Simple Summary
Three blends of attractants and pheromones (i.e., attractant 1, attractant 2, and attractant 3) were compared for monitoring adults of Xylotrechus chinensis. The experimental procedure was carried out for a two-year period. The attractants were baited in traps and placed in trees of three heavily infested areas of Athens (Greece). Traps were checked weekly, the captured X. chinensis adults were recorded, and they were removed from the traps. Adults of X. chinensis start flying in late spring and end in the middle of autumn (end of October). Attractant 3 performed better than attractant 1 and 2, even with low numbers of insects. Xylotrechus chinensis did not seem likely to be attracted to the basic part of the blend, which consisted of a blend of pheromones + ethanol. Overall, the inclusion of α-pinene and ipsenol increased the activity of the basic lure.
Abstract
The Asian coleopteran Xylotrechus chinensis (Chevrolat) (Cerambycidae: Cerambycinae) is an invasive species in several European countries, attacking mulberry trees. In the current research, we evaluated the performance of three mixtures consisting of pheromones and attractants for the monitoring of X. chinensis adults. Attractant 1 (i.e., geranyl acetone, fuscumol acetate, fuscumol, monochamol, 3-hydroxyhexan-2-one, 2-methyl-1-butanol, anti-2,3-hexanediol, prionic acid + ethanol), attractant 2 (i.e., geranyl acetone, fuscumol acetate, fuscumol, monochamol, 3-hydroxyhexan-2-one, 2-methyl-1-butanol, anti-2,3-hexanediol, prionic acid + α-pinene + ethanol) and attractant 3 (i.e., geranyl acetone, fuscumol acetate, fuscumol, monochamol, 3-hydroxyhexan-2-one, 2-methyl-1-butanol, anti-2,3-hexanediol, prionic acid + α-pinene + ipsenol + ethanol) were baited in multi-funnel traps and installed in mulberries for a two-year period in Athens (Greece). The flight activity of X. chinensis starts at the end of April and terminates at the end of October. The peaks of X. chinensis flight activity were observed on 16 August 2021 and on 6 July 2022. Attractant 3 proved to be the most effective blend, catching 953 adults, followed by attractant 2 (523 adults) and attractant 1 (169 adults), throughout the experimental period. It seems that the pest was not attracted to the basic part of the blend (i.e., pheromones + ethanol). The incorporation of α-pinene and ipsenol resulted in the elevated activity of the base lure. The elevated performance of attractant 3 may be attributed to only the α-pinene and the ipsenol, or possibly the α-pinene, ipsenol, and ethanol, because the pheromone blend did not contain any of the pheromone components of the target species. Overall, attractant 3 could be a useful tool to detect and track X. chinensis in new invasive areas, triggering early management strategies against further establishment of this species.
1. Introduction
The invasive species Xylotrechus chinensis (Chevrolat) (Coleoptera: Cerambycidae: Cerambycinae) is a destructive pest that feeds and grows on mulberries (Morus spp.) [1,2,3]. This species is native to the Eastern Palaearctic region (i.e., China, South Korea, and Japan) [4,5,6]. In the last decade, X. chinensis has become established in Europe, mainly in countries with a wide presence of Morus spp., such as France, Greece, and Spain [1,2,7,8,9,10]. Specifically, in Spain, X. chinensis was first recorded in 2013 in Catalonia, where in 2020 the infestation had expanded to 12 cities, covering an area of 378.1 km2 [11]. Meanwhile, a new outbreak of X. chinensis occurred in Valencia in 2018 [2]. In the same year, X. chinensis was reported for the first time in France (Gironde) [12,13], while in 2019 attacked Morus trees were found in the Occitanie region [8,14]. The first detection of this species in Greece was in 2017 in Crete, where approximately 200 mulberries (Morus spp.) were found infested, of which 15% were dead [1]. In 2019, an outbreak of X. chinensis was recorded in Athens, the capital of Greece, where 300 out of 1300 infested mulberry trees were dead [9,13]. The damage from X. chinensis spread further in Athens in the following years, making this species an important pest of mulberry trees throughout the city. In addition, X. chinensis has been intercepted twice in Germany, in 2007 (first record in Europe) and 2017, and once in Philadelphia, USA (2011) in containers transporting different wooden objects from China [6,11,15], indicating that wooden materials can be vehicles for its spread.
