Potential Global Distribution of Paracoccus marginatus, under Climate Change Conditions, Using MaxEnt
Abstract
Simple Summary
Abstract
1. Introduction
- (i)
- It uses presence-only occurrence data;
- (ii)
- It simultaneously uses continuous and categorical variables;
- (iii)
- It effectively controls the model fit through certain parameter settings;
- (iv)
- It relies on the present data, so that the sampling bias can be better dealt with [33].
- (1)
- To predict the trends in changes of suitable habitat areas under different climate change scenarios;
- (2)
- To identify the major climatic variables that restrict the potential distribution of papaya mealybug;
- (3)
- To provide a theoretical reference for policy makers to control and reduce invasive risks in future.
2. Materials and Methods
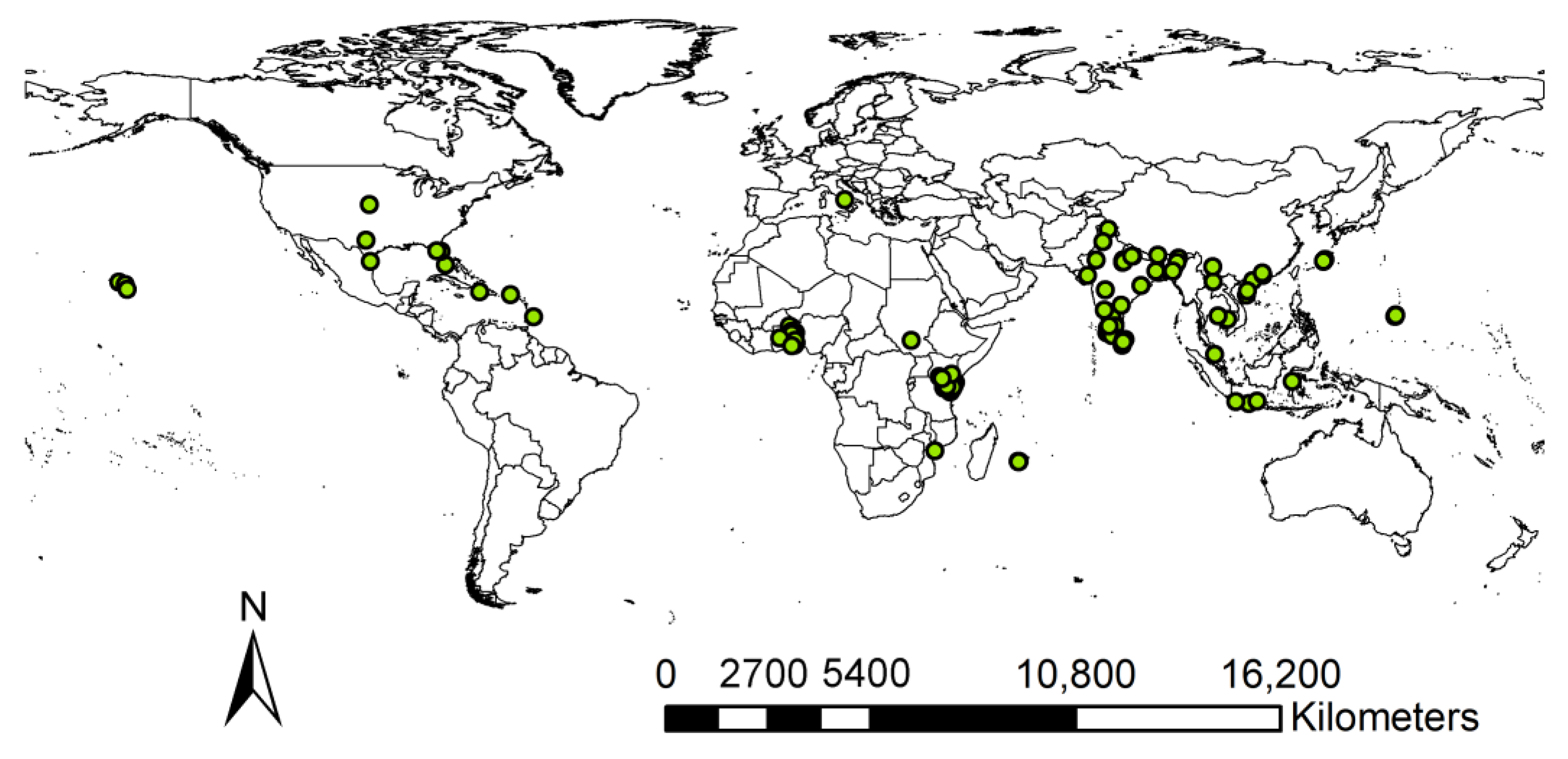
2.1. Occurrence Records Collection
2.2. Environmental Data
2.3. Variable Selection
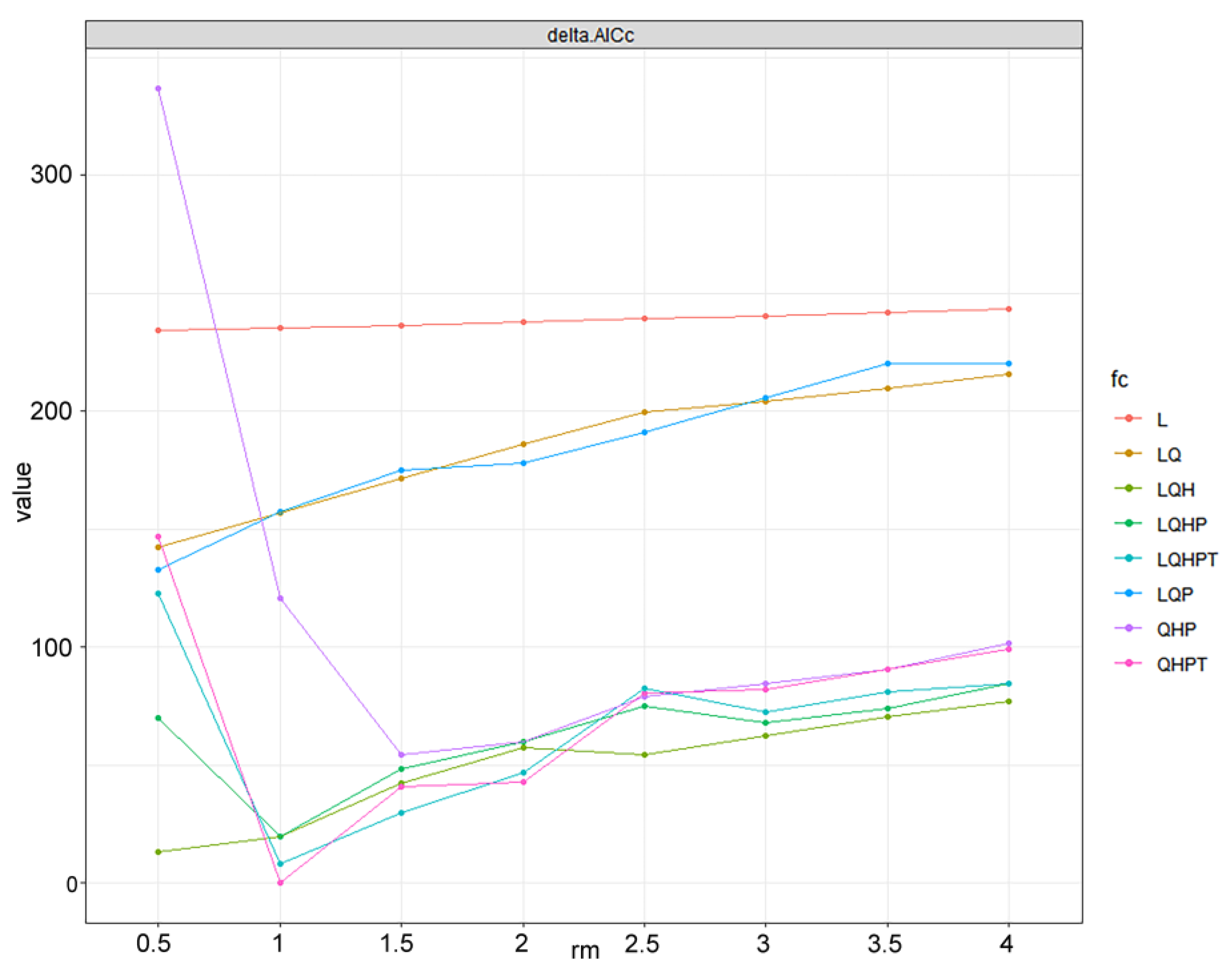
2.4. Modeling Procedure and Evaluation
3. Results
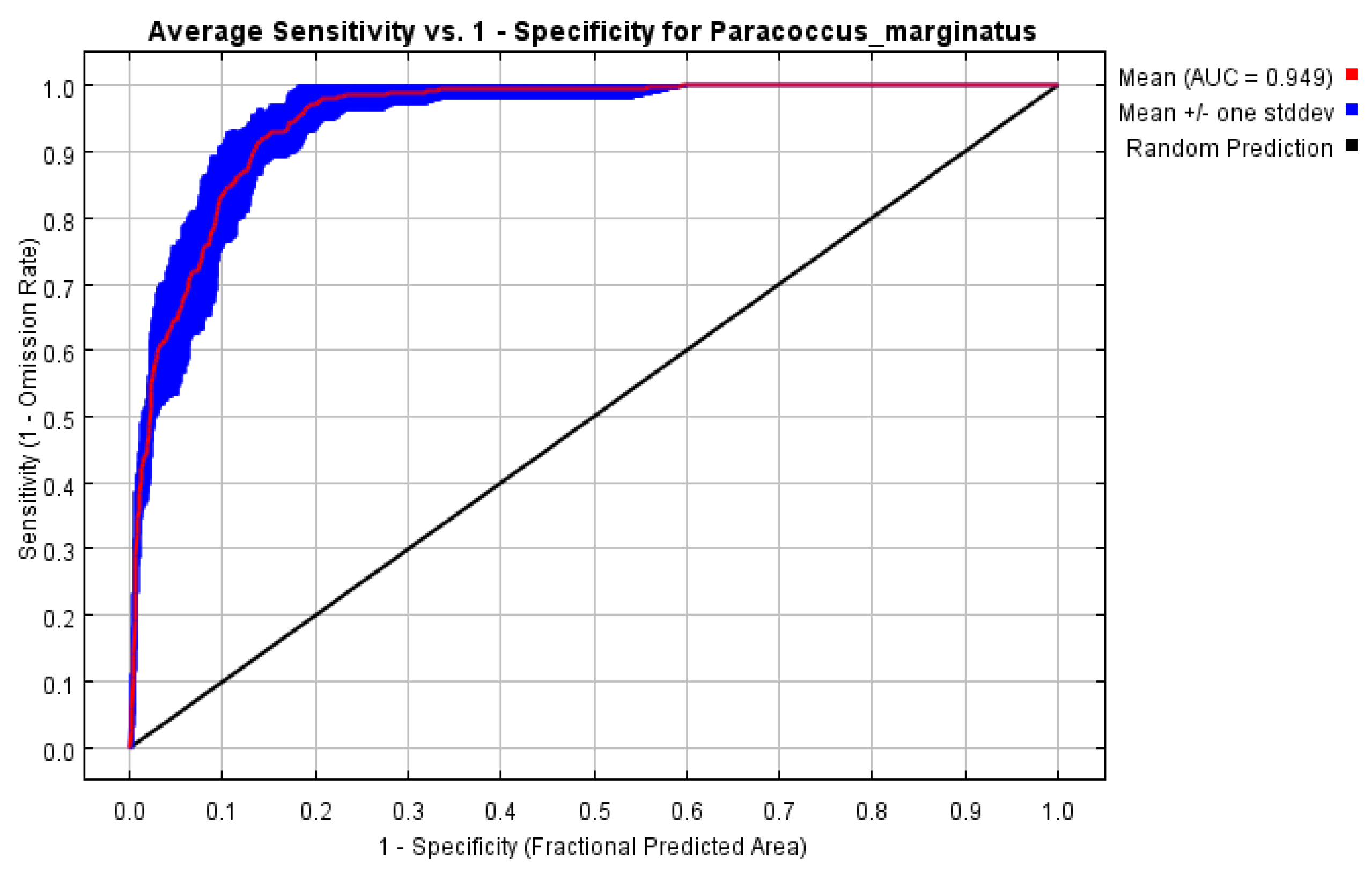
3.1. Model Performance and Contributions of Variables
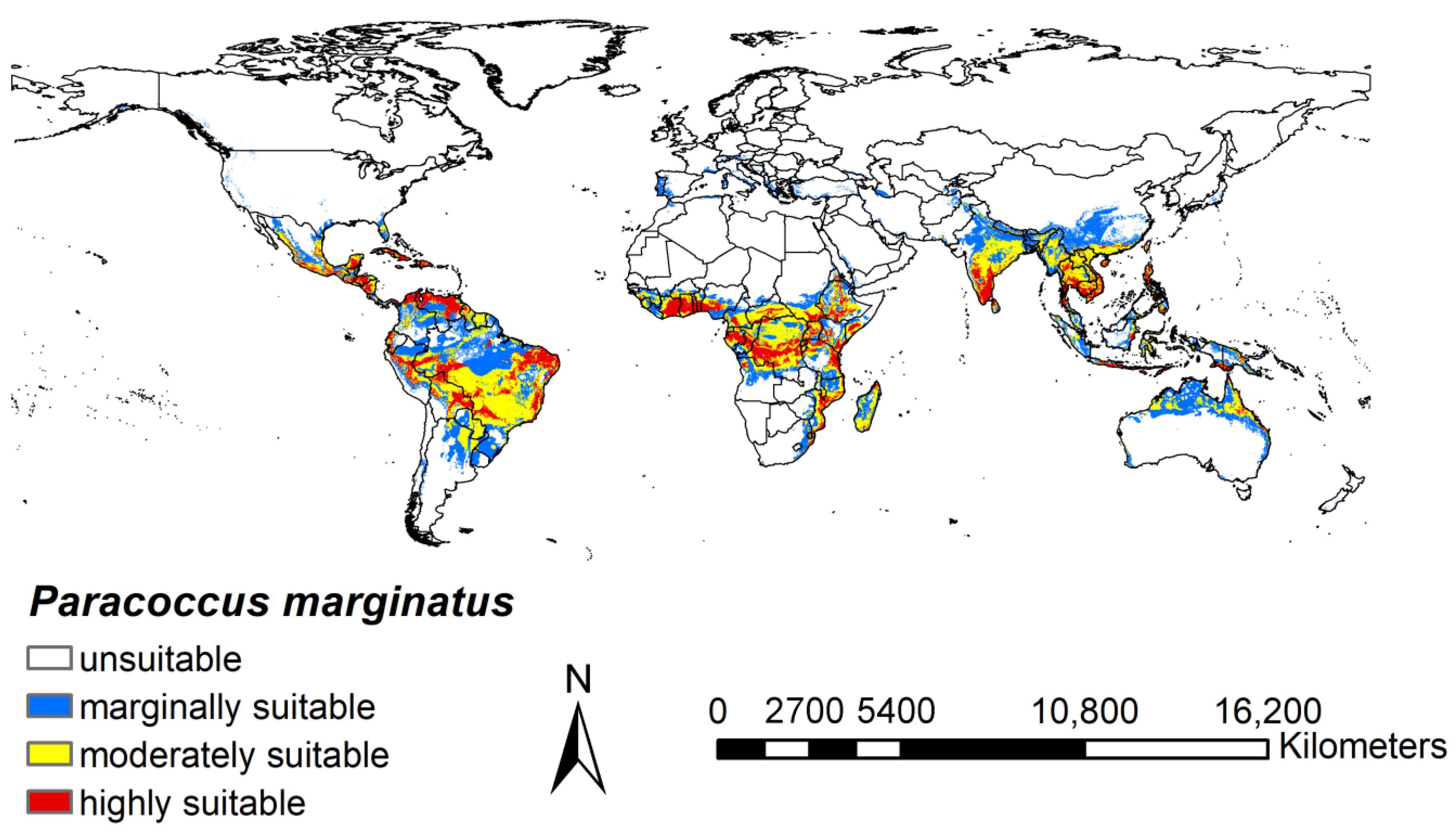
3.2. Potential Distribution under Current Climate
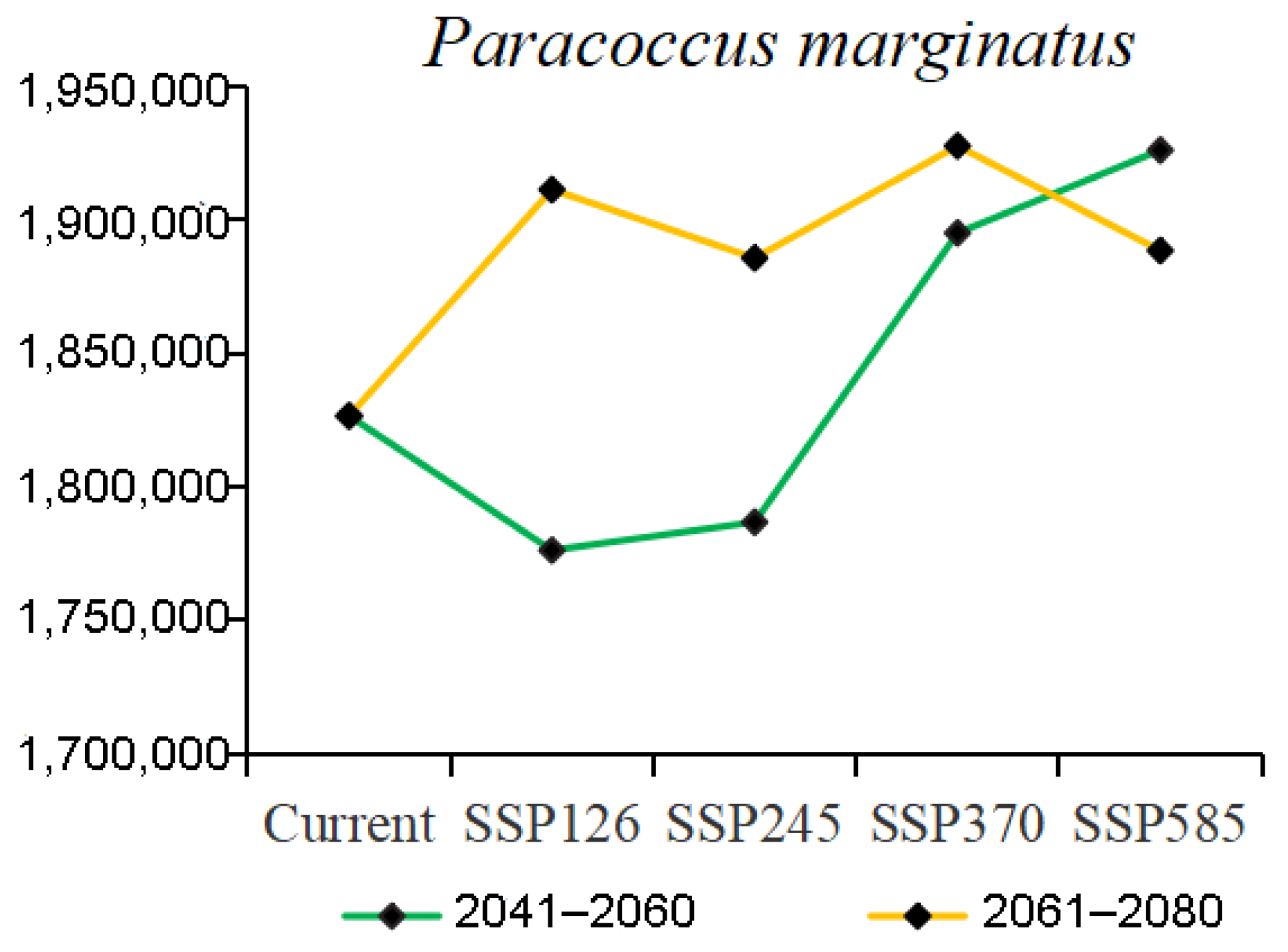
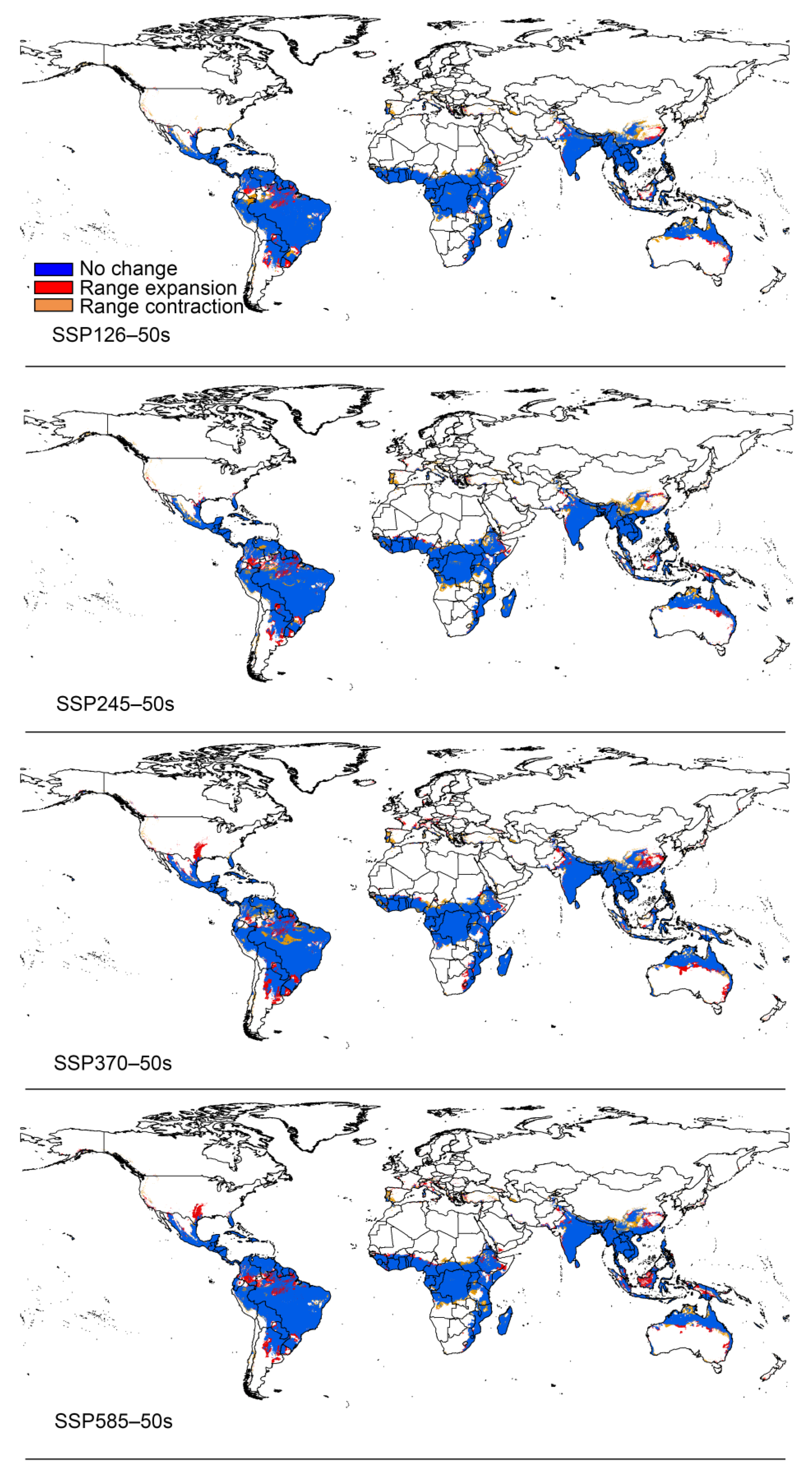
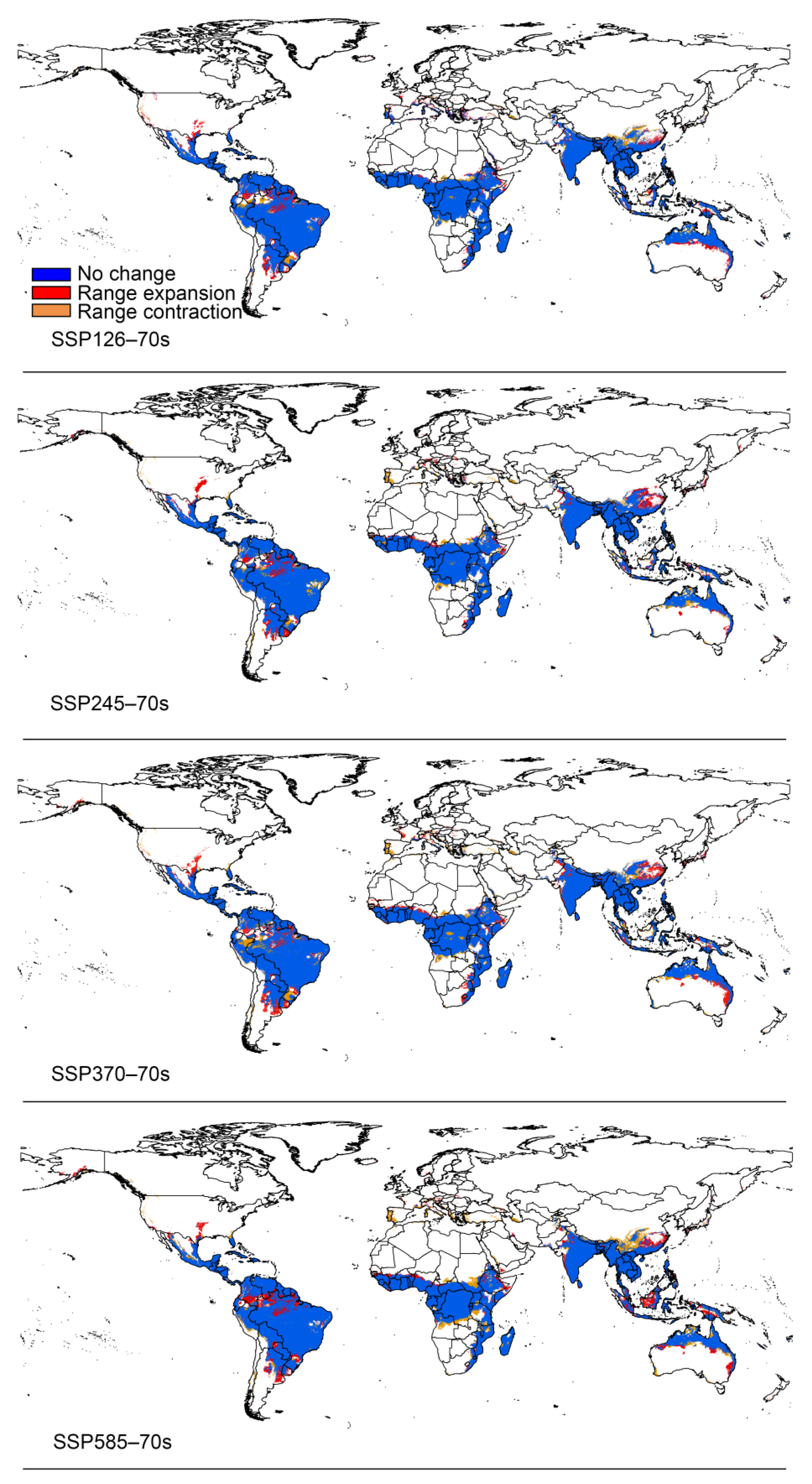
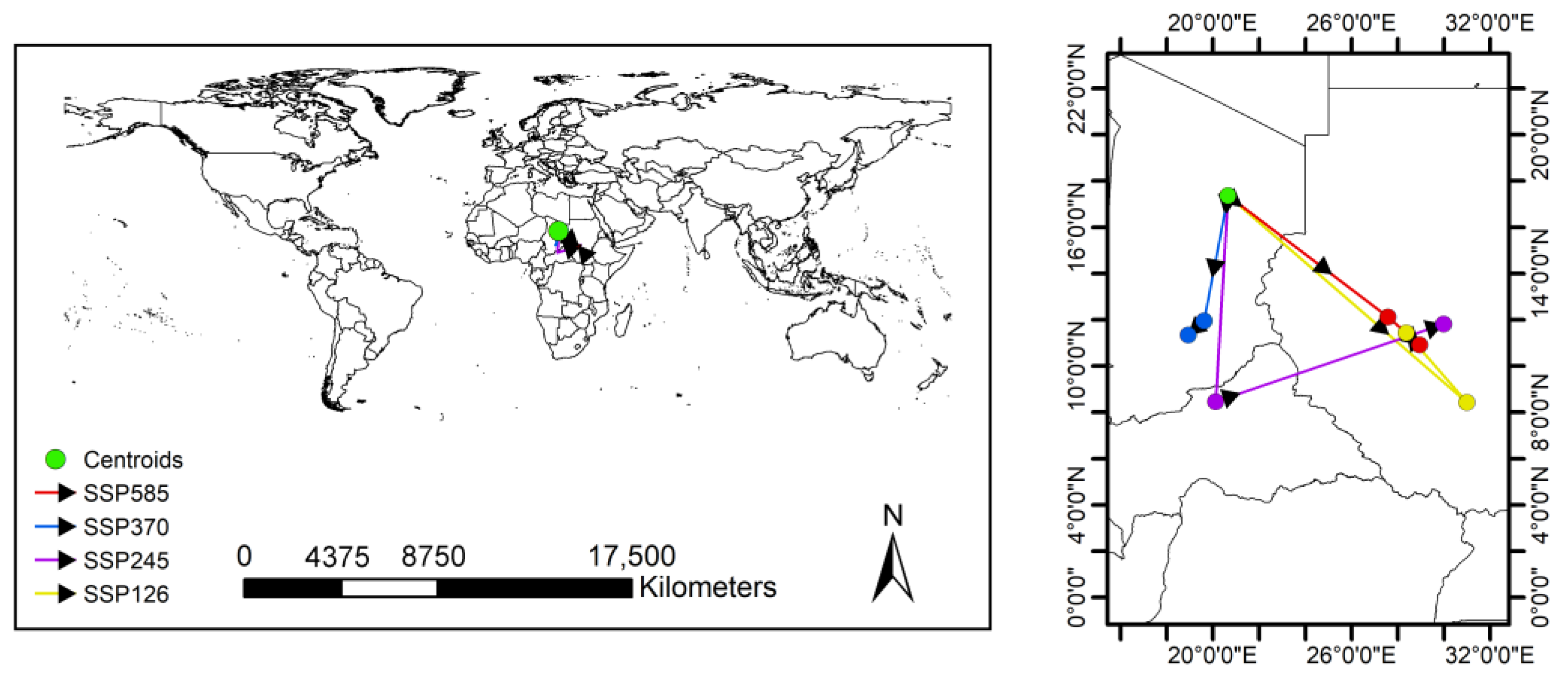
3.3. Potential Distribution under Climate Change
4. Discussion
4.1. Influence of Predictor Variables
4.2. Changes in the Distribution of Papaya Mealybug in the Future
4.3. The Limitation of the Current Model
4.4. A Comprehensive Control Strategy for P. marginatus
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Boyd, I.L.; Freer-Smith, P.; Gilligan, C.A.; Godfray, H.C.J. The consequence of tree pests and diseases for ecosystem services. Science 2013, 342, 1235773. [Google Scholar] [CrossRef] [PubMed]
