Breaking the Law: Is It Correct to Use the Converse Bergmann Rule in Ceroglossus chilensis? An Overview Using Geometric Morphometrics
Abstract
Simple Summary
Abstract
1. Introduction
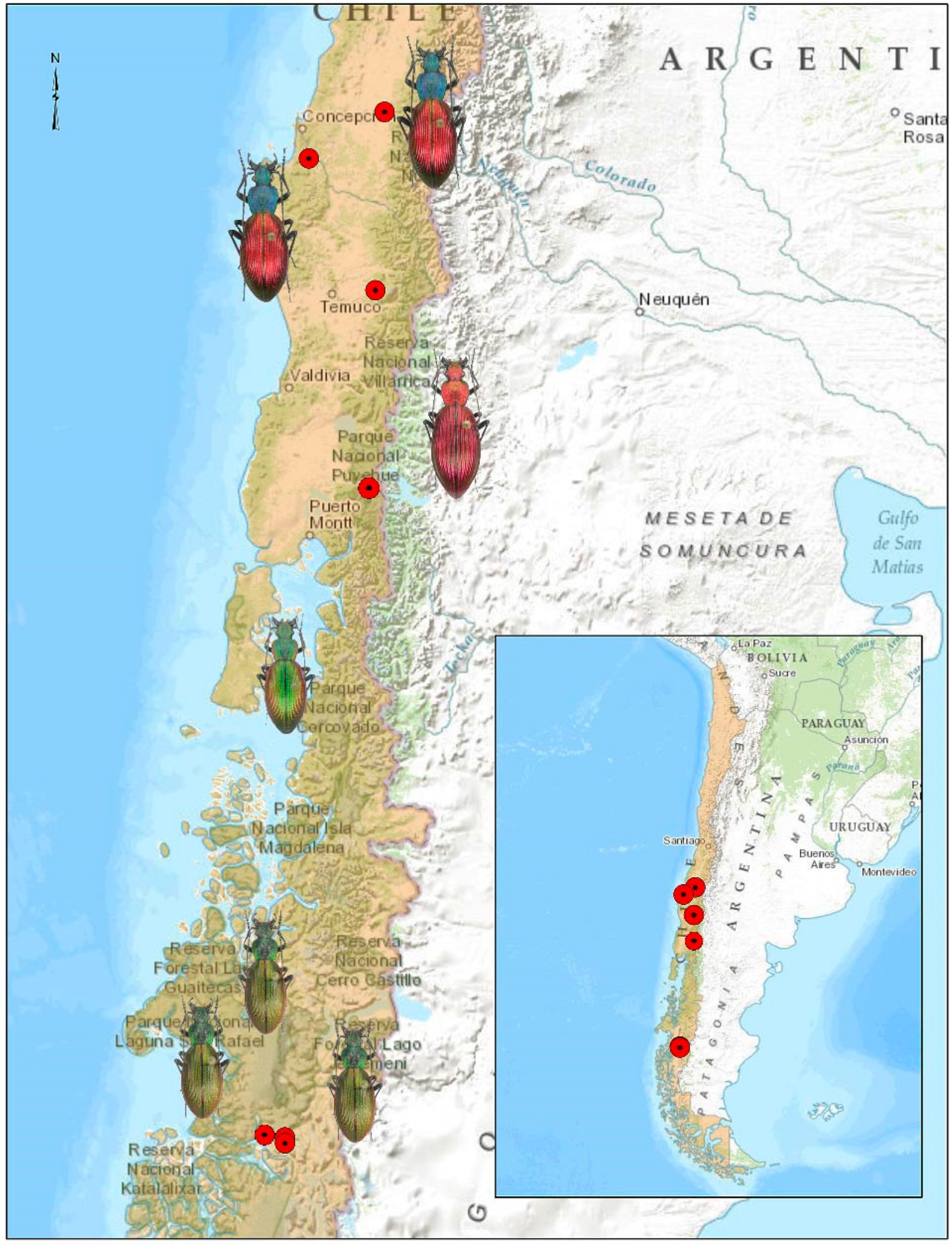
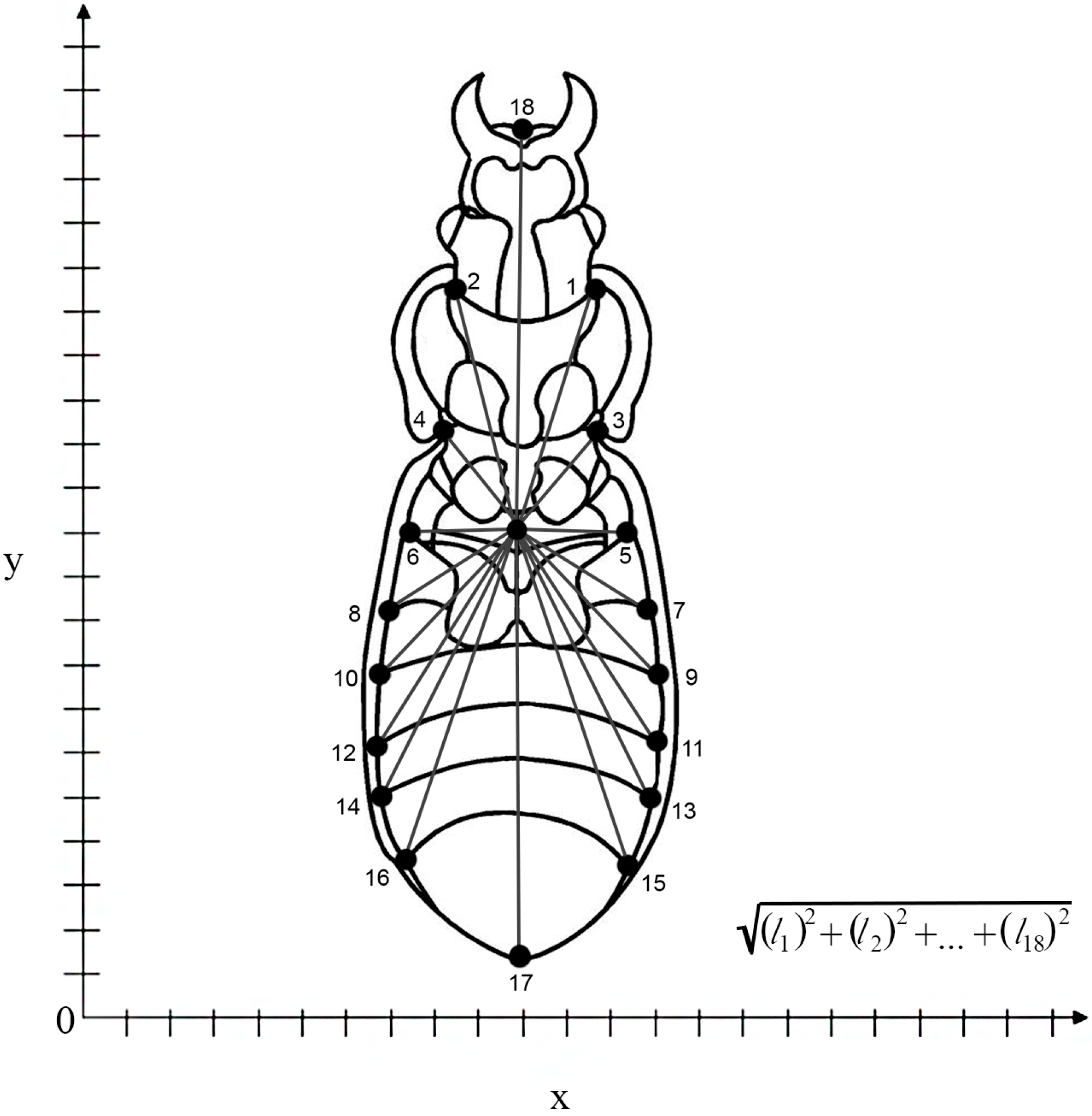
2. Materials and Methods
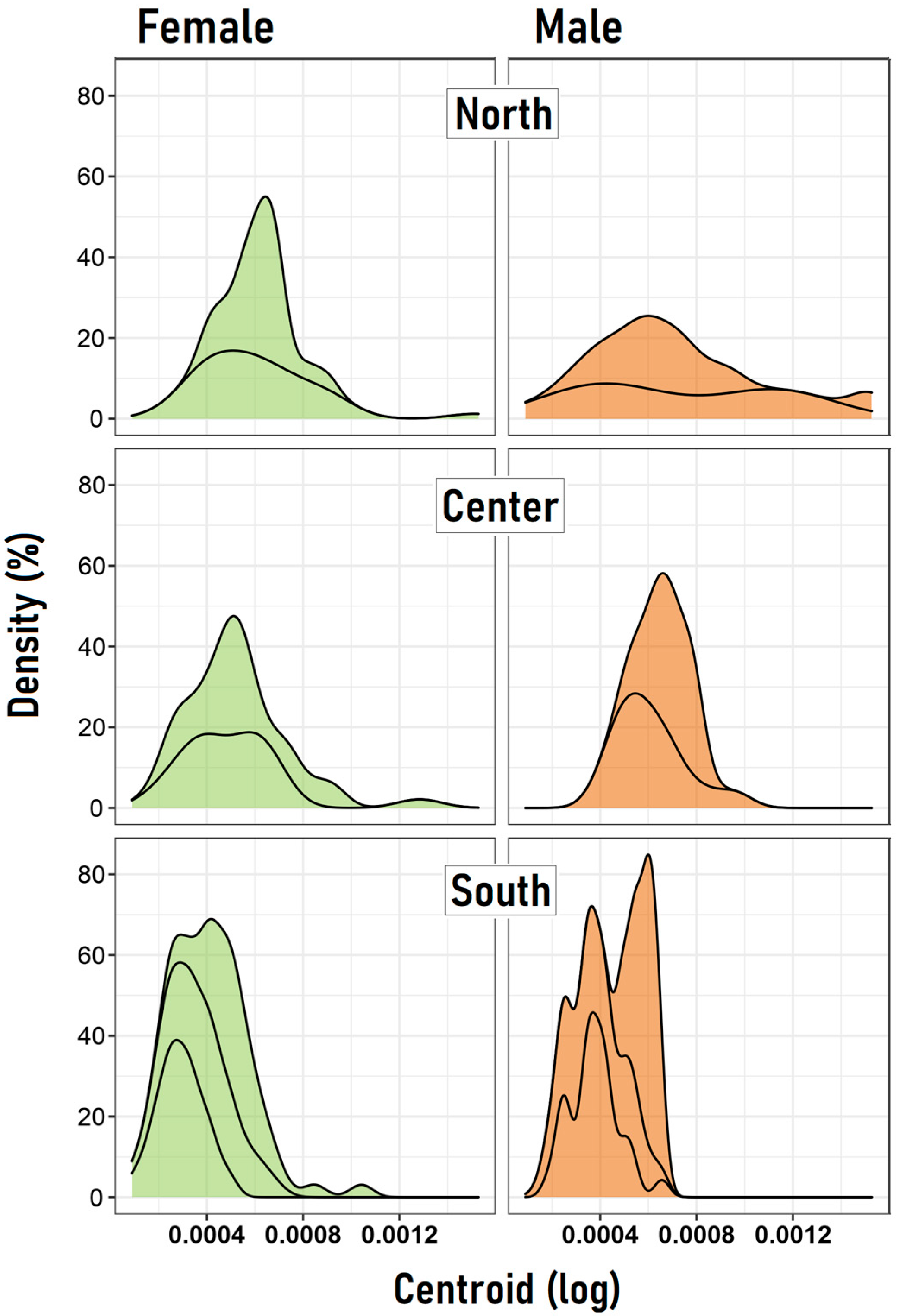
3. Results
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salewski, V.; Watt, C. Bergmann’s rule: A biophysiological rule examined in birds. Oikos 2017, 126. [Google Scholar] [CrossRef]
- Watt, C.; Mitchell, S.; Salewski, V. Bergmann’s rule; A concept cluster? Oikos 2010, 119, 89–100. [Google Scholar] [CrossRef]
- Shelomi, M. Where are we now? Bergmann’s rule sensu lato in insects. Am. Nat. 2012, 180, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Meiri, S. Bergmann’s Rule—What’s in a name? Glob. Ecol. Biogeogr. 2011, 20, 203–207. [Google Scholar] [CrossRef]
- Shelomi, M.; Zeuss, D. Bergmann’s and Allen’s rules in native European and Mediterranean Phasmatodea. Front. Ecol. Evol. 2017, 5, 25. [Google Scholar] [CrossRef]
- Baranovská, E.; Knapp, M. Steep converse Bergmann’s cline in a carrion beetle: Between-and within-population variation in body size along an elevational gradient. J. Zool. 2018, 304, 243–251. [Google Scholar] [CrossRef]
- Mousseau, T.A. Ectotherms follow the converse to Bergmann’s rule. Evolution 1997, 51, 630–632. [Google Scholar] [CrossRef]
- Lövei, G.L.; Magura, T. Body size and the urban heat island effect modulate the temperature–size relationship in ground beetles. J. Biogeogr. 2022, 49, 1618–1628. [Google Scholar] [CrossRef]
- Blanckenhorn, W.U.; Stillwell, R.C.; Young, K.A.; Fox, C.W.; Ashton, K.G. When Rensch meets Bergmann: Does sexual size dimorphism change systematically with latitude? Evolution 2006, 60, 2004–2011. [Google Scholar]
- Gérard, M.; Vanderplanck, M.; Franzen, M.; Kuhlmann, M.; Potts, S.G.; Rasmont, P.; Schweiger, O.; Michez, D. Patterns of size variation in bees at a continental scale: Does Bergmann’s rule apply? Oikos 2018, 127, 1095–1103. [Google Scholar] [CrossRef]
