Control Effects of Short-Term Heatwaves on a Holocyclic Aphid
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Aphids and Soybean Plants
2.2. Survival, Development, and Reproduction of A. glycines Virginoparae Exposed to Short-Term Heatwaves Beginning at Different Developmental Stages
2.3. Morph Differentiation of the Offspring Deposited by A. glycines Virginoparae Exposed to Short-Term Heatwaves Beginning at Different Developmental Stages
2.4. Data Analysis
3. Results
3.1. Survival, Development, and Reproduction of A. glycines Virginoparae Exposed to Short-Term Heatwaves, Beginning at different Developmental Stages
3.2. Morph Differentiation of Offspring Deposited by A. glycines Virginoparae When Exposed to Short-Term Heatwaves Beginning at Different Developmental Stages
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Blackman, R.L.; Eastop, V.F. Aphids on the World’s Crops, An Identification and Information Guide, 2nd ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2000. [Google Scholar]
- Ragsdale, D.W.; Voegtlin, D.J.; O’Neil, D.J. Soybean aphid biology in North America. Ann. Entomol. Soc. Am. 2004, 97, 204–208. [Google Scholar] [CrossRef]
- Wang, S.Y.; Bao, X.Z.; Sun, Y.J.; Chen, R.L.; Zhai, B.P. Effects of Soybean aphid on growth and Yield of soybean. Soybean Sci. (In Chinese). 1996, 15, 243–247. [Google Scholar]
- Koshimizu, Y.; Iizuka, N. Studies on soybean virus diseases in Japan. Bull. Tohoku Natl. Agric. Exp. Stn. 1963, 27, 1–103, (In Japanese with English Summary). [Google Scholar]
- Takahashi, K.; Tanaka, T.; Iida, W.; Tsuda, Y. Studies on virus diseases and causal viruses of soybean in Japan. Bull. Tohoku Natl. Agric. Exp. Stn. 1980, 62, 1–130, (In Japanese with English Summary). [Google Scholar]
- Hill, J.H.; Alleman, R.; Hogg, D.B. First report of transmission of Soybean mosaic virus and Alfalfa mosaic virus by Aphis glycines in the New World. Plant Dis. 2001, 85, 561. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, R.Y.; Ghabrial, S.A. Effect of Aphid Behavior on Efficiency of Transmission of Soybean mosaic virus by the Soybean–Colonizing Aphid, Aphis glycines. Plant Dis. 2002, 86, 1260–1264. [Google Scholar] [CrossRef] [PubMed]
- Irwin, M.E.; Ruesink, W.G.; Isard, S.A.; Kampmeier, G.E. Mitigating epidemics caused by non–persistently transmitted aphidborne viruses, the role of the pliant environment. Virus Res. 2000, 71, 185–211. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.R.; Deng, X.C.; Ying, L.J.; Song, Y.H.; Zhang, D.Y.; Shen, H.B. Analysis on factors of soybean aphid outbreak in Heilongjiang Province in 2004. Soybean Sci. Technol. 2005, 3, 19–20. (In Chinese) [Google Scholar]
- Lagos-Kutz, D.; Pawlowski, M.L.; Diers, B.W.; Pur, S.R.; Tilmon, K.J.; Hartman, G.L. Virulence of soybean aphid, Aphis glycines (Hemiptera, Aphididae) clones on detached leaves and whole plants. J. Kans. Entomol. Soc. 2020, 92, 497–511. [Google Scholar] [CrossRef]
- Zhang, G.X.; Zhong, T.S. Homoptera aphids. In Chinese Economic Entomology; Science Press: Beijing, China, 1983; Volume 25, pp. 214–215. [Google Scholar]
- Wang, C.R.; Chen, J.G.; Guo, T.R.; Gong, X.Y.; Xu, Z.F.; Lin, C. Occurrence regularity and control methods of soybean aphid in Heilongjiang Province. Soybean Sci. Technol. 1998, 6, 17. (In Chinese) [Google Scholar]
- Easterling, D.R.; Evans, J.L.; Groisman, P.Y.; Karl, T.R.; Kunkel, K.E.; Ambenje, P. Observed variability and trends in extreme climate events a brief review. Bull. Am. Meteorol. Soc. 2000, 81, 417–425. [Google Scholar] [CrossRef]
