1. Introduction
In recent years, strawberry production has been moving into greenhouses as protected cultivation offers several advantages over conventional field production. In addition to the obvious advantage of protecting the crop from extreme weather events, controlling the production environment allows growers to produce better quality fruits at faster harvest cycles, to optimize the water and nutrient use, and to provide supplemental lighting for out-of-season, i.e., winter, production [
1]. Demand for organically grown strawberries is also on the rise, and the potential profits of greenhouse-grown organic strawberries and greenhouse-grown conventional strawberries are as much as 9.5 times and 1.5 times greater than field production, respectively [
2].
Western flower thrips (WFT),
Frankliniella occidentalis Pergande (Thysanoptera: Thripidae), and two-spotted spider mites (TSSM),
Tetranychus urticae Koch (Acari: Tetranychidae), are some of the most difficult-to-control arthropod pests in greenhouse crops worldwide, due to their small size, high reproduction rate, cryptic habits, and ability to develop resistance to chemical pest-control compounds [
3,
4,
5,
6,
7]. In strawberry production, both species cause feeding damage on leaves and flowers and reduce the yield [
3,
8,
9], but WFT especially can cause damage to the fruits (bronzing, cracking, and cat-facing injuries) [
10]. Tactics for the biological control of these pests often include the application of phytoseiid predatory mites or entomopathogenic fungi [
5,
8,
9,
11,
12], and combinations of both have also been recommended [
13,
14].
Several insects from Nabidae, a predatory hemipteran family, have good potential as biological control agents due to their global distribution, natural occurrence in several crops, and wide range of pests they can attack, including prey much larger than themselves [
15]. In open-field strawberries, nabids were reported to feed on green strawberry aphids, strawberry root aphids, and potato aphids in Turkey [
16]; on European tarnished plant bugs in the UK [
17]; on tarnished plant bugs in eastern Canada [
18]; and on two-spotted spider mites in New Zealand [
19]. In a previous study, three nabid species commonly occurring in southern Ontario,
Nabis americoferus Carayon,
Nabis roseipennis Reuter, and
Hoplistoscelis pallescens (Reuter), were screened for their potential as new biological control agents in Canadian greenhouses by comparing life histories and examining their laboratory predation efficacy against WFT, TSSM, greenhouse whiteflies [
Trialeurodes vaporariorum (Westwood)], and green peach aphids [
Myzus persicae (Sulzer)]. As a result,
N. americoferus was determined to be the most suitable predator because this species had a relatively short life cycle, and was a significantly better predator of WFT, while showing similar efficacy against the rest of the pests tested [
20].
When scouting for natural enemies in field strawberry in southern Ontario,
N. americoferus was observed to be one of the most encountered predators (personal observation). This study examined if this predatory bug can be integrated with, and will add value to, a common biological control program for greenhouse strawberries.
Nabis americoferus is a generalist predator and has a large body size (10–13 mm as an adult). This, and their voracious nature, may cause adverse effects on the phytoseiid predatory mites commonly used on greenhouse strawberry, so it is important to determine the risk of intra-guild predation (IGP). IGP occurs when natural enemies share the same prey and attack each other, especially when the prey population is low, which can reduce the biological control program efficacy [
21]. In addition to phytoseiid predatory mites, microbial agents such as entomopathogenic fungi are also an important tool in greenhouse strawberry pest management, so the susceptibility of