- Blanckenhorn, W.; Demont, M. Bergmann and converse Bergmann latitudinal clines in arthropods: Two ends of a continuum? Integr. Comp. Biol. 2004, 44, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Meiri, S.; Dayan, T. On the validity of Bergmann’s rule. J. Biogeogr. 2003, 30, 331–351. [Google Scholar] [CrossRef]
- Partridge, L.; Coyne, J.A. Bergmann’s rule in ectotherms: Is it adaptive? Evolution 1997, 51, 632–635. [Google Scholar] [CrossRef] [PubMed]
- Rendoll-Cárcamo, J.; Gañán, M.; Madriz, R.; Convey, P.; Contador, T. Wing reduction and body size variation along a steep elevation gradient: A case study with Magellanic sub-Antarctic mayflies and stoneflies. Front. Ecol. Evol. 2023, 11, 1188889. [Google Scholar] [CrossRef]
- Ashton, K.G.; Feldman, C.R. Bergmann’s rule in nonavian reptiles: Turtles follow it, lizards and snakes reverse it. Evolution 2003, 57, 1151–1163. [Google Scholar] [PubMed]
- Rivas, J.; Quiero, A.; Penna, M.; Velásquez, N.A. Body-size variation across environmental gradients in an ectothermic organism: An intraspecific approach to ecogeographic patterns. Herpetologica 2018, 74, 191–198. [Google Scholar] [CrossRef]
- Ray, C. The application of Bergmann’s and Allen’s rules to the poikilotherms. J. Morphol. 1960, 106, 85–108. [Google Scholar] [CrossRef] [PubMed]
- Van Voorhies, W.A. Bergmann size clines: A simple explanation for their occurrence in ectotherms. Evolution 1996, 50, 1259–1264. [Google Scholar] [CrossRef]
- Karl, I.; Fischer, K. Why get big in the cold? Towards a solution to a life-history puzzle. Oecologia 2008, 155, 215–225. [Google Scholar] [CrossRef]
- Sanzana, M.-J.; Parra, L.E.; Sepulveda-Zuniga, E.; Benitez, H.A. Latitudinal gradient effect on the wing geometry of Auca coctei (Guerin) (Lepidoptera, Nymphalidae). Rev. Bras. Entomol. 2013, 57, 411–416. [Google Scholar] [CrossRef][Green Version]