Early detection of X. chinensis is a complex issue, since the immature stages develop inside the tree trunk [8]. The infestation is usually noticed mainly by the exit holes of the adults, as well as by other symptoms that are not so obvious, such as frass and the droppings of the beetles on the trunk [1,2,10]. The xylophagous larvae inhibit the movement of nutrients and water within the infested trees, resulting in their progressive drying [16]. The feeding habit of X. chinensis adults is not clear, since it is reported that adults infest leaves and stems of the tops of the mulberry trees [1,2] or they do not feed but drink water [17,18], indicating that the most destructive life stage of this species is larva. For all the above reasons, management of X. chinensis is challenging. In recent research efforts in Spain (Barcelona) and Greece (Athens), the trunk injection method was used to control X. chinensis larvae [10,11]. During spring 2018 in Barcelona, abamectin was injected into 107 mulberry trees. Almost a third of these treated trees (31 out of 107) showed new infestation symptoms in December, particularly exit holes, indicating a significant reduction in X. chinensis presence [11]. In Athens, after two years of application of abamectin, fipronil and imidacloprid via trunk injection to infested Morus spp. trees, the emergence holes of X. chinensis adults were reduced by 85.6%, 71.8%, and 76.1%, respectively [10]. Chemical control of this species has previously been proposed by spraying the trunks of mulberry trees with contact insecticides, targeting sites where females are resting and/or laying eggs, and newly emerged larvae are present [2]. Both methods were applied during summer [2,10,11]. The trunk injection method is generally safe and can be applied to urban trees [10,19], while trunk spraying is not suggested in urban areas due to concerns related to the exposure of citizens and environment to insecticides [20]. In addition, some preventive measures should be applied to heavily infested trees, including cutting down the affected mulberries, followed by burning or shredding the removed parts of the trees [2,8,12].
Recently, a new device that can monitor trees systematically against wood-boring pests was evaluated [21]. Trees infested by X. chinensis and Rhynchophorus ferrugineus Olivier (Coleoptera: Curculionidae) were used in the tests. This device automatically records the acoustic emissions generated by the activity of the larvae while feeding inside the trunk of the tree, thus detecting any tree infestation. It showed high accuracy in the detection of wood borer species and provided rapid inspection of many mulberries and palms without human interference, rendering it particularly suitable for trunk inspection in quarantine areas. The vibration recording device potentially contributes to a reduction in unnecessary management treatments and becoming a useful tool for monitoring of X. chinensis.
The three ingredients of male pheromones of X. chinensis, i.e., 2,3-octanediol, 2-hydroxy-3-octanone, and 3-hydroxy-2-octanone [22,23], have been identified and attract female individuals [24], but they have not yet been developed as a method of detection for this species [8]. Pheromone components could be used for mass trapping of X. chinensis females or for mating disruption [8]. Recently, traps containing a mixture of eight kairomones and pheromones, which attracted a wide spectrum of insects [25], were used in Catalonia (Spain), Crete (Greece), and Sète (France) [8,14]. Among the captured species of the Cerambycidae family were X. chinensis adult individuals.
In the present study, three mixtures of attractants that can attract a wide range of wood-boring insects were compared to monitor the population of X. chinensis in different areas of the Municipality of Athens. It is very important to know whether any of the components that make up each attractant can disrupt the attraction of X. chinensis [26]. A search of the global bibliography yielded no data on the seasonal occurrence of X. chinensis in Europe. There are sporadic reports on the emergence period of X. chinensis adults from the trunks of the mulberries, providing evidence about the beginning of the flight of this pest. In southern Greece, adults of X. chinensis appear from the end of spring to the beginning of summer and in northeastern Spain from mid- to late summer [1,2]. Thus, the aim of the current two year (2021–2022) research was to investigate the flight activity of X. chinensis, offering a useful monitoring tool for this species.