N. americoferus to this microbial agent also needs to be clarified.
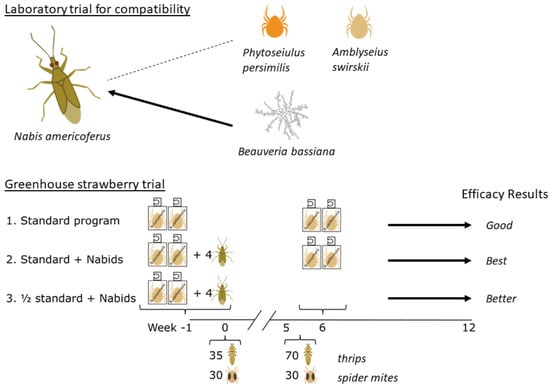
First, this study examined the compatibility of
N. americoferus with phytoseiid mites and a fungal bioinsecticide commonly used on Canadian greenhouse crops. Trials were carried out in the laboratory using the following species:
Phytoseiulus persimilis Athias-Henriot, a specialist phytoseiid mite used exclusively to control TSSM on foliage;
Amblyseius swirskii Athias-Henriot, a generalist phytoseiid mite used to manage WFT larvae and whitefly; and
Beauveria bassiana (Balsamo) GHA strain, an entomopathogenic fungus with a wide host range. Second, a greenhouse cage study was conducted in order to determine if it is beneficial to add
N. americoferus to the phytoseiid-mites-based biological control program for WFT and TSSM on greenhouse strawberry. The study focused on preventative biocontrol in the winter months (November–February), a period when greenhouse strawberries replace field-grown strawberries on the Canadian market. Hewitt et al. [
22] compared
A. swirskii and
Neoseiulus cucumeris (Oudemans), another generalist phytoseiid mite used to manage WFT larvae and TSSM, for WFT control in summer and winter climates, and concluded
N. cucumeris was a more cost-effective choice for winter months, whereas
A. swirskii performed better in the summer and was equally good under a winter climate.
Phytoseiulus persimilis is a TSSM specialist, and is less suited for preventative application, while
Neoseiulus californicus (McGregor), a generalist phytoseiid mite used to manage TSSM and WFT larvae, can survive on other mites, thrips, and pollen in the absence of TSSM [
23]. For those reasons,
N. cucumeris and
N. californicus were chosen for the greenhouse study.
2. Materials and Methods
2.1. Nabis Americoferus Colony
A colony of
N. americoferus was established from individuals collected in a grass field at the Vineland Research and Innovation Centre in Lincoln, ON, Canada in 2017. In later years, the colony was periodically supplemented with freshly caught individuals from the same location. Close-aged adult cohorts were reared as described in Saito et al. [
20]. The current study released approximately 50 females and 5 males in a rearing cage to start a cohort. All
N. americoferus adults used for testing were between 7 and 10 days old, after which they were assumed to have mated and have mature eggs. In all laboratory compatibility trials, each female was isolated in an individual polystyrene vial (Falcon™ 14 mL, 17 mm diam. Ø, BD Biosciences, Franklin Lakes, NJ, USA) containing a strip of paper and stoppered with a moist cotton plug for a pre-trial starvation period of 24 h prior to testing.
2.2. Compatibility Trial with Phytoseiid Mites
Laboratory trials were set up using one N. americoferus female versus multiple adult female phytoseiid mites to mimic the proportions of predators that are commonly encountered in a crop. Phytoseiulus persimilis were obtained from Koppert Canada Ltd. (Scarborough, ON, Canada) in a 100 mL bottle, containing a mixture of ca. 2000 adults and nymphs. Only adult female P. persimilis were used in this study. The bottle was stored at 10 °C for two days until used in the experiment. The TSSM were reared continuously in a growth chamber (16 L:8 D, 27 ± 1 °C, 60% RH) with bean plants (var. California Red Kidney, Stokes Seeds Canada Ltd., Thorold, ON, Canada). Adult female TSSM used for testing were removed directly from the colony.