- Benítez, H.A.; Sukhodolskaya, R.A.; Avtaeva, T.A.; Escobar-Suárez, S.; Órdenes-Claveria, R.; Laroze, D.; Hernández-P, R.; Vavilov, D.N. Quantifying elevational effect on the geometric body shape of Russian beetle Carabus exaratus (Coleoptera: Carabidae). Zool. Anz. 2023, 302, 30–36. [Google Scholar] [CrossRef]
- Vamosi, J.C.; Vamosi, S.M. Body size, rarity, and phylogenetic community structure: Insights from diving beetle assemblages of Alberta. Divers. Distrib. 2007, 13, 1–10. [Google Scholar] [CrossRef]
- Pallarés, S.; Lai, M.; Abellán, P.; Ribera, I.; Sánchez-Fernández, D. An interspecific test of Bergmann’s rule reveals inconsistent body size patterns across several lineages of water beetles (Coleoptera: Dytiscidae). Ecol. Entomol. 2019, 44, 249–254. [Google Scholar] [CrossRef]
- Ramírez-Delgado, V.H.; Sanabria-Urbán, S.; Serrano-Meneses, M.A.; Cueva del Castillo, R. The converse to Bergmann’s rule in bumblebees, a phylogenetic approach. Ecol. Evol. 2016, 6, 6160–6169. [Google Scholar] [CrossRef]
- Benítez, H.A.; Avaria-Llautureo, J.; Canales-Aguirre, C.B.; Jerez, V.; Parra, L.E.; Hernandez, C.E. Evolution of sexual size dimorphism and its relationship with sex ratio in carabid beetles of Genus Ceroglossus Solier. Curr. Zool. 2013, 59, 769–777. [Google Scholar] [CrossRef]
- Muñoz-Ramírez, C. The phylogenetic position of Ceroglossus ochsenii GERMAIN and Ceroglossus guerini GERMAIN (Coleoptera: Carabidae), two endemic ground beetles from the Valdivian forest of Chile. Rev. Chil. Entomol. 2015, 40, 14–21. [Google Scholar]
- López-López, A.; Acosta, V.; Rataj, L.; Galián, J. Evolution and diversification of the Southern Chilean genus Ceroglossus (Coleoptera, Carabidae) during the Pleistocene glaciations. Syst. Entomol. 2021, 46, 856–869. [Google Scholar] [CrossRef]
- Muñoz-Ramírez, C.P.; Bitton, P.-P.; Doucet, S.M.; Knowles, L.L. Mimics here and there, but not everywhere: Müllerian mimicry in Ceroglossus ground beetles? Biol. Lett. 2016, 12, 20160429. [Google Scholar] [CrossRef]
