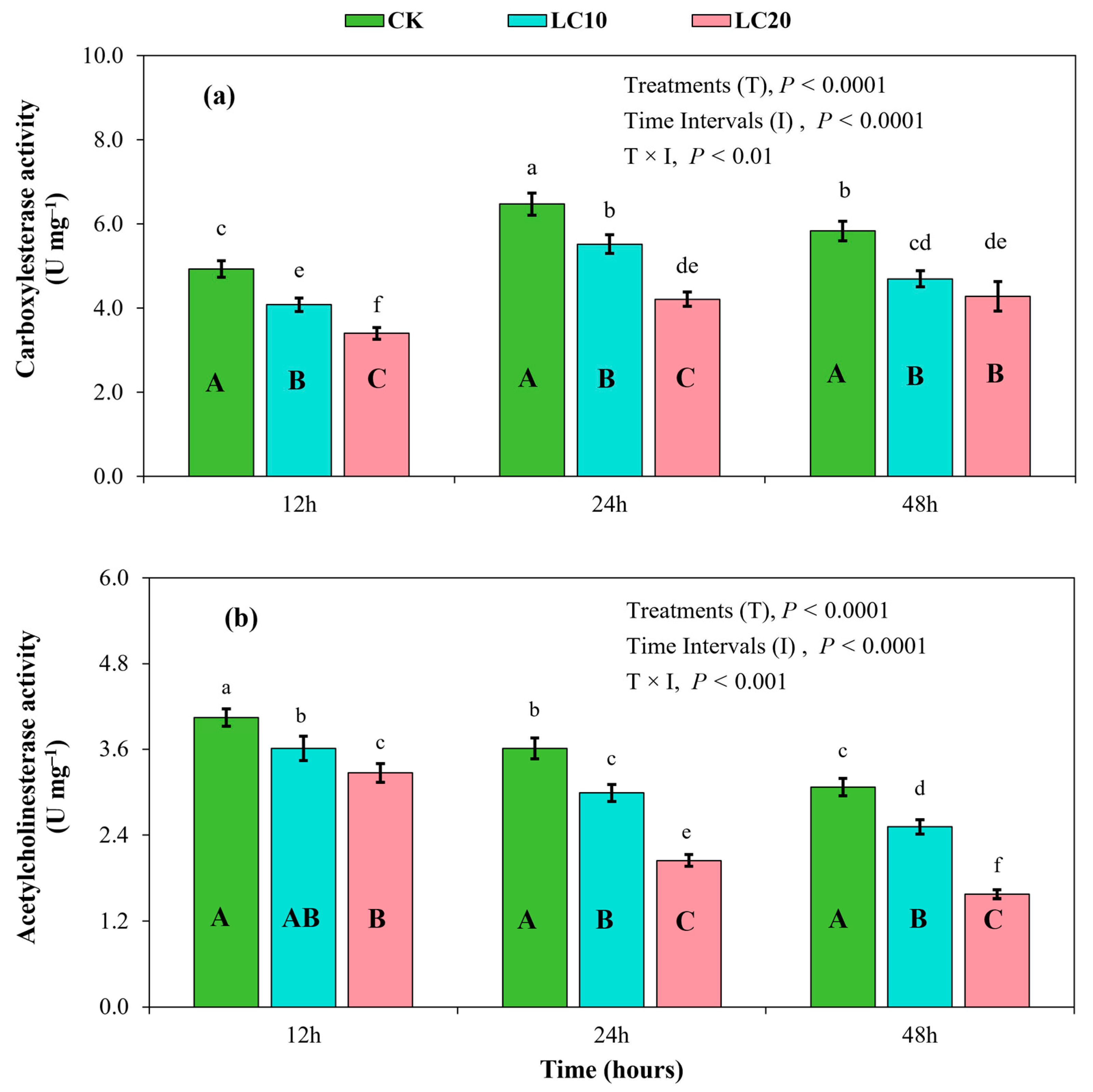
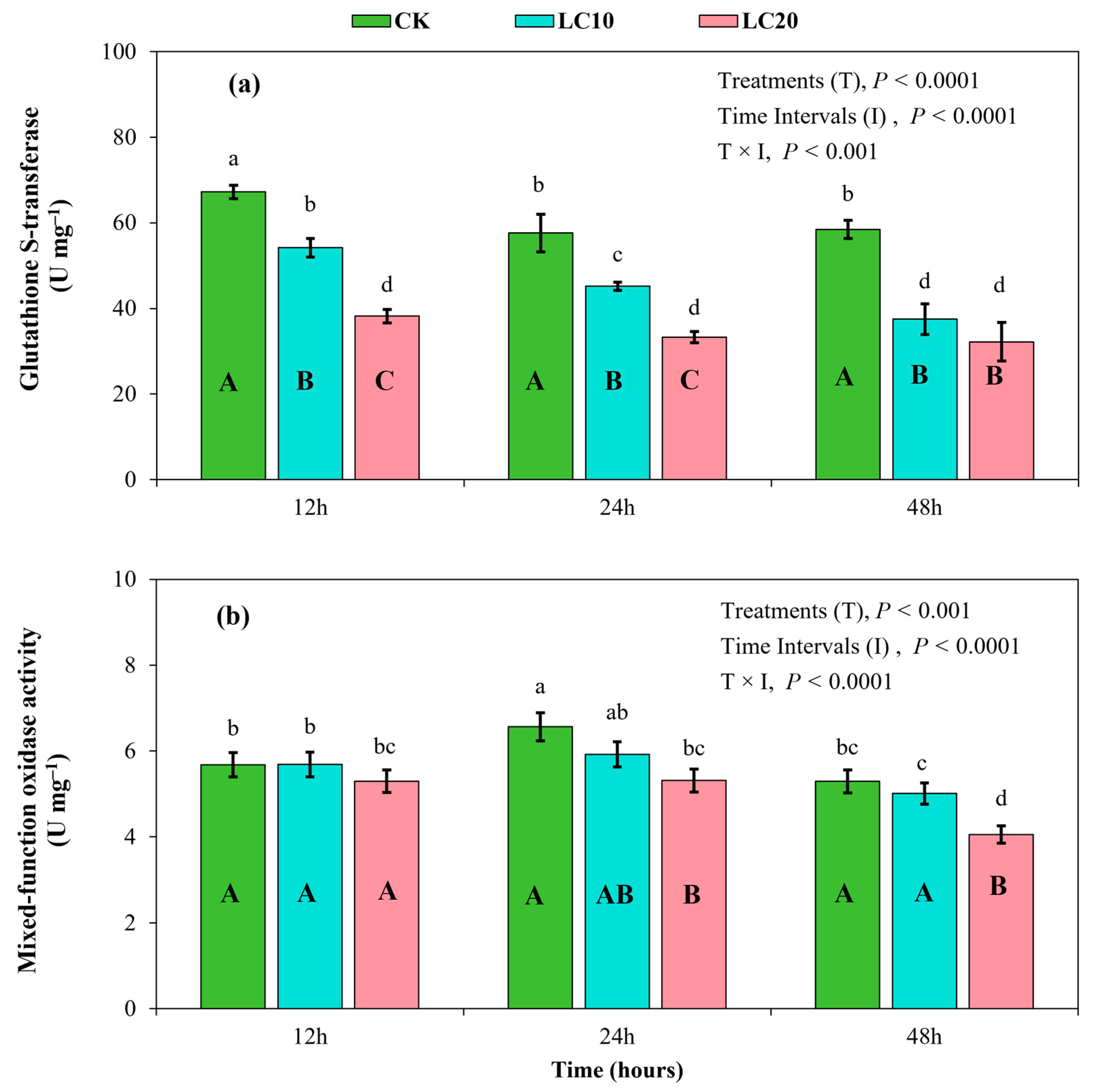
3.3. Spinetoram Sublethal Effects on the Detoxification Enzymes of T. absoluta Larvae
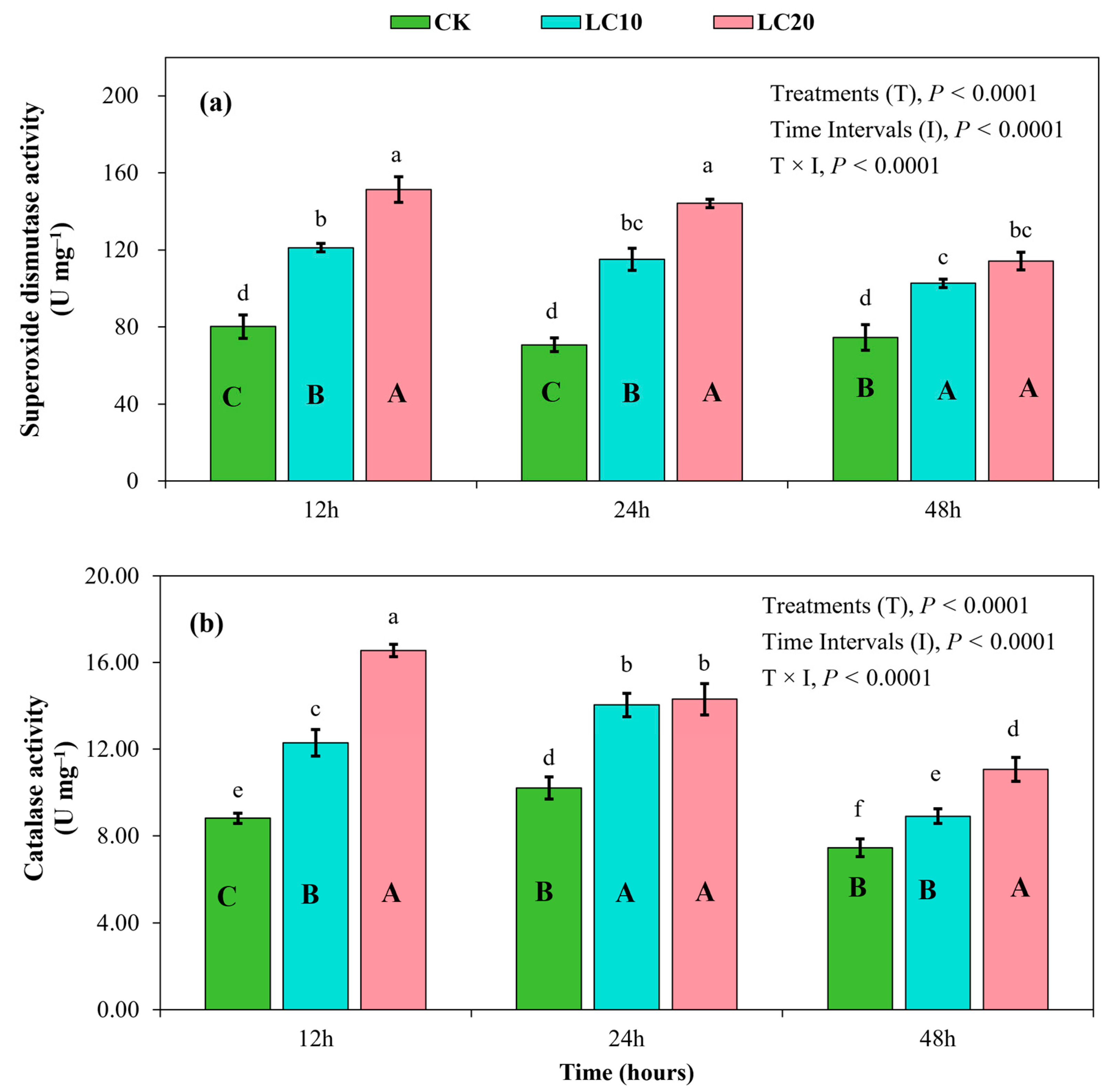
3.4. Spinetoram Sublethal Effect on Antioxidant Enzymes in T. absoluta Larvae
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Khalil, M.I.I.; Youssef, S.A.; Tartoura, K.A.; Eldesoky, A.A. Comparative Evaluation of Physiological and Biochemical Alteration in Tomato Plants Infected by Alternaria Alternata in Response to Trichoderma Viride and Chaetomium Globosum Application. Physiol. Mol. Plant Pathol. 2021, 115, 101671. [Google Scholar] [CrossRef]
- Zheng, M.; Deng, Y.; Zhou, Y.; Liu, R.; Liu, Y.; Wang, H.; Zhu, W.; Zhou, Z.; Diao, J. Multifaceted Effects of Difenoconazole in Tomato Fruit Ripening: Physiology, Flavour and Nutritional Quality. Plant Physiol. Biochem. 2023, 194, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Sharifi-Rad, R.; Sharopov, F.; Namiesnik, J.; Roointan, A.; Kamle, M.; Kumar, P.; Martins, N.; Sharifi-Rad, J. Beneficial Effects and Potential Risks of Tomato Consumption for Human Health: An Overview. Nutrition 2019, 62, 201–208. [Google Scholar] [CrossRef]
- Ahmed, S.S.; Kader, M.H.A.; Fahmy, M.A.M.; Abdelgawad, K.F. Control of Tuta Absoluta (Lepidoptera: Gelechiidae) by the New Trend of Photosensitizer and Nanocomposites and Their Effects on Productivity and Storability of Tomato. Int. J. Trop. Insect Sci. 2024, 44, 273–296. [Google Scholar] [CrossRef]
- FAO. World Food and Agriculture—Statistical Yearbook 2023; FAO: Rome, Italy, 2023. [Google Scholar] [CrossRef]
- Biondi, A.; Guedes, R.N.C.; Wan, F.H.; Desneux, N. Ecology, Worldwide Spread, and Management of the Invasive South American Tomato Pinworm, Tuta Absoluta: Past, Present, and Future. Annu. Rev. Entomol. 2018, 63, 239–258. [Google Scholar] [CrossRef] [PubMed]