Small plastic cups (opening 6 cm Ø, bottom 4 cm Ø, height 3 cm, volume 2 oz, Solo® cup B200, Dart Container Corporation, Mason, MI, USA) with a vent hole in the lid (2 cm Ø, covered with a thrips-proof mesh screen) were used in the trials. Detached tomato leaflets (var. Komeett, Stokes Seeds Canada Ltd.) were collected, and the petioles were dipped in 2% agar (cooled to approx. 40 °C) to prolong the freshness of the leaflets. Each cup contained a piece of wood wool (undyed Aspen fine excelsior, Uline Canada, Milton, ON, Canada) folded loosely, and the tomato leaflet was placed on the wood wool so that the arthropods could access both adaxial and abaxial sides of the leaflet. This setup provided more surface area and hiding places for the arthropods in the cup. Phytoseiulus persimilis were collected and transferred from the original bottles using a fine-tip paintbrush (size 000), and a pair of featherweight forceps were used to collect and transfer N. americoferus adult females (7–10 days old). There were two food items used, both of which were provided in excess: 35 TSSM adult females for P. persimilis and N. americoferus, and/or a frozen E. kuehniella egg strip (0.5 × 1.5 cm or coated with ca. 500 eggs) for N. americoferus. After the predators were placed inside the test cups, the snap-on lid was tightly placed. Six treatments were tested: 1. thirty-five adult female TSSM, no predators; 2. thirty-five adult female TSSM and two P. persimilis; 3. thirty-five adult female TSSM and one N. americoferus adult female; 4. thirty-five adult female TSSM, one E. kuehniella egg strip, and one N. americoferus; 5. thirty-five adult female TSSM, two P. persimilis, and one N. americoferus; and 6. thirty-five adult female TSSM, one E. kuehniella egg strip, two P. persimilis, and one N. americoferus. Water was not provided. The test containers were held in a growth chamber (16 L:8 D h, 25 ± 1 °C, 60% RH). The number of live and dead individuals was recorded 24 h later for all the species, as well as the number of P. persimilis eggs (laid on the tomato leaflet). Although the number of N. americoferus eggs (laid into the petioles and main leaf vain) was recorded, these data were omitted due to the inconsistency of oviposition occurring in the 24 h period. The first trial (n = 6 per treatment) was set up three days after receipt of P. persimilis. The whole trial was repeated once more using freshly ordered P. persimilis, yielding a total of n = 12 per treatment.
The number of dead TSSM (all treatments), dead P. persimilis (only treatments 2, 5, and 6), and P. persimilis eggs (only treatments 2, 5, and 6) was analyzed by a generalized linear mixed model using Proc GLIMMIX (α = 0.05), and Gaussian distribution was assumed (α = 0.05, SAS Studio, SAS Institute Inc., Cary, NC, USA) with the treatments as a fixed factor, and the trial repetition block as a random factor. A Tukey–Kramer multiple comparison was used to contrast the results.
Amblyseius swirskii were purchased from Koppert Canada Ltd. (Scarborough, ON, Canada) in mini sachets (Swirski-Mite Plus, 250 mites per sachet). When they arrived, the contents of three sachets were transferred into a 500 mL disposable plastic food container cup with a ventilation hole (6 cm Ø) in the lid covered with a thrips-proof screen, and it was stored at 15 °C to let the predatory mite population mature. Only female adults were used in this study. Frozen E. kuehniella egg strips (0.5 × 3.0 cm or ca. 1000 eggs) were used as food for both A. swirskii and N. americoferus. Three treatments were tested: 1. one egg strip and five A. swirskii; 2. one egg strip and one N. americoferus; and 3. one egg strip, five A. swirskii, and one N. americoferus. Water was not provided. The first trial (n = 10 per treatment) was set up two days after opening the sachet, and the whole trial was repeated two days later, yielding a total of n = 20 per treatment. The number of dead A. swirskii and A. swirskii eggs was analyzed in the same manner as in the P. persimilis trial.