- Jiroux, E. Le Genre Ceroglossus; Collection Systematique; Magellanes: Nice, France, 2006; Volume 14. [Google Scholar]
- Benítez, H.; Briones, R.; Jerez, V. Fluctuating asymmetry in two populations of Ceroglossus chilensis (Eschscholtz, 1829) (Coleoptera: Carabidae) in agroecosystem of Pinus radiata d. Don, Bio-Bio region, Chile. Gayana 2008, 72, 131–139. [Google Scholar]
- Benítez, H.A.; Briones, R.; Jerez, V. Intra and Inter-population morphological variation of shape and size of the Chilean magnificent beetle, Ceroglossus chilensis in the Baker River Basin, Chilean Patagonia. J. Insect Sci. 2011, 11, 94. [Google Scholar] [CrossRef][Green Version]
- Juache, A.; Ordenes, R.; Benítez, H.A. Quantifying the shape variation of the elytra in Patagonian populations of the ground beetle Ceroglossus chilensis (Coleoptera: Carabidae). Zool. Anz. 2018, 274, 123–126. [Google Scholar] [CrossRef]
- Benítez, H.A.; Sanzana, M.-J.; Jerez, V.; Parra, L.E.; Hernandez, C.E.; Canales-Aguirre, C.B. Sexual Shape and Size Dimorphism in Carabid Beetles of the Genus Ceroglossus: Is Geometric Body Size Similar between Sexes due to Sex Ratio? Zool. Sci. 2013, 30, 289–295. [Google Scholar] [CrossRef]
- Benítez, H.A.; Vidal, M.; Briones, R.; Jerez, V. Sexual Dimorphism and Morphological Variation in Populations of Ceroglossus chilensis (Eschscholtz, 1829) (Coleoptera: Carabidae). J. Entomol. Res. Soc. 2010, 12, 87–95. [Google Scholar]
- Briones, R.; Jerez, V.; Benítez, H.A. Vertical Diversity of Beetles (Insecta: Coleoptera) Associated with Lithraea caustica (Anacardiaceae) in Patches of Sclerophyllous Forest in Central Chile. J. Entomol. Res. Soc. 2013, 15, 41–52. [Google Scholar]
- Briones, R.; Flores, F.G.; Jerez, V. Insectos de Chile: Nativos, Introducidos y con Problemas de Conservación; Corporación Chilena de la Madera (CORMA): Concepción, Chile, 2012. [Google Scholar]