- Cuthbertson, A.G.S.; Mathers, J.J.; Blackburn, L.F.; Korycinska, A.; Luo, W.; Jacobson, R.J.; Northing, P. Population Development of Tuta Absoluta (Meyrick) (Lepidoptera: Gelechiidae) under Simulated UK Glasshouse Conditions. Insects 2013, 4, 185–197. [Google Scholar] [CrossRef]
- Silva, W.M.; Berger, M.; Bass, C.; Williamson, M.; Moura, D.M.N.; Ribeiro, L.M.S.; Siqueira, H.A.A. Mutation (G275E) of the Nicotinic Acetylcholine Receptor A6 Subunit Is Associated with High Levels of Resistance to Spinosyns in Tuta Absoluta (Meyrick) (Lepidoptera: Gelechiidae). Pestic. Biochem. Physiol. 2016, 131, 1–8. [Google Scholar] [CrossRef]
- Desneux, N.; Luna, M.G.; Guillemaud, T.; Urbaneja, A. The Invasive South American Tomato Pinworm, Tuta Absoluta, Continues to Spread in Afro-Eurasia and beyond: The New Threat to Tomato World Production. J. Pest Sci. 2011, 84, 403–408. [Google Scholar] [CrossRef]
- Birhan, A. Tomato Leafminer [(Tuta Absoluta Meyrick) (Lepidoptera: Gelechiidae)] and Its Current Ecofriendly Management Strategies: A Review. J. Agric. Biotechnol. Sustain. Dev. 2018, 10, 11–24. [Google Scholar] [CrossRef]
- Sparks, T.C.; Crouse, G.D.; Benko, Z.; Demeter, D.; Giampietro, N.C.; Lambert, W.; Brown, A.V. The Spinosyns, Spinosad, Spinetoram, and Synthetic Spinosyn Mimics—Discovery, Exploration, and Evolution of a Natural Product Chemistry and the Impact of Computational Tools. Pest Manag. Sci. 2021, 77, 3637–3649. [Google Scholar] [CrossRef]
- Campos, M.R.; Silva, T.B.M.; Silva, W.M.; Silva, J.E.; Siqueira, H.A.A. Spinosyn Resistance in the Tomato Borer Tuta Absoluta (Meyrick) (Lepidoptera: Gelechiidae). J. Pest Sci. 2015, 88, 405–412. [Google Scholar] [CrossRef]