- Hansen, A.; Bi, P.; Nitschke, M.; Ryan, P.; Pisaniello, D.; Tucker, G. The effect of heat waves on mental health in a temperate Australian city. Environ. Health Perspect. 2008, 116, 1369–1375. [Google Scholar] [CrossRef]
- David, J.R.; Araripe, L.O.; Chakir, M.; Legout, H.; Lemos, B.; Pétavy, G.; Rohmer, C.; Joly, D.; Moreteau, B. Male sterility at extreme temperatures a significant but neglected phenomenon for understanding Drosophila climatic adaptations. J. Evol. Biol. 2005, 18, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Karl, I.; Stoks, R.; Block, M.D.; Janowitz, S.A.; Fischer, K. Temperature extremes and butterfly fitness conflicting evidence from life history and immune function. Glob. Change Biol. 2011, 17, 676–687. [Google Scholar] [CrossRef]
- Arambourou, H.; Stoks, R. Combined effects of larval exposure to a heat wave and chlorpyrifos in northern and southern populations of the damselfly Ischnura elegans. Chemosphere 2015, 128, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Denlinger, D.L.; Yocum, G.D. Temperature Sensitivity in Insects and Application in Integrated Pest Management in Physiology of Heat Sensitivity; CRC Press: Boca Raton, FL, USA, 2019; pp. 7–53. [Google Scholar]
- Feder, M.E.; Hofmann, G.E. Heat–shock proteins, molecular chaperones, and the stress response, evolutionary and ecological physiology. Annu. Rev. Physiol. 1999, 61, 243–282. [Google Scholar] [CrossRef]
- Pan, H.S.; Zhang, G.H.; Xu, N.P. A preliminary analysis of climate warming in Heilongjiang province since the 1980s. Clim. Environ. Res. (In Chinese). 2003, 8, 348–355. [Google Scholar]
- Zhou, X.J.; Wang, F.L.; Wu, Y.Y.; Na, J.H.; Pan, H.S.; Wang, Y. Analysis of the temperature change characteristics of Heilongjiang Province, northeast China and whole country in recent 60 years. J. Nat. Disasters 2013, 22, 124–129. (In Chinese) [Google Scholar]
- Heilongjiang Meteorological Bureau. Ambient Temperature Data of Harbin from 2010 to 2020. 2020. [Google Scholar]
- Fehr, W.R.; Caviness, C.E.; Pennington, J.S. Stage of development descriptions for soybeans Glycine max (L.) Merrill. Crop. Sci. 1971, 11, 929–931. [Google Scholar] [CrossRef]
- Bai, B.; Tian, Z.Q.; Gao, B.; Liu, Z.; Wang, L.; Liu, J. Performance of Aphis glycines Matsumura reared under different methods. Fla. Entomol. 2022, 105, 211–218. [Google Scholar] [CrossRef]
- Huo, D.B.; Tian, Z.Q.; Liu, D.L.; Wu, C.R.; Wang, L.; Liu, J. Adaptability of two soybean aphid species to high diurnal temperatures. Environ. Entomol. 2022, 51, 1241–1248. [Google Scholar] [CrossRef]
- Chen, X.H.; Fan, Y.J.; Tian, Z.Q.; Liu, J.; Zhao, K.J. Soybean aphid, Aphis glycines (Hemiptera, Aphididae), developmental and reproductive capacity on white clover, Trifolium repens (Rosales, Leguminosae), in northeast China. Appl. Entomol. Zool. 2017, 52, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.H.; Fan, Y.J.; Tian, Z.Q.; Liu, J.; Zhao, K.J. Effects of Temperature and Four Plant Species on morphological Development of Soybean Aphid. J. Environmanyal Entomol. 2015, 379, 250–257. (In Chinese) [Google Scholar]
- Tian, Z.Q.; Wang, S.J.; Bai, B.; Gao, B.; Liu, J. Effects of temperature on survival, development, and reproduction of Aphis glycines (Hemiptera, Aphididae) autumnal morphs. Fla. Entomol. 2020, 103, 236–242. [Google Scholar] [CrossRef]
- Chi, H.; Liu, H. Two new methods for the study of insect population ecology. Bull. Inst. Zool Acad Sin. 1985, 24, 225–240. [Google Scholar]
- Chi, H. TWOSEX–MSChart, Computer Program for the Age Stage, Two Sex Life Table Analysis. 2023. Available online: http://140.120.197.173/Ecology/prod02.htm (accessed on 1 May 2023).