- Rohlf, F.J. TPSdig, v. 2.17; State University at Stony Brook: New York, NY, USA, 2013. [Google Scholar]
- Rohlf, F.J.; Slice, D. Extensions of the Procustes methods for the optimal superimposition of landmarks. Syst. Zool. 1990, 39, 40–59. [Google Scholar] [CrossRef]
- Dryden, I.L.; Mardia, K.V. Statistical Shape Analysis; Wiley: Chichester, UK, 1998; Volume 4. [Google Scholar]
- Fruciano, C. Measurement error in geometric morphometrics. Dev. Genes Evol. 2016, 226, 139–158. [Google Scholar] [CrossRef]
- Arnqvist, G.; Martensson, T. Measurement error in geometric morphometrics: Empirical strategies to assess and reduce its impact on measures of shape. Acta Zool. Acad. Sci. Hung. 1998, 44, 73–96. [Google Scholar]
- Rohlf, F.J.; Marcus, L.F. A revolution in morphometrics. Trends Ecol. Evol. 1993, 8, 129–132. [Google Scholar] [CrossRef]
- Bates, D. Mixed Models in R Using the lme4 Package Part 5: Generalized Linear Mixed Models; University of Wisconsin: Madison, WI, USA, 2011. [Google Scholar]
- Lo, S.; Andrews, S. To transform or not to transform: Using generalized linear mixed models to analyse reaction time data. Front. Psychol. 2015, 6, 1171. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. arXiv 2014, arXiv:1406.5823. [Google Scholar]
- Klingenberg, C.P. MorphoJ: An integrated software package for geometric morphometrics. Mol. Ecol. Resour. 2011, 11, 353–357. [Google Scholar] [CrossRef]
- Vinarski, M. On the applicability of Bergmann’s rule to ectotherms: The state of the art. Biol. Bull. Rev. 2014, 4, 232–242. [Google Scholar] [CrossRef]
- Meiri, S.; Thomas, G.H. The geography of body size–challenges of the interspecific approach. Glob. Ecol. Biogeogr. 2007, 16, 689–693. [Google Scholar] [CrossRef]
- Sota, T.; Takami, Y.; Kubota, K.; Ujiie, M.; Ishikawa, R. Interspecific body size differentiation in species assemblages of the carabid subgenus Ohomopterus in Japan. Popul. Ecol. 2000, 42, 279–291. [Google Scholar] [CrossRef]