- Nauen, R.; Slater, R.; Sparks, T.C.; Elbert, A.; Mccaffery, A. IRAC: Insecticide Resistance and Mode-of-action Classification of Insecticides. Mod. Crop Prot. Compd. 2019, 3, 995–1012. [Google Scholar]
- Fan, R.; Fan, Z.; Sun, Z.; Chen, Y.; Gui, F. Insecticide Susceptibility and Detoxification Enzyme Activity of Frankliniella Occidentalis under Three Habitat Conditions. Insects 2023, 14, 643. [Google Scholar] [CrossRef] [PubMed]
- Sparks, T.C.; Dripps, J.E.; Watson, G.B.; Paroonagian, D. Resistance and Cross-Resistance to the Spinosyns—A Review and Analysis. Pestic. Biochem. Physiol. 2012, 102, 1–10. [Google Scholar] [CrossRef]
- Fan, Z.; Qian, L.; Chen, Y.; Fan, R.; He, S.; Gao, Y.; Gui, F. Effects of Elevated CO2 on Activities of Protective and Detoxifying Enzymes in Frankliniella Occidentalis and F. Intonsa under Spinetoram Stress. Pest Manag. Sci. 2022, 78, 274–286. [Google Scholar] [CrossRef]
- Besard, L.; Mommaerts, V.; Abdu-Alla, G.; Smagghe, G. Lethal and Sublethal Side-Effect Assessment Supports a More Benign Profile of Spinetoram Compared with Spinosad in the Bumblebee Bombus Terrestris. Pest Manag. Sci. 2011, 67, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, F.; Haq, I.U.; Li, C.; Gou, Y.; Zhang, K.; Liu, H.; Liu, C. Comparative Toxicity and Enzymatic Detoxification Responses in Spodoptera Frugiperda (Lepidoptera: Noctuidae) to Two Insecticides. Ecotoxicol. Environ. Saf. 2024, 284, 116917. [Google Scholar] [CrossRef]
- Chen, L.; Wang, X.; Zhang, Y.; Yang, R.; Zhang, S.; Xu, X.; Zhu, M.; Gong, C.; Hasnain, A.; Shen, L.; et al. The Population Growth, Development and Metabolic Enzymes of the White-Backed Planthopper, Sogatella Furcifera (Hemiptera: Delphacidae) under the Sublethal Dose of Triflumezopyrim. Chemosphere 2020, 247, 125865. [Google Scholar] [CrossRef]
