Challenging Popular Belief, Mosquito Larvae Breathe Underwater
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Mosquitoes
2.2. Survival Experiments
2.3. Oxygen Consumption
2.4. Statistics
3. Results
3.1. Survival Experiments
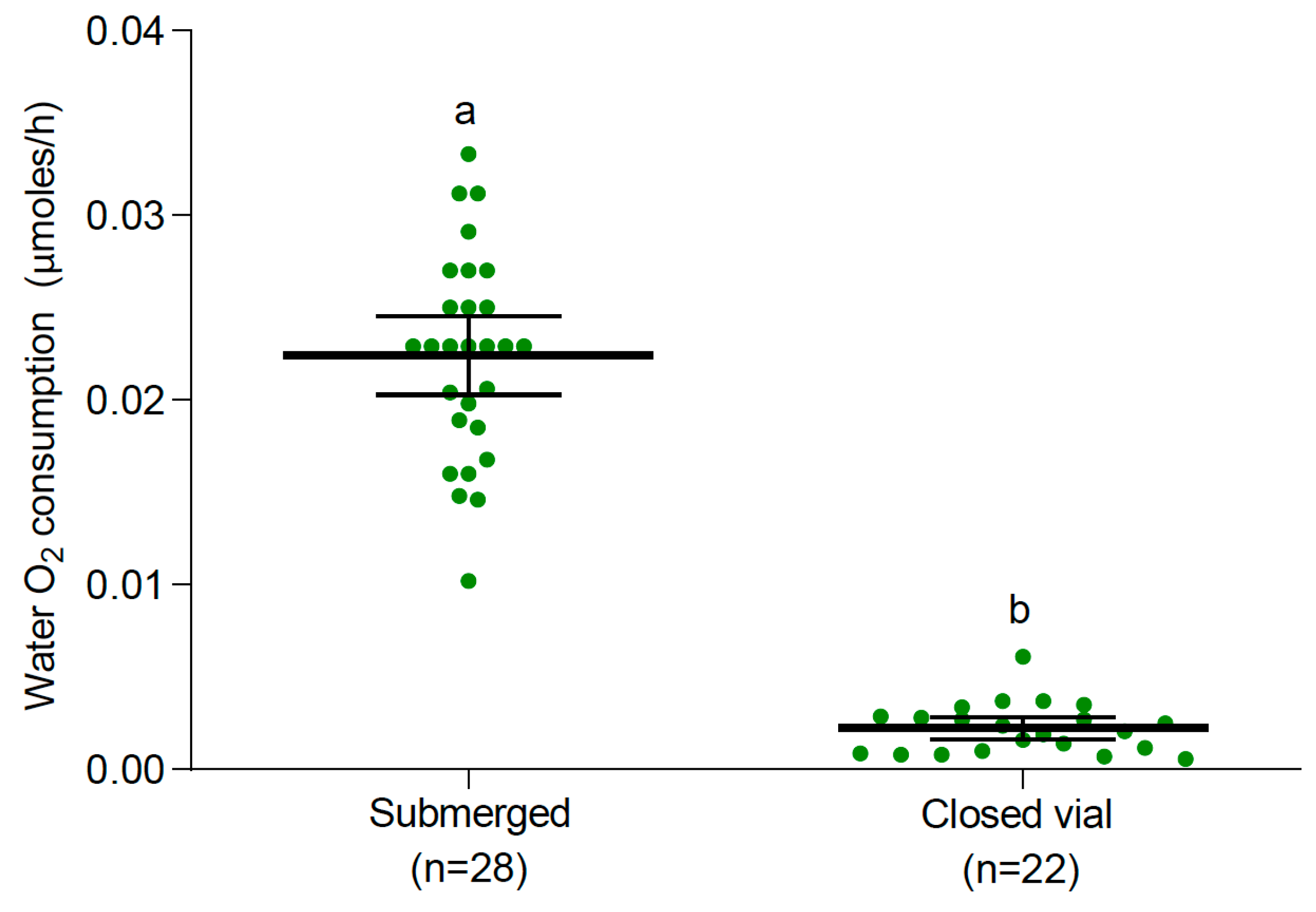
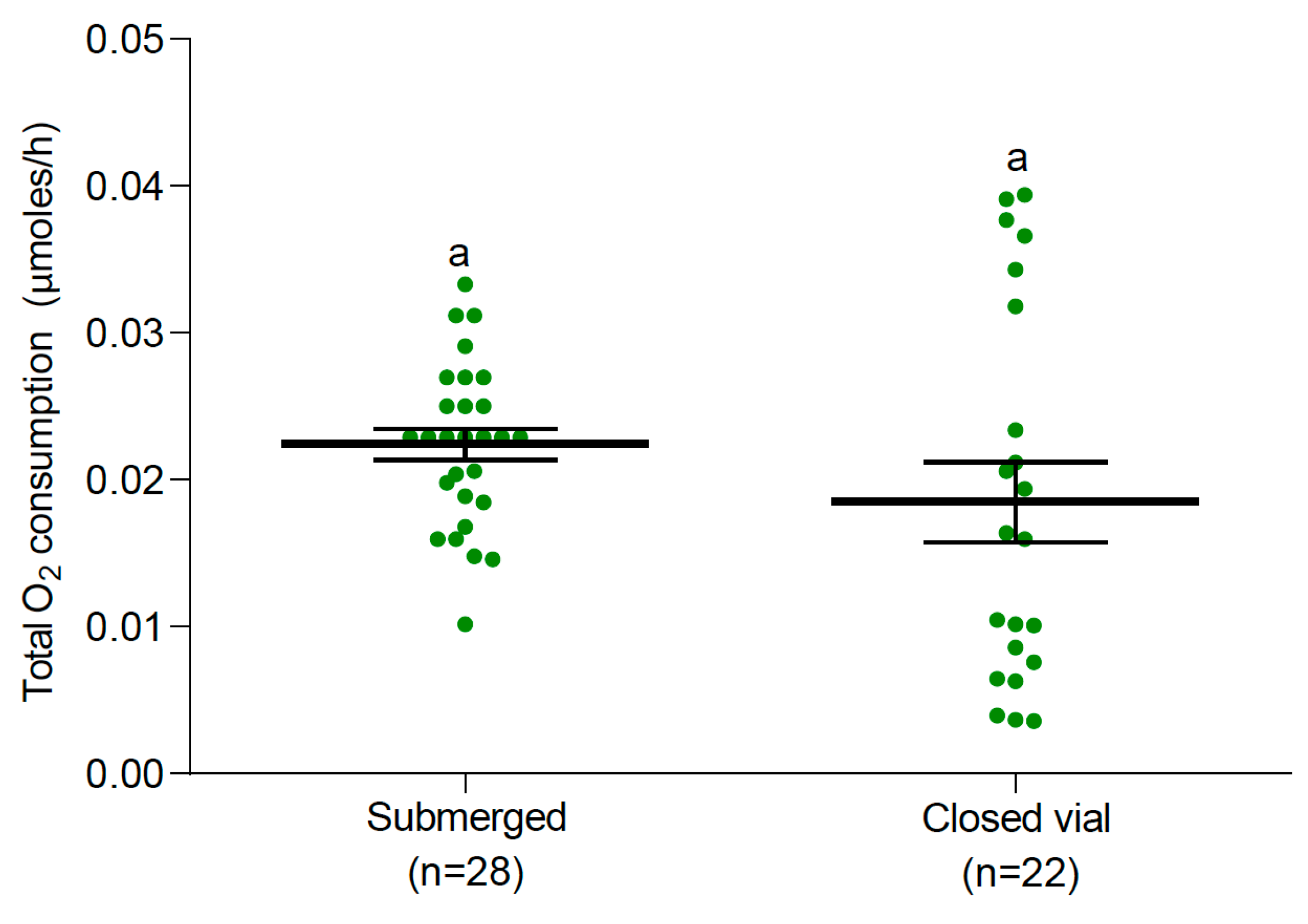
3.2. Oxygen Consumption
3.2.1. Larvae and Pupae at 25 °C
3.2.2. Effect of Temperature on Oxygen Consumption (Q10 of Larvae)
3.2.3. Aedes albopictus
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dejours, P. Respiration in Water and Air; Elsevier: Amsterdam, The Netherlands, 1988; p. 179. [Google Scholar]
- Wigglesworth, V.B. The Principles of Insect Physiology, 8th ed.; Chapman and Hall: London, UK, 1972; p. 836. [Google Scholar]