- Liebhold, A.M.; Yamanaka, T.; Roques, A.; Augustin, S.; Chown, S.L.; Brockerhoff, E.G.; Pyšek, P. Global compositional variation among native and non-native regional insect assemblages emphasizes the importance of pathways. Biol. Invasions 2016, 18, 893–905. [Google Scholar] [CrossRef]
- Kerdelhue, C.; Boivin, T.; Burban, C. Contrasted invasion processes imprint the genetic structure of an invasive scale insect across southern Europe. Heredity 2014, 113, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, C.M.; Krüger, A.P.; Nava, D.E.; Garcia, F.R.M. Global potential distribution of Anastrepha grandis (Diptera, Tephritidae) under climate change scenarios. Crop Prot. 2022, 151, 105836. [Google Scholar] [CrossRef]
- Teixeira, C.M.; Krüger, A.P.; Nava, D.E.; Garcia, F.R.M. Potential global distribution of the south American cucurbit fruit fly Anastrepha grandis (Diptera: Tephritidae). Crop Prot. 2021, 145, 105647. [Google Scholar] [CrossRef]
- Dos Santos, L.A.; Mendes, M.F.; Krüger, A.P.; Blauth, M.L.; Gottschalk, M.S.; Garcia, F.R. Global potential distribution of Drosophila suzukii (Diptera, Drosophilidae). PLoS ONE 2017, 12, e0174318. [Google Scholar] [CrossRef] [PubMed]
- Frank, S.D.; Just, M.G. Can Cities Activate Sleeper Species and Predict Future Forest Pests? A Case Study of Scale Insects. Insects 2020, 11, 142. [Google Scholar] [CrossRef]
- Łagowska, B.; Golan, K.; Kot, I.; Kmieć, K.; Gorska-Drabik, E.; Goliszek, K. Alien and invasive scale insect species in Poland and their threat to native plants. Bull. Insectol. 2015, 68, 13–22. [Google Scholar]
- García Morales, M.; Denno, B.D.; Miller, D.R.; Miller, G.L.; Ben-Dov, Y.; Hardy, N.B. ScaleNet: A literature-based model of scale insect biology and systematics. Database 2016, 2016, bav118. [Google Scholar]
- Miller, D.; Miller, G. Redescription of Paracoccus marginatus Williams & Granara De Willink (Hemiptera: Coccoidea; Pseudococcidae) including descriptions of the immature stages and adult male. Proc. Entomol. Soc. Wash. 2002, 104, 1–23. [Google Scholar]