- Liao, X.; Ali, E.; Li, W.; He, B.; Gong, P.; Xu, P.; Li, J.; Wan, H. Sublethal Effects of Sulfoxaflor on the Development and Reproduction of the Brown Planthopper, Nilaparvata Lugens (Stål). Crop Prot. 2019, 118, 6–14. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, H.; Wu, S.; Xia, F.; He, M.; Yang, L.; Li, R.; Liao, X.; Li, M. Sublethal Effects of Nitenpyram on the Biological Traits and Metabolic Enzymes of the White-Backed Planthopper, Sogatella Furcifera (Hemiptera: Delphacidae). Crop Prot. 2022, 155, 105931. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, X.; Gu, F.; Gong, C.; Chen, L.; Zhang, Y.; Hasnain, A.; Shen, L.; Jiang, C. Sublethal Effects of Triflumezopyrim on Biological Traits and Detoxification Enzyme Activities in the Small Brown Planthopper Laodelphax Striatellus (Hemiptera: Delphacidae). Front. Physiol. 2020, 11, 261. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.-H.; Wu, Q.-J.; Li, X.-F.; Zhang, Y.-J.; Xu, B.-Y. Sublethal Effects of Spinosad on Plutella Xylostella (Lepidoptera: Yponomeutidae). Crop Prot. 2008, 27, 1385–1391. [Google Scholar] [CrossRef]
- Wen, S.; Xue, Y.; Du, R.; Liu, C.; Wang, X.; Wang, Y.; Liu, C.; Wang, S.; Wang, J.; Xia, X. Toxicity and Sublethal Effects of Triflumezopyrim on the Development and Detoxification Enzymatic Activities in the Small Brown Planthopper (SBPH), Laodelphax Striatellus (Fallen). Crop Prot. 2021, 150, 105813. [Google Scholar] [CrossRef]
- Wang, D.; Gong, P.; Li, M.; Qiu, X.; Wang, K. Sublethal Effects of Spinosad on Survival, Growth and Reproduction of Helicoverpa Armigera (Lepidoptera: Noctuidae). Pest Manag. Sci. 2009, 65, 223–227. [Google Scholar] [CrossRef]
- Chen, Y.Z.; Zhang, B.W.; Yang, J.; Zou, C.S.; Li, T.; Zhang, G.C.; Chen, G. sheng Detoxification, Antioxidant, and Digestive Enzyme Activities and Gene Expression Analysis of Lymantria Dispar Larvae under Carvacrol. J. Asia. Pac. Entomol. 2021, 24, 208–216. [Google Scholar] [CrossRef]