2.3. Compatibility Trial with Beauveria Bassiana
The susceptibility of
N. americoferus to
B. bassiana was assessed in a laboratory bioassay, using BotaniGard
® 22WP (wettable powder formulation of GHA strain, Certis Biologicals, MD, USA). The viability of
B. bassiana conidia was determined prior to the assays as described in Saito and Brownbridge [
13] and used to adjust the dilution ratio to achieve the target test concentrations. The mean viability was 85% for the first trial and 82% for the second trial. Two test concentrations were prepared in deionized water for the bioassays: 1 × 10
5 and 1 × 10
7 viable conidia/mL, which are both lower than the recommended rate on the BotaniGard
® 22WP product label for WFT control (5.5 × 10
7–1.1 × 10
8 conidia/mL). The exposure arena consisted of a sterile tight-fit Petri dish (50 mm Ø, PALL Corporation, MI, USA) lined with a sterilized filter paper (Whatman™, #1, 55 mm Ø, Cytiva, Marlborough, MA, USA). The lid had a screened vent hole (20 mm Ø) and also an access hole (8 mm Ø, plugged with a foam ear plug) for introducing insects into the dish. Three treatments were tested: deionized water only as the control,
B. bassiana low rate 1 × 10
5 conidia/mL, and
B. bassiana high rate 1 × 10
7 conidia/mL. Twenty dishes were prepared for each treatment (
n = 20 per treatment, 10 males and 10 females). First, the filter paper was inoculated with 0.3 mL of the assigned fungal or control treatment; one
N. americoferus was then placed in a dish via the hole in the lid and was provided with frozen
E. kuehniella eggs (0.02 g per dish) scattered on the filter paper as food. Dishes were held in a growth chamber (16 L:8 D h, 25 ± 1 °C, 60% RH) for 48 h. After the exposure period had elapsed, using a pair of featherweight forceps (sterilized with 70% ethanol for each transfer), each surviving bug was transferred into an individual small plastic cup (see
Section 2.1), containing a piece of fresh organically grown French bean (purchased in a grocery store, triple water-rinsed, as a water source and oviposition substrate) and an
E. kuehniella egg strip (0.5 × 1.5 cm), allowing the bug to feed ad libitum. Survival and oviposition were assessed every 48 h for a further 12 days (14 days total observation period); a fresh bean piece and an egg strip were provided every 48 h. Dead individuals were placed on a glass slide in a Petri dish lined with a moist filter paper and incubated at 25 °C to promote outgrowth of fungi to confirm death by mycosis.
The whole trial was repeated once more, using a different mass-reared cohort, staggered seven days after the start of the first trial. The mortality data were analyzed with Fisher’s exact test (n = 40, α = 0.05, Proc FREQ). The odds ratios were used to contrast the results. The differences in the mortality between sexes were also compared within the same treatment using Fisher’s exact test (n = 20 per sex). To see the potential effect of the fungus on the fitness of the female N. americoferus, the eggs observed in every 48 h period as well as the total number of eggs observed per female from each treatment (n = 20) were analyzed by a generalized linear mixed model using Proc GLIMMIX (α = 0.05) with the treatments as a fixed factor, a Poisson distribution, and log link. Tukey’s multiple comparison was used to contrast the results.
2.4. Greenhouse Strawberry Trial
Greenhouse strawberry plugs (var. Albion) were purchased on 5 October 2022. They were transplanted into 4 L pots with peat-based standard growing media (one plant per pot). Additionally, one 3 L pot of banker plants (barley) was seeded in each of the cages preassigned for N. americoferus treatments, on which bird-cherry oat aphids were inoculated as soon as the seedlings emerged. There were 24 walk-in dome-shaped cages (160 cm × 160 cm × 180 cm, BugDorm©, MegaView Science, Taichung, Taiwan) placed in four rows of six cages in a greenhouse compartment. The greenhouse conditions were set to 24–26 °C daytime, 20–22 °C nighttime, and supplemental HPS lights were used to maintain a photoperiod of 16:8 h light:dark. These settings ensured that venting and cooling occurred on sunny days and additional heating was applied at night during the Canadian winter. Using a randomized complete block design, each cage containing six potted strawberries elevated on plant pot risers represented one replicate.