- Seni, A.; Sahoo, A. Biology of the papaya mealybug, Paracoccus marginatus williams and granara de willink (Hemiptera: Pseudococcidae). Int. J. Agric. Environ. Biotechnol. 2014, 7, 875. [Google Scholar]
- Pantoja, A.; Abreu, E.; Peña, J.; Robles, W. Paracoccus marginatus Williams and Granara de Willink (Homoptera: Pseudococcidae) affecting papaya in Puerto Rico. J. Agric. Univ. Puerto Rico 2007, 91, 223. [Google Scholar] [CrossRef]
- Finch, E.A.; Beale, T.; Chellappan, M.; Goergen, G.; Gadratagi, B.G.; Khan, M.A.M.; Rehman, A.; Rwomushana, I.; Sarma, A.K.; Wyckhuys, K.A.J.P.M.S. The potential global distribution of the papaya mealybug, Paracoccus marginatus, a polyphagous pest. Pest Manag. Sci. 2021, 77, 1361–1370. [Google Scholar] [CrossRef] [PubMed]
- Macharia, I.; Kimani, E.; Koome, F.; Kosiom, T.; Heya, H.; Otipa, M.; Oronje, M. First report and distribution of the papaya mealybug, Paracoccus marginatus, in Kenya1. J. Agric. 2017, 33, 142–150. [Google Scholar] [CrossRef]
- Muniappan, R.; Shepard, B.; Watson, G.; Carner, G.; Rauf, A.; Sartiami, D.; Hidayat, P.; Afun, J.; Goergen, G.; Rahman, A.K.M.Z. New records of invasive insects (hemiptera: Sternorrhyncha) in Southeast Asia and West Africa. J. Agric. Urban Entomol. 2009, 26, 167–174. [Google Scholar] [CrossRef]
- Galanihe, L.D.; Jayasundera, M.U.P.; Vithana, A.; Asselaarachchi, N.; Watson, G.W. Occurrence, distribution and control of papaya mealybug, Paracoccus marginatus (Hemiptera: Pseudococcidae), an invasive alien pest in Sri Lanka. Trop. Agric. Res. Ext. 2011, 13, 81–86. [Google Scholar] [CrossRef]
- Miller, D.R.; Williams, D.J.; Hamon, A.B. Notes on a new mealybug (Hemiptera: Coccoidea: Pseudococcidae) pest in Florida and the Caribbean: The papaya mealybug, Paracoccus marginatus Williams and Granara de Willink. Insecta Mundi 1999, 13, 179–181. [Google Scholar]
- Williams, D.J.; Granara de Willink, M.C.; CAB International. Mealybugs of Central and South America; CAB International: Wallingford, UK, 1992. [Google Scholar]