- Zou, C.S.; Lv, C.H.; Wang, Y.J.; Cao, C.W.; Zhang, G.C. Larvicidal Activity and Insecticidal Mechanism of Chelidonium Majus on Lymantria Dispar. Pestic. Biochem. Physiol. 2017, 142, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.-D.; Xia, F.; Lin, Q.-S.; Chen, H.-Y.; Li, Z.-Y.; Fei, Y.I.N.; Liang, P.; Gao, X.-W. Biochemical Mechanism of Chlorantraniliprole Resistance in the Diamondback Moth, Plutella Xylostella Linnaeus. J. Integr. Agric. 2014, 13, 2452–2459. [Google Scholar] [CrossRef]
- Zhang, X.M.; Hu, C.X.; Zhao, H.X.; Zhang, H.R.; Gui, F.R.; Li, Z.Y. Effects of Imidacloprid Stress on Development and Sex Ratio of Frankliniella Occidentalis Populations. J. Environ. Entomol 2017, 39, 870–878. [Google Scholar]
- Yao, S.; Yang, Y.; Xue, Y.; Zhao, W.; Liu, X.; Du, M.; Yin, X.; Guan, R.; Wei, J.; An, S. New Insights on the Effects of Spinosad on the Development of Helicoverpa Armigera. Ecotoxicol. Environ. Saf. 2021, 221, 112452. [Google Scholar] [CrossRef]
- Fahmy, N.; Dahi, H. Changes in Detoxifying Enzymes and Carbohydrate Metabolism Associated with Spinetoram in Two Field-Collected Strains of Spodoptera Littoralis (Biosd.). Egypt. Acad. J. Biol. Sci. F. Toxicol. Pest Control 2009, 1, 17–26. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, L.; Yang, S.; Xie, B.; An, S.; Liang, G. Assessment of the Lethal and Sublethal Effects by Spinetoram on Cotton Bollworm. PLoS ONE 2018, 13, e0204154. [Google Scholar] [CrossRef] [PubMed]
- Ullah, F.; Güncan, A.; Gul, H.; Hafeez, M.; Zhou, S.; Wang, Y.; Zhang, Z.; Huang, J.; Ghramh, H.A.; Guo, W.; et al. Spinosad-Induced Intergenerational Sublethal Effects on Tuta Absoluta: Biological Traits and Related Genes Expressions. Entomol. Gen. 2024, 44, 395–404. [Google Scholar] [CrossRef]