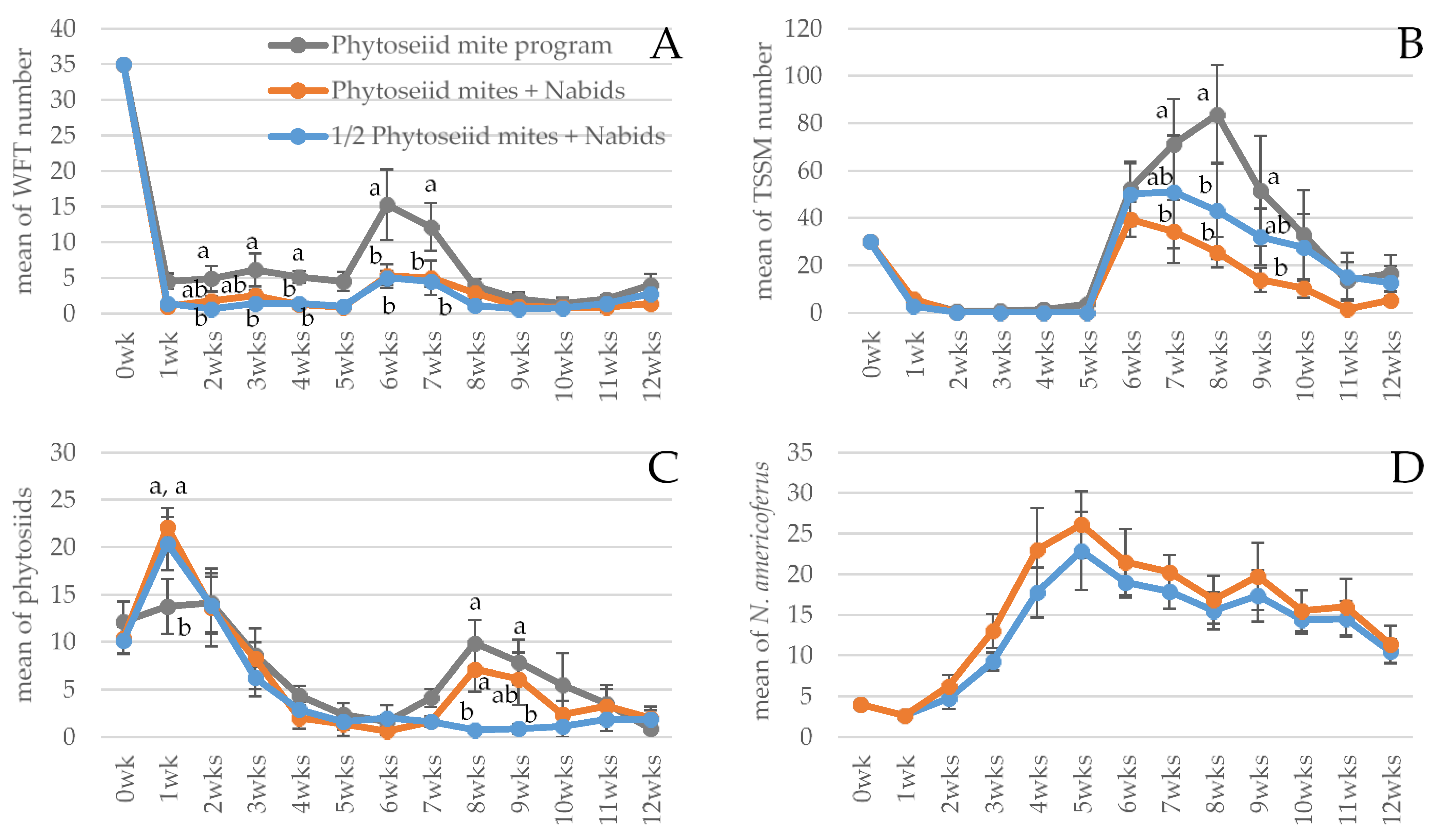
The plants were left to grow for one week, and then all the predators were released preventatively, one week prior to the pest release (−1 week). The mite sachets were slow-release breeding sachets containing feeder mites for the predatory mites, and N. americoferus had the aphid banker plant to keep them fed and allow them to start reproducing. A pre-treatment count of predators was conducted just before the pest release at week 0. Pests were released in two inundative waves: the first release was at week 0 at the rate of 35 WFT (30 females, 5 males) and 30 TSSM females; the second release took place immediately after week 5 data were taken, at the rate of 70 WFT (60 females, 10 males) and 30 TSSM females. Arthropod populations (adult and juvenile stages combined) were visually assessed weekly up to 12 weeks on all six strawberry plants in each cage.