- Kansiime, M.K.; Rwomushana, I.; Mugambi, I.; Makale, F.; Lamontagne-Godwin, J.; Chacha, D.; Kibwage, P.; Oluyali, J.; Day, R. Crop losses and economic impact associated with papaya mealybug (Paracoccus marginatus) infestation in Kenya. Int. J. Pest Manag. 2023, 69, 150–163. [Google Scholar] [CrossRef]
- Watson, G. Paracoccus marginatus (Papaya Mealybug); CAB International: Wallingford, UK, 2022. [Google Scholar]
- Cyprian, E. Biology, host range and management options for Papaya mealybug in Africa. Res. J. Agric. For. 2019, 7, 49–57. [Google Scholar]
- Mendel, Z.; Watson, G.; Protasov, A.; Spodek, M. First record of the papaya mealybug, Paracoccus marginatus Williams & Granara de Willink (Hemiptera: Coccomorpha: Pseudococcidae), in the Western Palaearctic. EPPO Bull. 2016, 46, 580–582. [Google Scholar]
- Amarasekare, K.G.; Mannion, C.M.; Osborne, L.S.; Epsky, N.D. Life history of Paracoccus marginatus (Hemiptera: Pseudococcidae) on four host plant species under laboratory conditions. Environ. Entomol. 2008, 37, 630–635. [Google Scholar] [CrossRef][Green Version]
- Wei, J.; Peng, L.; He, Z.; Lu, Y.; Wang, F. Potential distribution of two invasive pineapple pests under climate change. Pest Manag. Sci. 2020, 76, 1652–1663. [Google Scholar] [CrossRef]
- Li, J.; Fan, G.; He, Y. Predicting the current and future distribution of three Coptis herbs in China under climate change conditions, using the MaxEnt model and chemical analysis. Sci. Total Environ. 2020, 698, 134141. [Google Scholar] [CrossRef] [PubMed]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudík, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.; et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Booth, T.H.; Nix, H.A.; Busby, J.R.; Hutchinson, M.F. BIOCLIM: The first species distribution modelling package, its early applications and relevance to most current MAXENT studies. Divers. Distrib. 2014, 20, 1–9. [Google Scholar] [CrossRef]