- Srivastava, M.; Bosco, L.; Funderburk, J.; Weiss, A. Spinetoram is Compatible with the Key Natural Enemy of Frankliniella Species Thrips in Pepper. Plant Health Prog. 2008, 9, 30. [Google Scholar] [CrossRef]
- Abbas, A.; Zhao, C.R.; Arshad, M.; Han, X.; Iftikhar, A.; Hafeez, F.; Aslam, A.; Ullah, F. Sublethal Effects of Spinetoram and Emamectin Benzoate on Key Demographic Parameters of Fall Armyworm, Spodoptera Frugiperda (Lepidoptera: Noctuidae) under Laboratory Conditions. Environ. Sci. Pollut. Res. 2023, 30, 82990–83003. [Google Scholar] [CrossRef] [PubMed]
- Rabea, E.I. Comparative Toxicity of Five Pesticides Against Tetranychus Urticae (Koch), Myzus Persicae (Sulzer) and Aphis Nerii (Boyer de Fonscolombe). Alexandria Sci. Exch. J. Int. Q. J. Sci. Agric. Environ. 2009, 30, 412–418. [Google Scholar] [CrossRef]
- Desneux, N.; Rafalimanana, H.; Kaiser, L. Dose-Response Relationship in Lethal and Behavioural Effects of Different Insecticides on the Parasitic Wasp Aphidius Ervi. Chemosphere 2004, 54, 619–627. [Google Scholar] [CrossRef]
- Schneider, M.I.; Smagghe, G.; Pineda, S.; Viñuela, E. Action of Insect Growth Regulator Insecticides and Spinosad on Life History Parameters and Absorption in Third-Instar Larvae of the endoparasitoid Hyposoter didymator. Biol. Control 2004, 31, 189–198. [Google Scholar] [CrossRef]
- Gao, Z.; Chen, Y.; He, K.; Guo, J.; Wang, Z. Sublethal Effects of the Microbial-Derived Insecticide Spinetoram on the Growth and Fecundity of the Fall Armyworm (Lepidoptera: Noctuidae). J. Econ. Entomol. 2021, 114, 1582–1587. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.S.; Wang, Y.J.; Zou, H.; Ding, N.; Geng, N.N.; Cao, C.W.; Zhang, G.C. Sanguinarine in Chelidonium Majus Induced Antifeeding and Larval Lethality by Suppressing Food Intake and Digestive Enzymes in Lymantria Dispar. Pestic. Biochem. Physiol. 2019, 153, 9–16. [Google Scholar] [CrossRef]