- Efron, B.; Tibshirani, R.J. In Monographs on statistics and applied probability. In An Introduction to the Bootstrap; Chapman Hall/CRC Press: Boca Raton, FL, USA, 1993; pp. 49–54. [Google Scholar]
- Huang, Y.B.; Chi, H. Age–stage, two–sex life tables of Bactrocera cucurbitae (Coquillett) (Diptera, Tephritidae) with a discussion on the problem of applying female age–specific life tables to insect populations. Insect Sci. 2011, 19, 263–273. [Google Scholar] [CrossRef]
- Yu, L.Y.; Chen, Z.Z.; Zheng, F.Q.; Shi, A.J.; Guo, T.T.; Yeh, B.H.; Chi, H.; Xu, Y.Y. Demographic analysis, a comparison of the jackknife and bootstrap methods, and predation projection, a case study of Chrysopa pallens (Neuroptera, Chrysopidae). J. Econ. Entomol. 2013, 106, 1–9. [Google Scholar] [CrossRef]
- Wei, M.F.; Chi, H.; Guo, Y.F.; Li, X.W.; Zhao, L.L.; Ma, R.Y. Demography of Cacopsylla chinensis (Hemiptera, Psyllidae) reared on four cultivars of Pyrus bretschneideri (Rosales, Rosaceae) and P. communis pears with estimations of confidence intervals of specific life table statistics. J. Econ. Entomol. 2020, 113, 2343–2353. [Google Scholar] [CrossRef]
- SAS Institute. SAS Version 8.1 SAS Software. United States of America. Cary, North Carolina. 2000. Available online: https://www.sas.com/zh_cn/learn/academic–programs/software.html (accessed on 10 September 2002).