- Stockwell, D. The GARP modelling system: Problems and solutions to automated spatial prediction. Int. J. Geogr. Inf. Sci. 1999, 13, 143–158. [Google Scholar] [CrossRef]
- Aljaryian, R.; Kumar, L.; Taylor, S. Modelling the current and potential future distributions of the sunn pest Eurygaster integriceps (Hemiptera: Scutelleridae) using CLIMEX. Pest Manag. Sci. 2016, 72, 1989–2000. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.N.; Seo, C.; Thorne, J.; Nelson, J.K.; Erwin, S.; O’Brien, J.M.; Schwartz, M.W. Using species distribution models to predict new occurrences for rare plants. Divers. Distrib. 2009, 15, 565–576. [Google Scholar] [CrossRef]
- Prasad, A.M.; Iverson, L.R.; Liaw, A. Newer classification and regression tree techniques: Bagging and random forests for ecological prediction. Ecosystems 2006, 9, 181–199. [Google Scholar] [CrossRef]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M.; Schapire, R.E. A maximum entropy approach to species distribution modeling. In Proceedings of the Twenty-First International Conference on Machine Learning, Banff, AB, Canada, 4–8 July 2004; p. 83. [Google Scholar]
- Zhang, K.; Yao, L.; Meng, J.; Tao, J. Maxent modeling for predicting the potential geographical distribution of two peony species under climate change. Sci. Total Environ. 2018, 634, 1326–1334. [Google Scholar] [CrossRef]
- Ju, R.; Gao, L.; Wei, S.; Li, B. Spring warming increases the abundance of an invasive specialist insect: Links to phenology and life history. Sci. Rep. 2017, 7, 14805. [Google Scholar] [CrossRef] [PubMed]
- Heya, H.M.; Khamis, F.M.; Onyambu, G.K.; Akutse, K.S.; Mohamed, S.A.; Kimathi, E.K.; Ombura, F.L.O.; Ekesi, S.; Dubois, T.; Subramanian, S.; et al. Characterization and risk assessment of the invasive papaya mealybug, Paracoccus marginatus, in Kenya under changing climate. J. Appl. Entomol. 2020, 144, 442–458. [Google Scholar] [CrossRef]