- Da Costa Lima, A. Contributions to the study of the Biology of the Culicidae. Mem. Inst. Oswaldo Cruz 1914, 6, 18–35. [Google Scholar]
- Macfie, J.W.S. The limitations of kerosene as a larvicide, with some observations on the cutaneous respiration of mosquito larvae. Bull. Entomol. Res. 1917, 7, 277–295. [Google Scholar] [CrossRef][Green Version]
- Ramsey, G.C.; Carpenter, J.A. An investigation of petroleum oils for malaria control purposes. Rec. Malaria Surv. India 1932, 3, 203–218. [Google Scholar]
- Wang, L.S. A Comparative study of the oxygen requirements of mosquito larvae. Chin. Med. J. Suppl. 1938, 2, 487–493. [Google Scholar]
- Richards, A.G. Differentiation between toxic and suffocating effects of petroleum oils on larvae of the house mosquito (Culex pipiens L.) (Diptera). Trans. Am. Entomol. Soc. 1941, 67, 161–196. [Google Scholar]
- Sen, S.K. Observation on respiration of Culicidae. Indian J. Med. Res. 1914, 2, 681–697. [Google Scholar]
- Da Costa Lima, A. Contribuição para o estudo da biolojia dos culicidas: Observações sobre a respiração nas larvas. Mem. Inst. Oswaldo Cruz 1916, 8, 44–49. [Google Scholar] [CrossRef]
- Koch, H.J. The absorption of chloride ions by the anal papillae of Diptera larvae. J. Exp. Biol. 1937, 15, 152–160. [Google Scholar] [CrossRef]
- Wigglesworth, V.B. The function of the anal gills of the mosquito larva. J. Exp. Biol. 1933, 10, 16–26. [Google Scholar] [CrossRef]
- Wigglesworth, V.B. On the function of the so-called “rectal glands” of insects. J. Cell Sci. 1932, 2, 131–150. [Google Scholar] [CrossRef]
- Bradley, T.J. Physiology of osmoregulation in mosquitoes. Annu. Rev. Entomol. 1987, 32, 439–462. [Google Scholar] [CrossRef]