- Sota, T.; Ishikawa, R. Phylogeny and life-history evolution in Carabus (subtribe Carabina: Coleoptera, Carabidae) based on sequences of two nuclear genes. Biol. J. Linn. Soc. 2004, 81, 135–149. [Google Scholar] [CrossRef]
- Bjørnstad, O.N.; Nelson, W.A.; Tobin, P.C. Developmental synchrony in multivoltine insects: Generation separation versus smearing. Popul. Ecol. 2016, 58, 479–491. [Google Scholar] [CrossRef]
- Nijhout, H. The control of body size in insects. Dev. Biol. 2003, 261, 1–9. [Google Scholar] [CrossRef]
- Koyama, T.; Mirth, C.K. Unravelling the diversity of mechanisms through which nutrition regulates body size in insects. Curr. Opin. Insect Sci. 2018, 25, 1–8. [Google Scholar] [CrossRef]
- Koyama, T.; Mendes, C.C.; Mirth, C.K. Mechanisms regulating nutrition-dependent developmental plasticity through organ-specific effects in insects. Front. Physiol. 2013, 4, 263. [Google Scholar] [CrossRef]
- Nijhout, H.F.; Riddiford, L.M.; Mirth, C.; Shingleton, A.W.; Suzuki, Y.; Callier, V. The developmental control of size in insects. Wiley Interdiscip. Rev.: Dev. Biol. 2014, 3, 113–134. [Google Scholar] [CrossRef]
- Makarieva, A.M.; Gorshkov, V.G.; Li, B.-L. Body size, energy consumption and allometric scaling: A new dimension in the diversity–stability debate. Ecol. Complex. 2004, 1, 139–175. [Google Scholar] [CrossRef]
- Gallé, R.; Geppert, C.; Földesi, R.; Tscharntke, T.; Batáry, P. Arthropod functional traits shaped by landscape-scale field size, local agri-environment schemes and edge effects. Basic Appl. Ecol. 2020, 48, 102–111. [Google Scholar] [CrossRef]
- Davidowitz, G.; D’Amico, L.J.; Nijhout, H.F. The effects of environmental variation on a mechanism that controls insect body size. Evol. Ecol. Res. 2004, 6, 49–62. [Google Scholar]
- Lira, A.F.; Andrade, A.R.; Foerster, S.I. Latitudinal Trends in Scorpion Assemblages of Brazilian Atlantic Forest: Do the Rapoport’s and Bergmann’s Rules Apply? In Neotropical Gradients and Their Analysis; Springer: Berlin/Heidelberg, Germany, 2023; pp. 179–203. [Google Scholar]