- Germain, J.-F.; Sookar, P.; Buldawoo, I.; Permalloo, S.; Quilici, S. Trois espèces de Cochenilles potentiellement invasives nouvelles pour l’île Maurice (Hemiptera, Coccoidea, Pseudococcidae). Bull. Société Entomol. Fr. 2014, 119, 27–29. [Google Scholar] [CrossRef]
- Meyerdirk, D.; Muniappan, R.; Warkentin, R.; Bamba, J.; Reddy, G.J.P.P.Q. Biological control of the papaya mealybug, Paracoccus marginatus (Hemiptera: Pseudococcidae) in Guam. Plant Prot. Q. 2004, 19, 110–114. [Google Scholar]
- Goergen, G.; Tamo, M.; Kyofa-Boamah, M.E.; Bokonon-Ganta, A.; Neuenschwander, P. Papaya Mealybug: A New Invading Pest in West Africa. Biocontrol News Inf. 2011, 32, 9N–10N. [Google Scholar]
- Muniappan, R.; Shepard, B.; Watson, G.; Carner, G.; Sartiami, D.; Rauf, A.; Hammig, M. First Report of the Papaya Mealybug, Paracoccus marginatus (Hemiptera: Pseudococcidae), in Indonesia and India. J. Agric. Urban Entomol. 2008, 25, 37–40. [Google Scholar] [CrossRef]
- Tanaka, H.; Uesato, T.; Kamitani, S. A record of Paracoccus marginatus Williams & Granara de Willink, 1992 (Hemiptera, Coccomorpha, Pseudococcidae) from Okinawa Island, Japan. Jpn. J. Entomol. 2021, 24, 36–37. [Google Scholar] [CrossRef]
- Nasari, S.P.; Treydte, A.; Ndakidemi, P.A.; Mbega, E.R. Towards conservation of Apefly (Spalgis lemolea. Druce) for managing papaya mealybug (Paracoccus marginatus Williams and Granara de Willink) in Sub Saharan Africa. Sci. Afr. 2020, 7, e00236. [Google Scholar] [CrossRef]
- EFSA Panel on Plant Health; Bragard, C.; Baptista, P.; Chatzivassiliou, E.; Di Serio, F.; Gonthier, P.; Jaques Miret, J.A.; Justesen, A.F.; Magnusson, C.S.; Milonas, P.; et al. Pest categorisation of Paracoccus marginatus. EFSA J. 2023, 21, e07899. [Google Scholar] [CrossRef] [PubMed]
- Zizka, A.; Silvestro, D.; Andermann, T.; Azevedo, J.; Ritter, C.D.; Edler, D.; Farooq, H.; Herdean, A.; Ariza, M.; Scharn, R.; et al. CoordinateCleaner: Standardized cleaning of occurrence records from biological collection databases. Methods Ecol. Evol. 2019, 10, 744–751. [Google Scholar] [CrossRef]