- Mccornack, B.P.; Ragsdale, D.W.; Venette, R.C. Demography of soybean aphid (Homoptera Aphididae) at summer temperatures. J. Econ. Entomol. 2004, 97, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Xu, G.Q.; Liu, P.B.; Chen, Y.; Wang, X.Y.; Zhao, T.H. Effects of temperature on growth development and reproduction of Soybean aphid. Chin. J. Oil Crop Sci. 2011, 33, 189–192, (In Chinese with English Abstract). [Google Scholar]
- Liu, J.; Bai, B.; Gao, B.; Liu, Z. Effects of Constant High Temperature on Survival Development and Reproduction of Aphis glycines Matsumura. J. Northeast. Agric. Univ. 2020, 27, 19–27, (English Edition). [Google Scholar]
- Tian, Z.Q.; Wang, S.J.; Bai, B.; Liu, J.; Zhao, K.J. A morphological study on autumnal morphs of Aphis glycines (Hemiptera, Aphididae). J. Asia Pac. Entomol. 2018, 21, 731–736. [Google Scholar] [CrossRef]
- Oka, Y.; Kagami–Yashima, C.; Kagawa, K.; Sonoda, S.; Murai, T. Clonal variation of sexual morph production in response to temperature and photoperiod in soybean aphid, Aphis glycines (Hemiptera, Aphididae). Appl. Entomol. Zool. 2018, 53, 509–517. [Google Scholar] [CrossRef]