- Romero, G.Q.; Gonçalves-Souza, T.; Roslin, T.; Marquis, R.J.; Marino, N.A.C.; Novotny, V.; Cornelissen, T.; Orivel, J.; Sui, S.; Aires, G.; et al. Climate variability and aridity modulate the role of leaf shelters for arthropods: A global experiment. Glob. Chang. Biol. 2022, 28, 3694–3710. [Google Scholar] [CrossRef]
- Lee, C.Y.; Kim, M.K.; Kim, D.-G. Ecological Responses of Nannophya koreana (Odonata: Libellulidae) to Temperature: Following Converse Bergmann’s Rule. Biology 2022, 11, 830. [Google Scholar]


| Trait | Factor | b | SE | t | p |
|---|---|---|---|---|---|
| All | Intercept | −7.516 | 0.140 | −53.54 | <0.0001 |
| Sex (female–male) | 0.098 | 0.111 | 0.87 | 0.3804 | |
| Zone (C–N) | 0.137 | 0.175 | 0.78 | 0.4320 | |
| Zone (C–S) | −0.322 | 0.155 | −2.07 | 0.0378 | |
| Zone (N–S) | −0.460 | 0.140 | −3.28 | 0.0010 | |
| North | Intercept | −7.414 | 0.065 | −113.61 | <0.0001 |
| Sex (female–male) | 0.077 | 0.085 | 0.90 | 0.3660 | |
| Population (PC–CC) | −0.055 | 0.081 | −0.68 | 0.4960 | |
| Center | Intercept | −7.592 | 0.068 | −110.53 | <0.0001 |
| Sex (female–male) | 0.270 | 0.085 | 3.17 | 0.0015 | |
| Population (PM–MZ) | −0.081 | 0.085 | −0.96 | 0.3290 | |
| South | Intercept | −7.575 | 0.051 | −145.80 | <0.0001 |
| Sex (female–male) | 0.094 | 0.048 | 1.96 | 0.049 | |
| Population (B2–B1) | −0.384 | 0.059 | −6.42 | <0.0001 | |
| Population (B3–B1) | −0.529 | 0.064 | −8.14 | <0.0001 | |
| Population (B3–B2) | 0.140 | 0.056 | 2.48 | 0.0131 |
| Zone | Population | Mean Female | Mean Male | Mean Diff | CI-Low | CI-High | t | d.f. | p |
|---|---|---|---|---|---|---|---|---|---|
| North | CC | 0.000616 | 0.000701 | −8.55 × 10−5 | −2.3 × 10−4 | 6.1 × 10−5 | −1.199 | 25.62 | 0.2411 |
| PC | 0.000612 | 0.000725 | −1.13 × 10−4 | −4.7 × 10−4 | 2.5 × 10−4 | −0.727 | 7.12 | 0.4904 | |