- Aiello-Lammens, M.E.; Boria, R.A.; Radosavljevic, A.; Vilela, B.; Anderson, R.P. spThin: An R package for spatial thinning of species occurrence records for use in ecological niche models. Ecography 2015, 38, 541–545. [Google Scholar] [CrossRef]
- Byeon, D.H.; Kim, S.H.; Jung, J.M.; Jung, S.; Kim, K.H.; Lee, W.H. Climate-based ensemble modelling to evaluate the global distribution of Anoplophora glabripennis (Motschulsky). Agric. For. Entomol. 2021, 23, 569–583. [Google Scholar] [CrossRef]
- Srivastava, V.; Roe, A.D.; Keena, M.A.; Hamelin, R.C.; Griess, V.C. Oh the places they’ll go: Improving species distribution modelling for invasive forest pests in an uncertain world. Biol. Invasions 2021, 23, 297–349. [Google Scholar] [CrossRef]
- Ayyasamy, R.; Regupathy, A. Need and scope for insecticide resistance management for the invasive papaya mealy bug Paracoccus marginatus Williams and Granara de Willink in small scale papaya farming system in Tamil Nadu, India. Resist. Pest Manag. Newsl. 2010, 19, 23–28. [Google Scholar]
- Warren, D.L.; Seifert, S.N. Ecological niche modeling in Maxent: The importance of model complexity and the performance of model selection criteria. Ecol. Appl. 2011, 21, 335–342. [Google Scholar] [CrossRef]
- Radosavljevic, A.; Anderson, R.P. Making better Maxent models of species distributions: Complexity, overfitting and evaluation. J. Biogeogr. 2014, 41, 629–643. [Google Scholar] [CrossRef]
- Muscarella, R.; Galante, P.J.; Soley-Guardia, M.; Boria, R.A.; Kass, J.M.; Uriarte, M.; Anderson, R.P. ENM eval: An R package for conducting spatially independent evaluations and estimating optimal model complexity for Maxent ecological niche models. Methods Ecol. Evol. 2014, 5, 1198–1205. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M.; Schapire, R.E. Maxent Software for Modeling Species Niches and Distributions (Version 3.4. 1). Available online: http://biodiversityinformatics.amnh.org/open_source/maxent/ (accessed on 22 September 2023).