- Yang, S.; Liu, J.; Dai, C.C.; Zhao, K.J. Morphological variantion for growth and development of soybean aphid collected from different geographical zones. Chin. J. Appl. Entomol. 2010, 47, 67–71. [Google Scholar]
- Dixon, A.F.G. Aphid Ecology, An Optimization Approach; Springer Science & Business Media: Berlin, Germany, 1998. [Google Scholar]
- Ma, C.S.; Hau, B.; Poehling, H.M. The effect of heat stress on the survival of the rose grain aphid Metopolophium dirhodum (Hemiptera, Aphididae). Eur. J. Entomol. 2004, 101, 327–332. [Google Scholar] [CrossRef]
- Wang, C.L.; Xiang, L.Y.; Zhang, G.X.; Zhu, H.X. Studies on soybean aphid Aphis glycines Matsumura. Acta Entomol. Sin. (In Chinese). 1962, 11, 31–44. [Google Scholar]
- Yang, G.; Nie, S.S.; Hang, J.N.; Li, H.Y.; Fang, Z.M. Research progress of melatonin in plant growth and development and stress response. J. Mt. Agric. Biol. 2022, 41, 37–46. (In Chinese) [Google Scholar]
- Gao, N.; Hardie, J. Melatonin and the pea aphid, Acyrthosiphon pisum. J. Insect Physiol. 1997, 43, 615–620. [Google Scholar] [CrossRef]
- Liu, X.L.; Li, X.M.; Liu, C.L.; Wang, K.Q.; Wang, S.; Xia, J.X.; Yang, F.; Shao, T.Y.; Xu, W.J. Population dynamics and spatial distribution pattern of Aphis glycines in Heilongjiang provines. Chin. J. Appl. Entomol. 2011, 48, 1608–1612. [Google Scholar]