Treatments were:
The phytoseiid full recommended rate: one N. cucumeris sachet (Thripex-plus, 1000 mites per sachet, Koppert Canada Ltd.) and one N. californicus sachet (Spical-plus, 100 mites per sachet, Koppert Canada Ltd.) per cage at −1 week; repeated at 6 weeks, just after the second wave of pests was released.
The phytoseiid full rate plus N. americoferus—same as treatment 1, plus two female and two male N. americoferus at −1 week.
The phytoseiid half rate plus N. americoferus—initial treatment the same as treatment 2, but the predatory mite sachets were NOT repeated.
The observed numbers of arthropods from each cage (n = 8 cages per treatment, WFT, TSSM, phytoseiid mites, and N. americoferus) were analyzed with a generalized linear mixed model using Proc GLIMMIX (α = 0.05, SAS Studio, SAS Institute Inc., Cary, NC, USA) with repeated measures. The treatment and time, and their interaction were considered as fixed factors. An AR(1) covariance structure was also chosen to consider the dependency between observations taken over time on the same cage. Separate models were created for each arthropod category. A least-squares means test was used to evaluate the significance of main effects. A Tukey–Kramer multiple comparison was used to evaluate post-hoc differences among the treatments in each sampling week.
4. Discussion
The laboratory trials examined the compatibility of N. americoferus with phytoseiid mites and a fungal bioinsecticide commonly used in Canadian greenhouse crops: P. persimilis, a TSSM specialist phytoseiid; A. swirskii, a generalist phytoseiid for managing WFT larvae and whitefly; and B. bassiana GHA strain, an entomopathogenic fungus with a wide host range. A greenhouse cage study was then conducted during the winter months, to showcase a scenario of out-of-season strawberry production. Although it would have been more straightforward to test the same species throughout this study, alternative phytoseiid species were used in the greenhouse study in order to reflect current recommendations for preventative biocontrol in winter: i.e., N. cucumeris, a more cost-effective alternative to A. swirskii; and N. californicus, a generalist alternative to P. persimilis.
Nabis americoferus is about 100 times larger than phytoseiid mites, which clearly makes it an intra-guild predator. Indeed, both phytoseiid mite species suffered unidirectional IGP in a small container. Despite this, the corrected mortality of P. persimilis and A. swirskii was only ca. 25%, which is a good indication that the IGP in a real crop setting could be minimal as it is often the case that IGP is worse in laboratory settings than it would be in greenhouse environments with a real crop.
The
P. persimilis trial also showed that
N. americoferus is 4× more efficacious per individual than
P. persimilis. One
P. persimilis adult female consumed about 4.5 TSSM in 24 h, which was similar to 3.2 TSSM in 24 h reported from another laboratory study [
25]. The slightly better efficacy found in our study may be due to environmental differences between the trials such as temperature.