- Thorpe, W.H. Tracheal and blood gills in aquatic insect larvae. Nature 1933, 131, 549–550. [Google Scholar] [CrossRef]
- Krogh, A. Comparative Physiology of Respiratory Mechanisms; University of Pennsylvania Press: Philadelphia, PA, USA, 1941; p. 172. [Google Scholar]
- Fraenkel, G.; Herford, G.V.B. The respiration of insects through the skin. J. Exp. Biol. 1938, 15, 266–280. [Google Scholar] [CrossRef]
- Christophers, S. Aedes aegypti (L.) the Yellow Fever Mosquito. Its Life History, Bionomics, and Structure; Cambridge University Press: Cambridge, UK, 1960; p. 752. [Google Scholar]
- Bates, M. The Natural History of Mosquitoes; Macmillan Company: New York, NY, USA, 1949; p. 379. [Google Scholar]
- Jenkins, D.W.; Carpenter, S.J. Ecology of the tree hole breeding mosquitoes of Nearctic North America. Ecol. Monogr. 1946, 16, 31–47. [Google Scholar] [CrossRef]
- Bradshaw, W.E.; Holzapfel, C.M. Seasonal development of tree-hole mosquitoes (Diptera: Culicidae) and chaoborids in relation to weather and predation. J. Med. Entomol. 1984, 21, 366–378. [Google Scholar] [CrossRef]
- Hearle, E. The life history of Aedes flavescens Müller. Trans. Roy. Soc. Can. 1929, 23, 85–101. [Google Scholar]
- Clements, A.N. The Biology of Mosquitoes; CABI Publishing: New York, NY, USA, 1992; Volume 1, p. 536. [Google Scholar]
- Wesenberg-Lund, C. Contributions to the Biology of the Danish Culicidae; AF Høst & Søn: Copenhagen, Denmark, 1920; p. 264. [Google Scholar]
- Lewis, D.J. Tracheal gills in some African Culicine mosquito larvae. Proc. Roy. Soc. Lond. 1949, 24, 60–66. [Google Scholar] [CrossRef]