- Sun, L.H.; Wan, L.; Li, W.; Chen, X.Y.; Chen, Y.; Li, X.C.; Zou, X.K.; Jiang, Y.D.; Zeng, H.L.; Ai, W.X. Climatic charateristics review over China in 2022. China Flood Drought Manag. 2023, 33, 8–15+21. [Google Scholar]
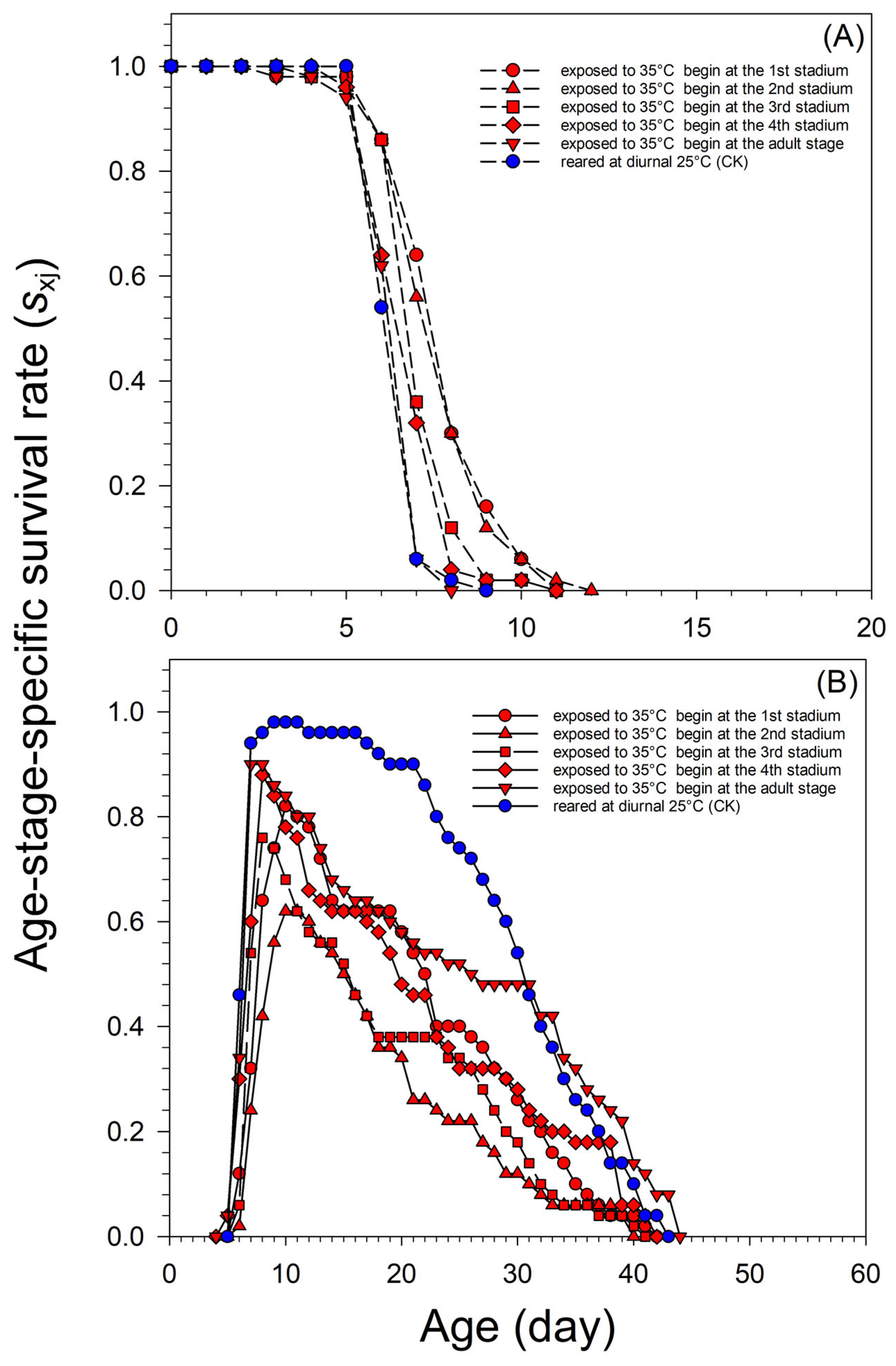
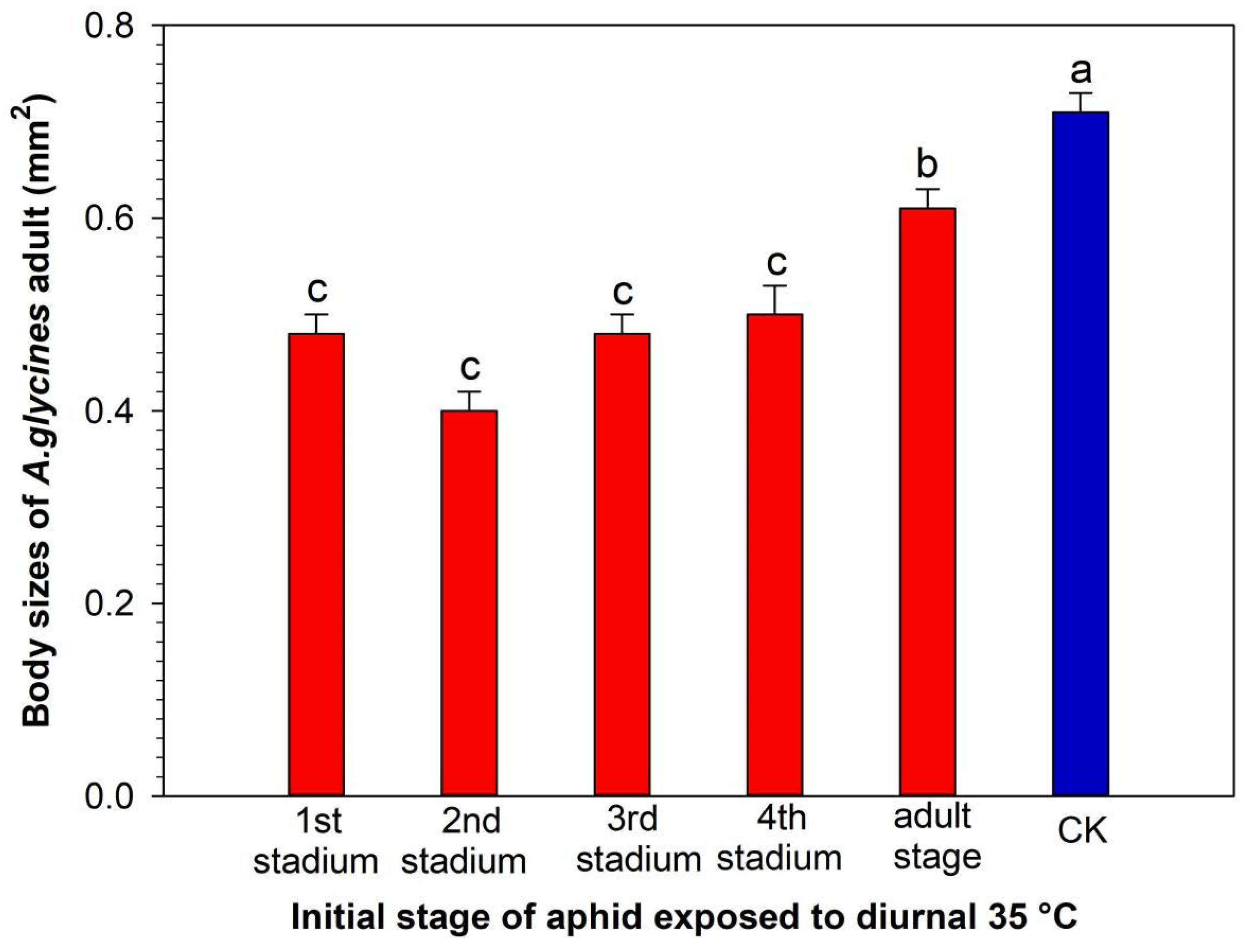
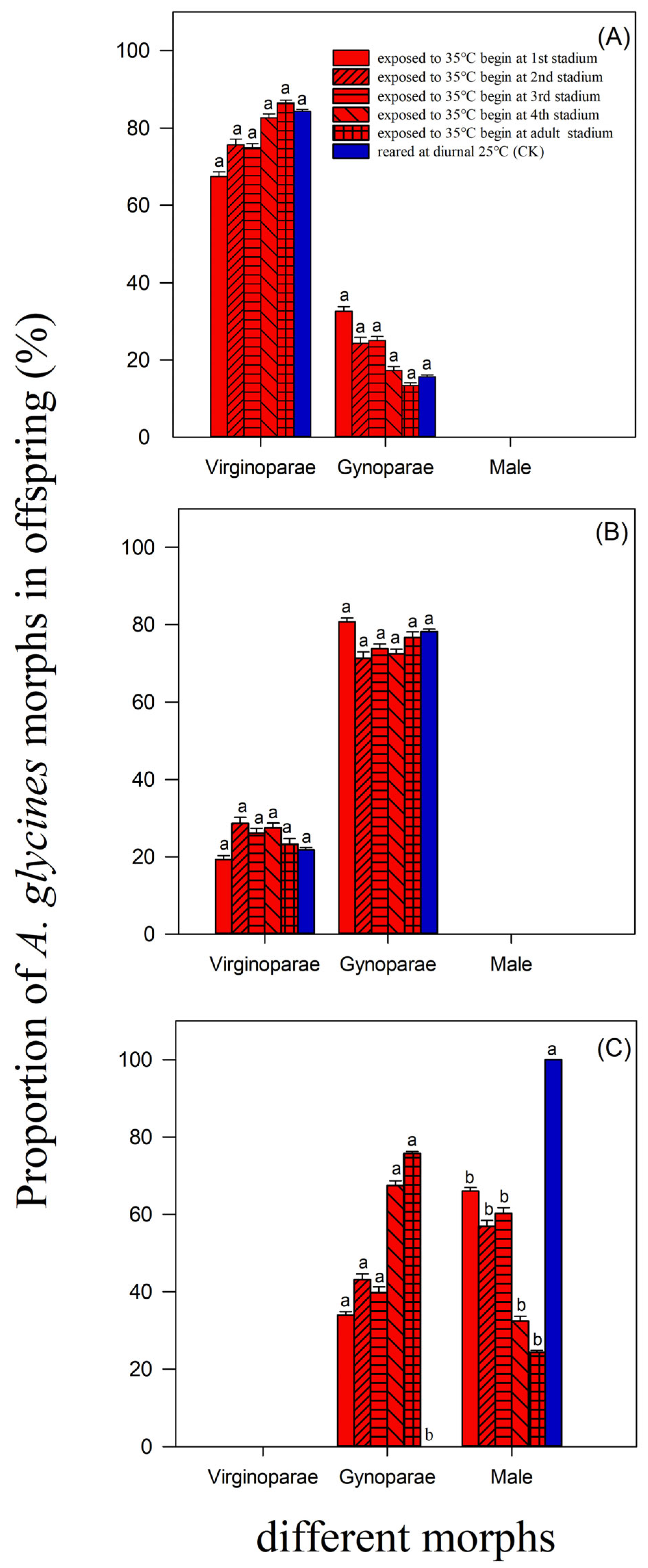
| Initial Stage of Aphids Exposed to Diurnal 35 °C | n | Nymph Stage Duration (Day) | Adult Lifespan (Day) | Total Lifespan (Day) |
|---|---|---|---|---|
| 1st–stadium | 45 | 8.00 ± 0.20 a | 15.80 ± 1.39 bc | 22.78 ± 1.45 bc |
| 2nd–stadium | 37 | 8.24 ± 0.21 a | 12.43 ± 1.48 c | 20.73 ± 1.50 cd |
| 3rd–stadium | 46 | 7.50 ± 0.14 b | 12.30 ± 1.47 c | 19.22 ± 1.47 cd |
| 4th–stadium | 46 | 6.98 ± 0.14 c | 16.11 ± 1.58 bc | 21.93 ± 1.60 bcd |
| Adult stage | 49 | 6.65 ± 0.10 d | 19.53 ± 1.82 b | 24.76 ± 1.77 ab |
| Aphids reared at diurnal 25 °C (CK) | 50 | 6.62 ± 0.09 d | 23.68 ± 1.10 a | 29.40 ± 1.10 a |
| Initial Stage of Aphids Exposed to Diurnal 35 °C | n | Adult Reproduction Duration (Day) | Adult Fecundity (Offspring per Female) | Intrinsic Rate of Increase (Day−1) |
|---|---|---|---|---|
| 1st–stadium | 45 | 11.25 ± 1.03 b | 19.91 ± 2.29 b | 0.2055 ± 0.0093 d |