- Tuanmu, M.-N.; Viña, A.; Roloff, G.J.; Liu, W.; Ouyang, Z.; Zhang, H.; Liu, J. Temporal transferability of wildlife habitat models: Implications for habitat monitoring. J. Biogeogr. 2011, 38, 1510–1523. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Fielding, A.H.; Bell, J.F. A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ. Conserv. 1997, 24, 38–49. [Google Scholar] [CrossRef]
- Peterson, A.T.; Soberón, J.; Pearson, R.G.; Anderson, R.P.; Martínez-Meyer, E.; Nakamura, M.; Araújo, M.B. Ecological Niches and Geographic Distributions; Princeton University Press: Princeton, NJ, USA, 2011. [Google Scholar]
- Gaikwad, S.; Pavan, S.; Patil, S.K. Seasonal incidence of papaya mealybug, Paracoccus marginatus Williams and Granara de Willink and its natural enemies with weather parameters. Pest Manag. Sci. 2018, 7, 2649–2652. [Google Scholar]
- Hazarika, M.; Dutta, S. Population dynamics of Papaya mealybug, Paracoccus marginatus Williams and Granara de Willink on three different host plant. J. Entomol. Zool. Stud. 2020, 8, 291–296. [Google Scholar]
- Song, Z.; Qing, Y.; Ma, F.; Li, Y.; Lu, G.; Sun, H.; Li, Z.; Zhan, G. Predicting the potential geographical distribution of Paracoccus marginatus (Hemiptera:Pseudococcidae) based on MaxEnt. Plant Quar. 2019, 33, 73–78. [Google Scholar]
- Gu, Y.; Qi, G. Precaution of a new invasive pest, papaya mealybug Paracoccus marginatus. J. Biosaf. 2015, 24, 39–44. [Google Scholar]
- Massamby, A.; Cugala, D.; Santos, L.; Sidumo, A. Assessment of current and potential geographic distribution of the papaya mealybug, Paracoccus marginatus (Hemiptera: Pseudococcidae) in Mozambique. In Proceedings of the Fifth African Higher Education Week and RUFORUM Biennial Conference 2016, “Linking Agricultural Universities with Civil Society, the Private Sector, Governments and Other Stakeholders in Support of Agricultural Development in Africa”, Cape Town, South Africa, 17–21 October 2016; pp. 151–157. [Google Scholar]
- Dong, Z.; Ge, F. The fitness of insects in response to climate warming. Plant Quar. 2011, 48, 1141–1148. [Google Scholar]
- Kriticos, D.; Maywald, G.; Yonow, T.; Zurcher, E.; Herrmann, N.; Sutherst, R. CLIMEX. Version 4. Exploring the Effects of Climate on Plants, Animals and Diseases; CSIRO: Clayton, VIC, Australia, 2015. [Google Scholar]
- Piirainen, S.; Lehikoinen, A.; Husby, M.; Kålås, J.A.; Lindström, Å.; Ovaskainen, O. Species distributions models may predict accurately future distributions but poorly how distributions change: A critical perspective on model validation. Divers. Distrib. 2023, 29, 654–665. [Google Scholar] [CrossRef]
- Banks, N.C.; Paini, D.R.; Bayliss, K.L.; Hodda, M. The role of global trade and transport network topology in the human-mediated dispersal of alien species. Ecol. Lett. 2015, 18, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Vall-llosera, M.; Woolnough, A.P.; Anderson, D.; Cassey, P. Improved surveillance for early detection of a potential invasive species: The alien Rose-ringed parakeet Psittacula krameri in Australia. Biol. Invasions 2017, 19, 1273–1284. [Google Scholar] [CrossRef]
- Le, K.H.; Tran, T.H.D.; Tran, D.H.; Nguyen, T.D.; Van Doan, C. Parasitoid Wasp Acerophagus papayae: A Promising Solution for the Control of Papaya Mealybug Paracoccus marginatus in Cassava Fields in Vietnam. Insects 2023, 14, 528. [Google Scholar] [CrossRef]
- Sakthivel, N. Effectiveness of three introduced encyrtid parasitic wasps (Acerophagus papayae, Anagyrus loecki and Pseudleptomastix mexicana) against papaya mealybug, Paracoccus marginatus, infesting mulberry in Tamil Nadu. J. Biopestic. 2013, 6, 71. [Google Scholar]

| Variables | Percentage Contribution (%) |
|---|---|
| Min temperature of coldest month (Bio6) | 46.8 |
| Precipitation of wettest month (Bio13) | 31.1 |
| Precipitation of coldest quarter (Bio19) | 13.1 |
| Precipitation of driest quarter (Bio17) | 7.3 |
| Slope | 1.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Q.; Li, H.; Chen, C.; Fan, S.; Wei, J.; Cai, B.; Zhang, H. Potential Global Distribution of Paracoccus marginatus, under Climate Change Conditions, Using MaxEnt. Insects 2024, 15, 98. https://doi.org/10.3390/insects15020098
Zhao Q, Li H, Chen C, Fan S, Wei J, Cai B, Zhang H. Potential Global Distribution of Paracoccus marginatus, under Climate Change Conditions, Using MaxEnt. Insects. 2024; 15(2):98. https://doi.org/10.3390/insects15020098
Chicago/Turabian StyleZhao, Qing, Huiping Li, Chao Chen, Shiyu Fan, Jiufeng Wei, Bo Cai, and Hufang Zhang. 2024. "Potential Global Distribution of Paracoccus marginatus, under Climate Change Conditions, Using MaxEnt" Insects 15, no. 2: 98. https://doi.org/10.3390/insects15020098
APA StyleZhao, Q., Li, H., Chen, C., Fan, S., Wei, J., Cai, B., & Zhang, H. (2024). Potential Global Distribution of Paracoccus marginatus, under Climate Change Conditions, Using MaxEnt. Insects, 15(2), 98. https://doi.org/10.3390/insects15020098