| Center | MZ | 0.000531 | 0.000688 | −1.56 × 10−4 | −2.6 × 10−4 | −4.6 × 10−5 | −2.922 | 24.91 | 0.0072 |
| PM | 0.000512 | 0.000605 | −9.25 × 10−5 | −2.2 × 10−4 | 3.8 × 10−5 | −1.444 | 32.15 | 0.1581 | |
| South | B3 | 0.000291 | 0.000375 | −8.34 × 10−5 | −1.3 × 10−4 | −3.0 × 10−5 | −3.137 | 53.72 | 0.0027 |
| B2 | 0.000395 | 0.000382 | 1.34 × 10−5 | −4.3 × 10−5 | 7.0 × 10−5 | 0.471 | 77.81 | 0.6389 | |
| B1 | 0.000527 | 0.000579 | −5.18 × 10−5 | −1.1 × 10−4 | 1.4 × 10−5 | −1.587 | 32.95 | 0.1219 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benítez, H.A.; Muñoz-Ramírez, C.; Correa, M.; Acuña-Rodríguez, I.S.; Villalobos-Leiva, A.; Contador, T.; Velásquez, N.A.; Suazo, M.J. Breaking the Law: Is It Correct to Use the Converse Bergmann Rule in Ceroglossus chilensis? An Overview Using Geometric Morphometrics. Insects 2024, 15, 97. https://doi.org/10.3390/insects15020097
Benítez HA, Muñoz-Ramírez C, Correa M, Acuña-Rodríguez IS, Villalobos-Leiva A, Contador T, Velásquez NA, Suazo MJ. Breaking the Law: Is It Correct to Use the Converse Bergmann Rule in Ceroglossus chilensis? An Overview Using Geometric Morphometrics. Insects. 2024; 15(2):97. https://doi.org/10.3390/insects15020097
Chicago/Turabian StyleBenítez, Hugo A., Carlos Muñoz-Ramírez, Margarita Correa, Ian S. Acuña-Rodríguez, Amado Villalobos-Leiva, Tamara Contador, Nelson A. Velásquez, and Manuel J. Suazo. 2024. "Breaking the Law: Is It Correct to Use the Converse Bergmann Rule in Ceroglossus chilensis? An Overview Using Geometric Morphometrics" Insects 15, no. 2: 97. https://doi.org/10.3390/insects15020097
APA StyleBenítez, H. A., Muñoz-Ramírez, C., Correa, M., Acuña-Rodríguez, I. S., Villalobos-Leiva, A., Contador, T., Velásquez, N. A., & Suazo, M. J. (2024). Breaking the Law: Is It Correct to Use the Converse Bergmann Rule in Ceroglossus chilensis? An Overview Using Geometric Morphometrics. Insects, 15(2), 97. https://doi.org/10.3390/insects15020097