| 2nd–stadium | 37 | 5.81 ± 0.79 d | 5.38 ± 1.10 d | 0.1133 ± 0.0151 f |
| 3rd–stadium | 46 | 6.63 ± 0.85 d | 9.98 ± 1.74 c | 0.1782 ± 0.0102 e |
| 4th–stadium | 46 | 7.71 ± 0.84 cd | 15.33 ± 1.96 b | 0.2339 ± 0.0090 c |
| Adult stage | 49 | 9.24 ± 0.70 bc | 17.18 ± 1.26 b | 0.2723 ± 0.0069 b |
| Aphids reared at diurnal 25 °C (CK) | 50 | 15.62 ± 0.59 a | 50.86 ± 1.94 a | 0.3408 ± 0.0056 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.; Liu, D.; Gu, C.; Tian, Z.; Zhang, X.; Liu, J. Control Effects of Short-Term Heatwaves on a Holocyclic Aphid. Insects 2024, 15, 100. https://doi.org/10.3390/insects15020100
Wu C, Liu D, Gu C, Tian Z, Zhang X, Liu J. Control Effects of Short-Term Heatwaves on a Holocyclic Aphid. Insects. 2024; 15(2):100. https://doi.org/10.3390/insects15020100
Chicago/Turabian StyleWu, Cirui, Dailin Liu, Chengxu Gu, Zhenqi Tian, Xinxin Zhang, and Jian Liu. 2024. "Control Effects of Short-Term Heatwaves on a Holocyclic Aphid" Insects 15, no. 2: 100. https://doi.org/10.3390/insects15020100
APA StyleWu, C., Liu, D., Gu, C., Tian, Z., Zhang, X., & Liu, J. (2024). Control Effects of Short-Term Heatwaves on a Holocyclic Aphid. Insects, 15(2), 100. https://doi.org/10.3390/insects15020100