- Salem, S.A.R.; Alhousini, E.M.E.; Al-Amgad, Z.; Mahmoud, M.A.B. Efficiency of Spinetoram on Biological, Biochemical, and Histological Parameters in the Invasive Fall Armyworm Spodoptera Frugiperda (Lepidoptera: Noctuidae) in Egypt. J. Plant Dis. Prot. 2024, 131, 489–499. [Google Scholar] [CrossRef]
- Hafeez, M.; Liu, S.; Jan, S.; Ali, B.; Shahid, M.; Fernández-Grandon, G.M.; Nawaz, M.; Ahmad, A.; Wang, M. Gossypol-induced Fitness Gain and Increased Resistance to Deltamethrin in Beet Armyworm, Spodoptera Exigua (Hübner). Pest Manag. Sci. 2019, 75, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ma, Y.; Wang, F.; Yang, Y.; Wu, S.; Wu, Y. Disruption of Nicotinic Acetylcholine Receptor A6 Mediated by CRISPR/Cas9 Confers Resistance to Spinosyns in Plutella Xylostella. Pest Manag. Sci. 2020, 76, 1618–1625. [Google Scholar] [CrossRef] [PubMed]
- Salgado, V.L.; Sparks, T.C. The Spinosyns: Chemistry, Biochemistry, Mode of Action, and Resistance. In Comprehensive Molecular Insect Science; Gilbert, L.I., Ed.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 137–173. ISBN 978-0-444-51924-5. [Google Scholar]
- Jeon, J.H.; Kim, S.-J.; Lee, H.S.; Cha, S.-S.; Lee, J.H.; Yoon, S.-H.; Koo, B.-S.; Lee, C.-M.; Choi, S.H.; Lee, S.H. Novel Metagenome-Derived Carboxylesterase That Hydrolyzes β-Lactam Antibiotics. Appl. Environ. Microbiol. 2011, 77, 7830–7836. [Google Scholar] [CrossRef] [PubMed]
- Mrdaković, M.; Ilijin, L.; Vlahović, M.; Todorović, D.; Gavrilović, A.; Mrkonja, A.; Perić-Mataruga, V. Effects of Fluoranthene on the Fitness-Related Traits and Antioxidative Defense in Lymantria Dispar L. Environ. Sci. Pollut. Res. 2015, 22, 10367–10374. [Google Scholar] [CrossRef]