Nabis americoferus performed better in eating TSSM (17.5 TSSM in this study) than they did in our previous study (8.5 TSSM) [
20]. The current study employed an arena with three-dimensional surfaces, while the previous study used a two-dimensional test arena. The more complex environment may have facilitated
N. americoferus’s hunting of TSSM in this study. However, having access to
E. kuehniella eggs reduced their predation efficacy on TSSM by half.
Nabis americoferus did eat most of the
E. kuehniella eggs presented, which suggests that when
N. americoferus find a patch of prey (‘egg strips’), they tend to stay in the prey patch until the patch is depleted, and may prefer
E. kuehniella eggs over TSSM.
The corrected mortality of
P. persimilis due to IGP was reduced to nil when
N. americoferus had access to
E. kuehniella eggs. The nabids were most likely satiated enough to not cause an increase in the mortality of
P. persimilis. Because the
A. swirskii IGP trial did not have alternative food sources, it is possible that the mortality of
A. swirskii could be reduced if another food source such as live WFT larvae was also available. Our findings were similar to those of Cloutier and Johnson [
26], who subjected another generalist hemipteran predator,
Orius tristicolor (White), to a similar test using WFT, TSSM, and
P. persimilis.
Orius tristicolor did feed on both WFT and
P. persimilis, but the mortality was reduced when TSSM were also available as alternative food. The authors noted it seemed that any phytoseiids present in the microhabitat where
O. tristicolor were searching for normal prey would be readily attacked. Chow et al. [
27] tested the compatibility of a generalist hemipteran predator,
Orius insidiosus (Say), with
A. swirskii. They found
O. insidiosus had no preference for
A. swirskii over WFT, but
O. insidiosus always switched to the more abundant prey. Similarly, our study also found no obvious preference for any of the biocontrol agents over the targeted pests and/or alternative food; actually, it seemed
N. americoferus readily attacked any arthropods in the vicinity of where it was searching for food. This generalist character of
N. americoferus could be a nuisance in situations where multiple agents are needed for multiple pests, but a theoretical work by Ikegawa et al. [
28] showed that, even when IGP by one of the natural enemies is severe, it may still be beneficial to use multiple natural enemies if the generalist switches the main prey, depending on the relative abundance of prey species. Cloutier and Johnson [
26] suggested that if the generalist predator attacking the specialist predator was dependent on prey density, increasing predation by a generalist on a specialist could possibly prevent a population crash of the specialist toward the end of a prey infestation.
There is one stage that phytoseiid mites could attack in the life cycle of
N. americoferus, which is the egg stage. Vangansbeke et al. [
29] looked at the possibility of phytoseiids feeding on the eggs of hemipteran predators inserted inside plant tissues and concluded the phytoseiids did not affect the egg-hatching rate of anthocorid and mirid predators. This is probably the case for
N. americoferus eggs as well, which are inserted into plant tissues but are also larger than the anthocorid and mirid eggs.
According to the classification system for side-effects of pesticides on natural enemies developed by the International Organisation for Biological Control [
30], the
B. bassiana GHA strain in BotaniGard 22WP may be considered ‘harmless to slightly harmful’ to
N. americoferus in our low concentration as the pathogen caused 9.68% mortality, and ‘moderately harmful’ in our high concentration as the pathogen caused 64.5% mortality. However, the number of confirmed mycosis cases clearly shows that
N. americoferus is susceptible to this pathogen. Although few other laboratory studies have looked at the compatibility of hemipteran predators and
B. bassiana, a similar laboratory dish assay confirmed that
O. insidiosus was susceptible to the
B. bassiana GHA strain (BotaniGard ES) with similar mortality rates to those observed in our study [
31]. There are numerous studies reporting hemipteran pests being susceptible to the
B. bassiana GHA strain: for example,
Lygus hesperus Knight [
32],
Lygus lineolaris (Palisot de Beauvois) [
33],
Nasonovia ribisnigri (Mosley) [
34],
Lycorma delicatula (White) [
35],
Nezara viridula (L.) [
36],
Piezodorus guildinii (Westwood) [
37], and
Halyomorpha halys (Stål) [
38]. Contrastingly, the oviposition data showed that exposure to
B. bassiana increased oviposition in
N. americoferus females. A similar observation was made by Ramírez-Ordorica et al. [
39], reporting that the volatiles from
B. bassiana triggered female moths to lay more eggs. They suggested that the stimulation of oviposition behavior could be an ecological adaptive advantage in which the entomopathogen stimulates the insect population growth to ensure the host availability. Overall, we concluded that