- Yap, H.H.; Cutkomp, L.K.; Halberg, F. Circadian rhythms in rate of oxygen consumption by larvae of the mosquito, Aedes aegypti (L). Chronobiologia 1974, 1, 54–61. [Google Scholar]
- Barbosa, P. Some Effects of Overcrowding on the Respiration of Larval Aedes aegypti (L.). Doctoral Dissertation, University of Massachusetts, Amherst, MA, USA, October 1971. [Google Scholar]
- Barbosa, P.; Peters, T.M. Some effects of overcrowding on pupal respiration in Aedes aegypti (L.). Can. J. Zool. 1972, 50, 1179–1181. [Google Scholar] [CrossRef]
- Barbosa, P.; Peters, T.M. Some effects of overcrowding on the respiration of larval Aedes aegypti. Ent. Exp. Appl. 1973, 16, 146–156. [Google Scholar] [CrossRef]
- Berry, W.O.; Brammer, J.D. The effect of temperature on oxygen consumption in Aedes aegypti pupae. Ann. Entomol. Soc. Am. 1975, 68, 198–300. [Google Scholar] [CrossRef]
- Reynolds, S.E. Integration of behaviour and physiology in ecdysis. Adv. Insect Phys. 1980, 15, 475–595. [Google Scholar] [CrossRef]
- Hagstrum, D.W. Simultaneous measurement of aerial and aquatic respiration and study of mode of action of petroleum oils. Ann. Entomol. Soc. Am. 1970, 63, 1466–1467. [Google Scholar] [CrossRef]
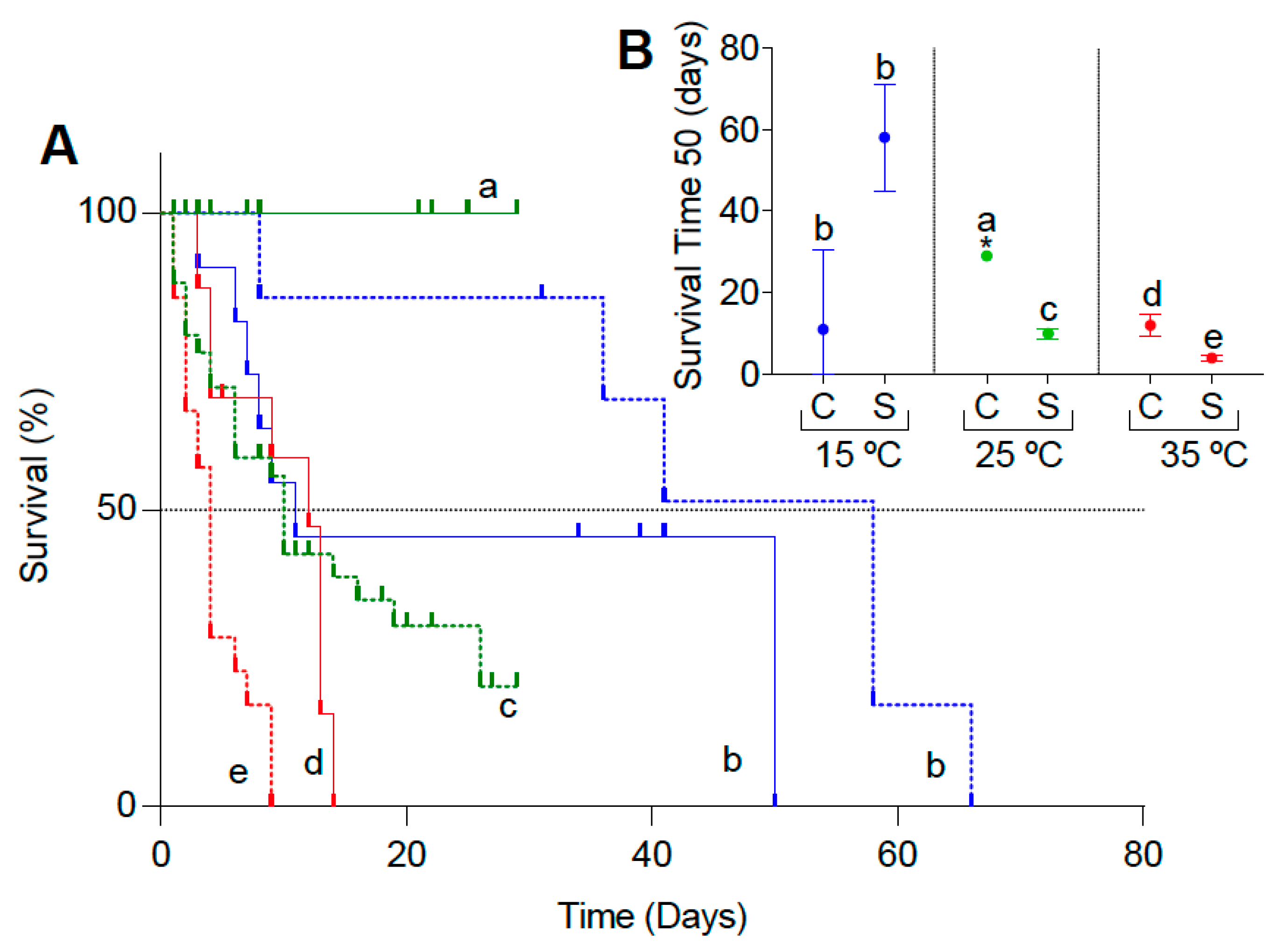
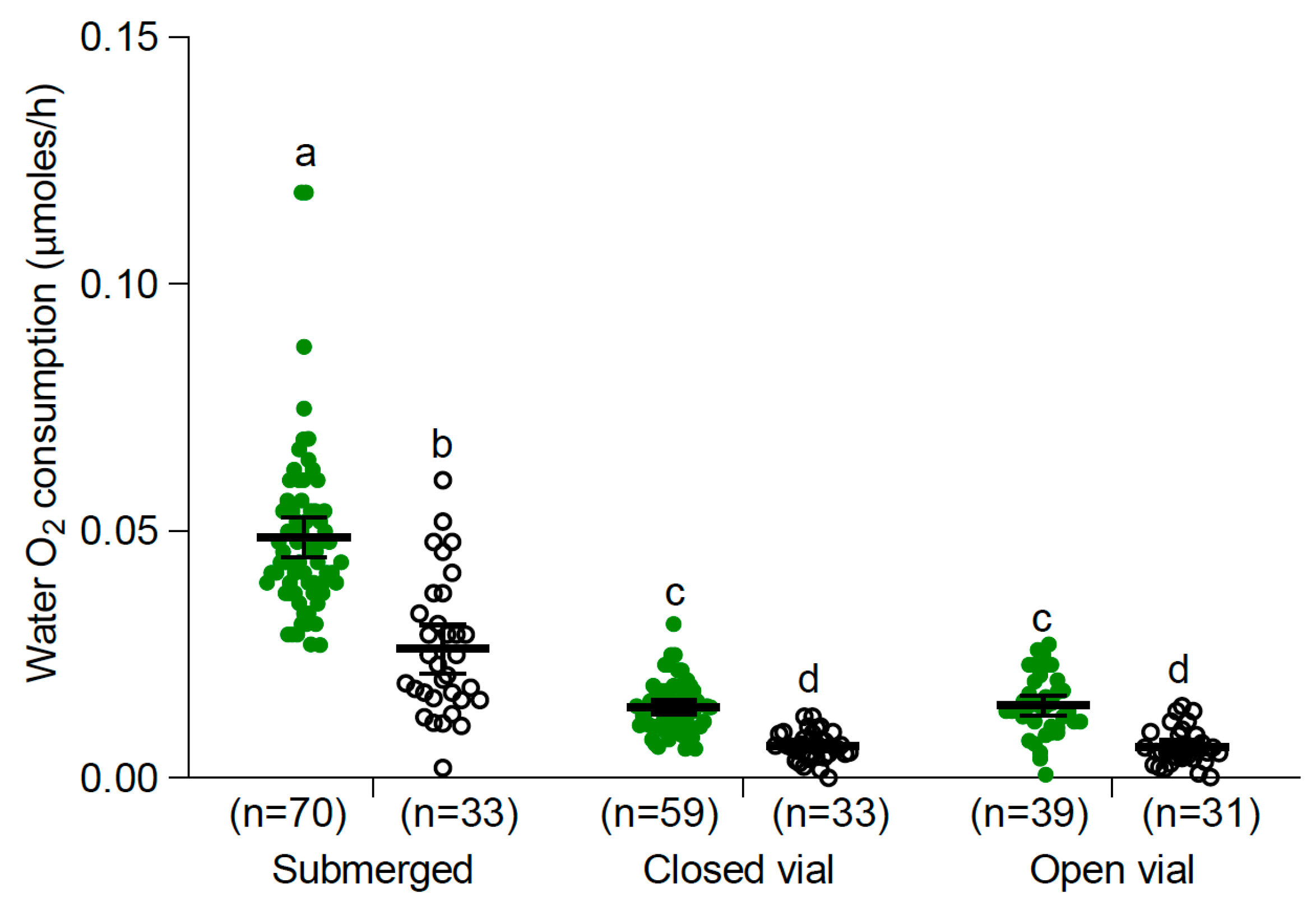
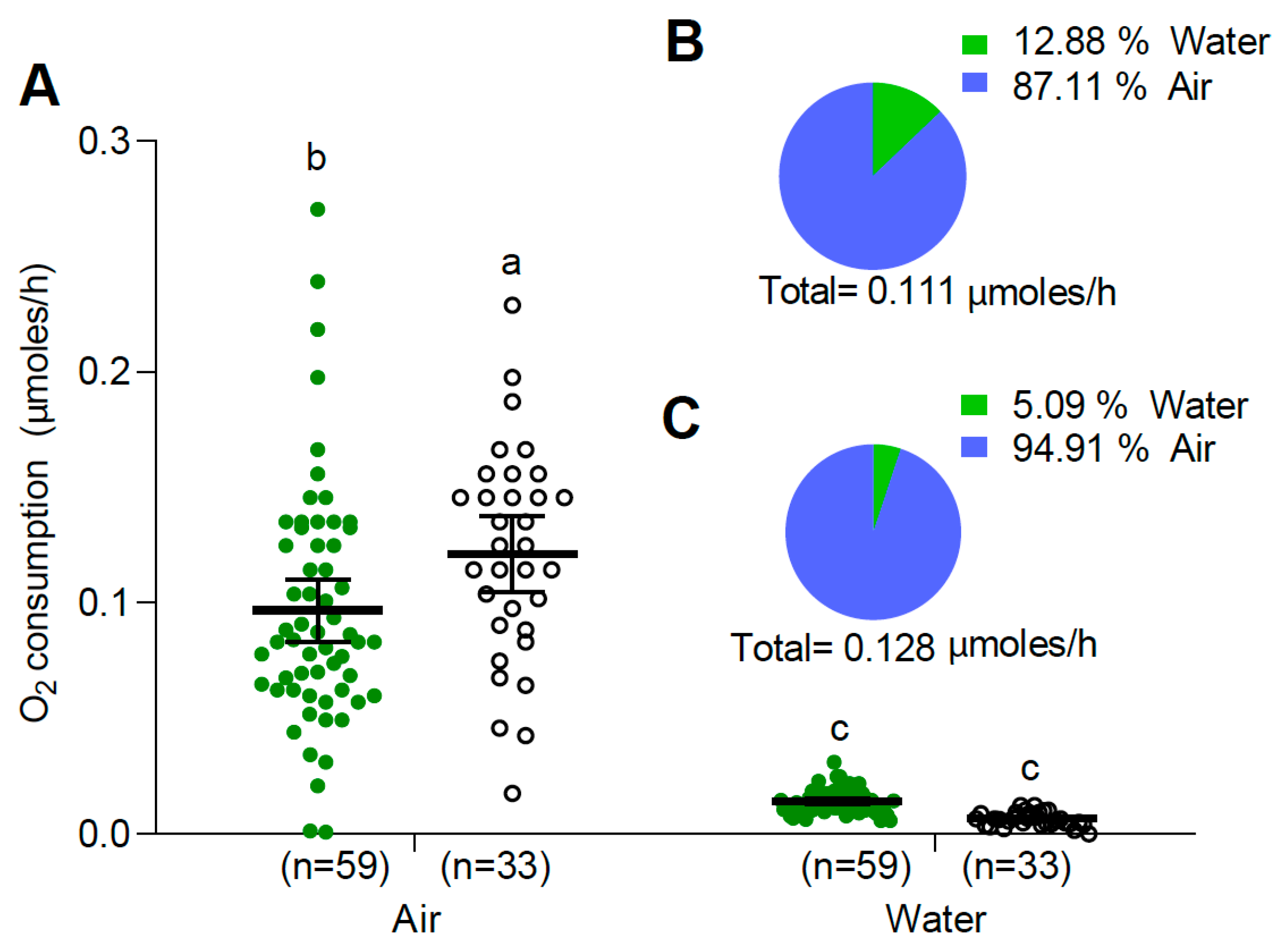
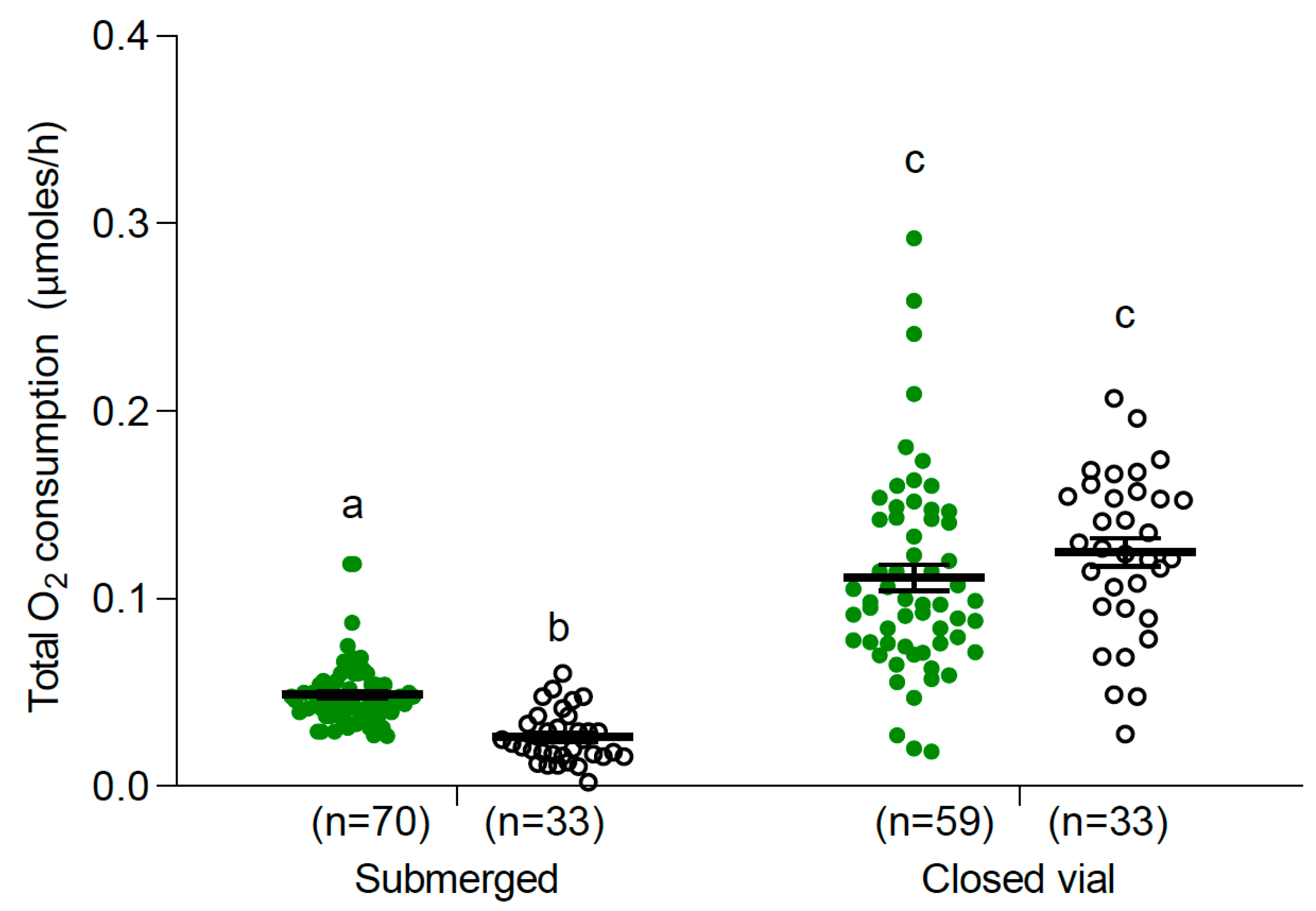

| Temperature (°C) | Control | Submerged |
|---|---|---|
| 15° | 9.09% (n = 11) | 0 (n = 7) |
| 25° | 52.38% (n = 21) | 0 (n = 36) |
| 35° | 18.75% (n = 16) | 0 (n = 21) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvarez-Costa, A.; Leonardi, M.S.; Giraud, S.; Schilman, P.E.; Lazzari, C.R. Challenging Popular Belief, Mosquito Larvae Breathe Underwater. Insects 2024, 15, 99. https://doi.org/10.3390/insects15020099
Alvarez-Costa A, Leonardi MS, Giraud S, Schilman PE, Lazzari CR. Challenging Popular Belief, Mosquito Larvae Breathe Underwater. Insects. 2024; 15(2):99. https://doi.org/10.3390/insects15020099
Chicago/Turabian StyleAlvarez-Costa, Agustin, Maria Soledad Leonardi, Silvère Giraud, Pablo E. Schilman, and Claudio R. Lazzari. 2024. "Challenging Popular Belief, Mosquito Larvae Breathe Underwater" Insects 15, no. 2: 99. https://doi.org/10.3390/insects15020099
APA StyleAlvarez-Costa, A., Leonardi, M. S., Giraud, S., Schilman, P. E., & Lazzari, C. R. (2024). Challenging Popular Belief, Mosquito Larvae Breathe Underwater. Insects, 15(2), 99. https://doi.org/10.3390/insects15020099