- Krishnan, N.; Kodrík, D. Antioxidant Enzymes in Spodoptera Littoralis (Boisduval): Are They Enhanced to Protect Gut Tissues during Oxidative Stress? J. Insect Physiol. 2006, 52, 11–20. [Google Scholar] [CrossRef]
| Insecticide | Concentrations (mg L−1) | Goodness of Fit | ||||
|---|---|---|---|---|---|---|
| LC10 (95% CI) a | LC20 (95% CI) | LC50 (95% CI) | Slope ± SE b | χ2 (df) c | p | |
| Spinetoram | 0.06 (0.03–0.08) | 0.10 (0.06–0.14) | 0.32 (0.24–0.41) | 1.69 ± 0.20 | 1.6 (6) | 0.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, M.; Qian, X.; Zhou, Z.; Liu, Y.; Zhang, M.; Yang, Y. Impacts of Sublethal Doses of Spinetoram on the Biological Traits and Detoxifying Enzymes of the Tomato Leaf Miner, Tuta absoluta (Lepidoptera: Gelechiidae). Insects 2024, 15, 990. https://doi.org/10.3390/insects15120990
Jiang M, Qian X, Zhou Z, Liu Y, Zhang M, Yang Y. Impacts of Sublethal Doses of Spinetoram on the Biological Traits and Detoxifying Enzymes of the Tomato Leaf Miner, Tuta absoluta (Lepidoptera: Gelechiidae). Insects. 2024; 15(12):990. https://doi.org/10.3390/insects15120990
Chicago/Turabian StyleJiang, Mingjun, Xiujuan Qian, Zhaoxu Zhou, Yueying Liu, Meijiao Zhang, and Yaxian Yang. 2024. "Impacts of Sublethal Doses of Spinetoram on the Biological Traits and Detoxifying Enzymes of the Tomato Leaf Miner, Tuta absoluta (Lepidoptera: Gelechiidae)" Insects 15, no. 12: 990. https://doi.org/10.3390/insects15120990
APA StyleJiang, M., Qian, X., Zhou, Z., Liu, Y., Zhang, M., & Yang, Y. (2024). Impacts of Sublethal Doses of Spinetoram on the Biological Traits and Detoxifying Enzymes of the Tomato Leaf Miner, Tuta absoluta (Lepidoptera: Gelechiidae). Insects, 15(12), 990. https://doi.org/10.3390/insects15120990