N. americoferus is susceptible to the
B. bassiana GHA strain, and that it is not recommended to apply
B. bassiana while
N. americoferus is present in a crop. Therefore,
B. bassiana was excluded from our greenhouse study.
Prior to the strawberry trial, preliminary greenhouse trials were conducted using potted sweet peppers and tomatoes in order to determine the optimum release strategy for
N. americoferus. The
N. americoferus population increased the most using the aphid banker plants and the nabid was strongly affiliated to the banker plant, almost neglecting the tall crop plants. In the greenhouse trial,
N. americoferus was observed to be affiliated with strawberry plants as strongly as with the banker plant. This was expected since the literature suggests that
N. americoferus prefers crops that are closer to the ground such as alfalfa, soybeans, and grasses [
40]. In our current study, the nabids initially reproduced in the banker plants, because the bird-cherry oat aphids were already abundant when the nabids were released, one week prior to the pest release. However, the nabids quickly spread among the strawberry plants as their population was building up. According to the raw data (not shown), the population age demographic of
N. americoferus indicated that the second generation appeared 8–14 days post-release, took three weeks to mature, and started to decline nine weeks post-release. This aligned well with our previous data on their life history where the egg period was 8.35 days; the total nymphal period was 18.34 days; and adult longevity was 28–38.75 days [
20]. The nabids are known to cannibalize, as older larger individuals readily prey on younger smaller ones [
15]. The slight population decrease between 5 weeks and 6 weeks was likely due to the increasing cannibalism, because the prey population was diminishing while the
N. americoferus population was rapidly increasing. The second generation of fresh adult nabids appeared to have quickly responded to the second wave of adult WFT as soon as the thrips were introduced after 5 weeks. They suppressed the pest population significantly better compared to the phytoseiid-mites-alone treatment, as shown by the large margin of difference at 6 weeks. The phytoseiid-alone treatment was slower to reduce the second wave of WFT, probably because phytoseiids attack mainly the first instar WFT larvae [
23,
41], whereas the nabid can attack all the mobile stages of WFT on the foliage, including adults [
20]. The third generation of young nabid nymphs appeared after 8 weeks, and they were often observed walking on the webbing produced by TSSM and eating TSSM. The continuous presence of
N. americoferus compared to the population fluctuations of the phytoseiid mites likely caused the large difference compared to the phytoseiid-alone treatment in the TSSM population at 8 weeks. The pest populations in the phytoseiid-alone treatment started to decrease at 8 weeks when the phytoseiid population on the foliage peaked as a result of the second application. The strawberry fruit yield data were omitted because mice were causing feeding damage periodically. However, some observations could be made: the nabids were often observed patrolling on the flowers and fruits, but no obvious deformations were observed, and only one nabid egg was found inserted into a fruit.
Although all biocontrol treatments brought both WFT and TSSM under control, the best pest control was achieved by the combination of the repeated phytoseiid mite sachets application plus N. americoferus. However, it is notable that the phytoseiid half rate plus nabid treatment provided better control of both WFT and TSSM, compared to the phytoseiid repeated application alone treatment. Neither the phytoseiids nor N. americoferus numbers were significantly affected by the presence of each other, indicating that they are functionally compatible. Combined with our laboratory compatibility trials with other phytoseiid species, this study demonstrated that the addition of N. americoferus to greenhouse strawberry biological control programs based on phytoseiid mite sachets is beneficial, not only potentially reducing the number of sachet applications and the associated costs, but also providing better pest control than phytoseiid mites alone.