2. Materials and Methods
2.1. Experimental Areas-Trees
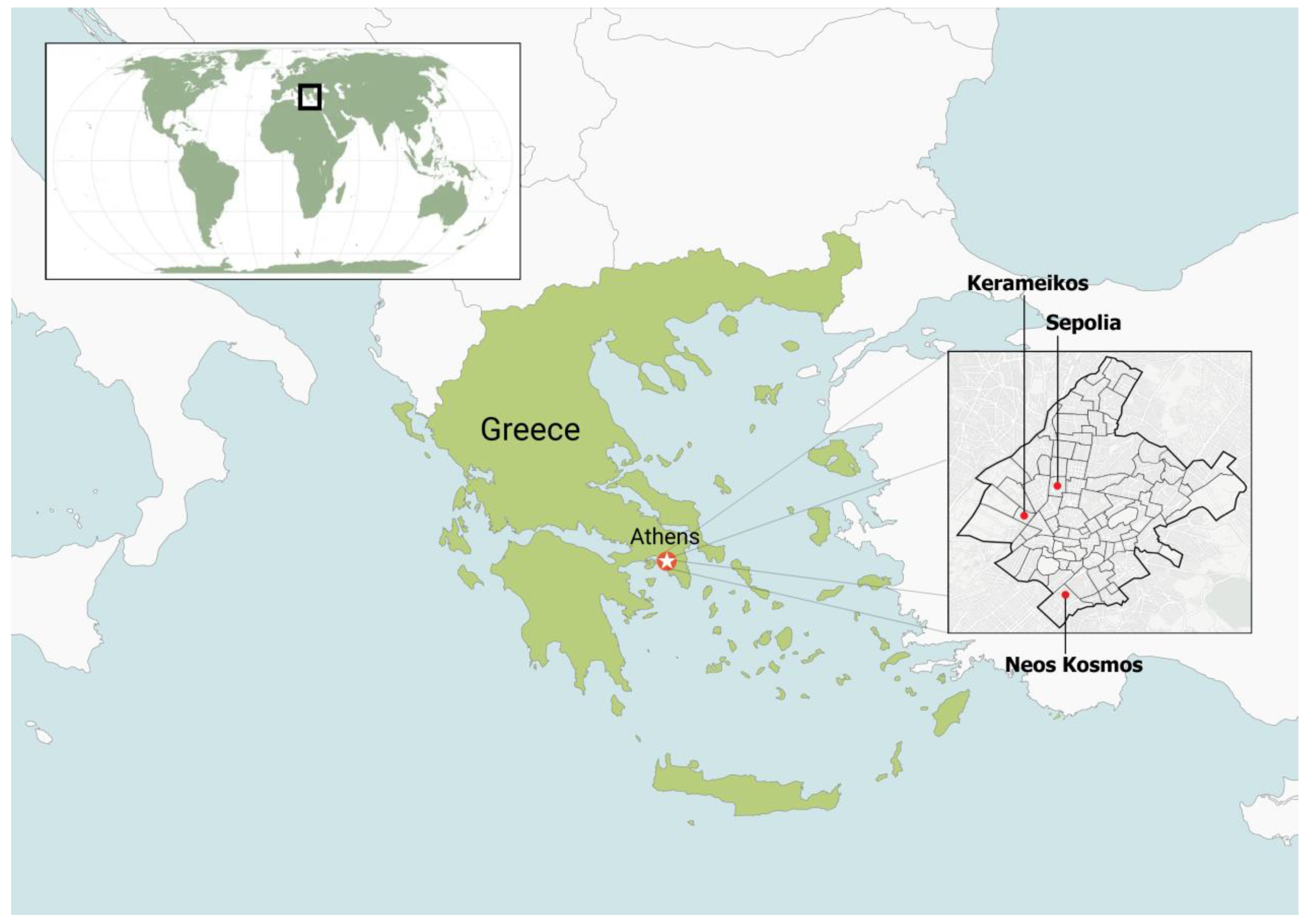
Three experimental areas were selected within the Municipality of Athens: Neos Kosmos (37° 03′ 60.00″ N 22° 25′ 59.99″ E), Kerameikos (37° 58′ 25.19″ N 23° 43′ 4.19″ E), and Sepolia (38° 00′ 9.99″ N 23° 42′ 48.58″ E) (Figure 1). The three selected areas have Morus spp. trees (Morus alba and Morus nigra). Based on the existence of numerous exit holes in mulberry trees, preliminary samplings (through installation of baited traps) indicated that mulberry trees suffered high infestation levels of X. chinensis in these areas.
Figure 1.
Map of Greece with the three experimental areas: Neos Kosmos, Kerameikos, and Sepolia, in the Municipality of Athens.
2.2. Attractants
The following three blends of pheromones and attractants were used during the experimental period: (i) geranyl acetone, fuscumol acetate, fuscumol, monochamol, 3-hydroxyhexan-2-one, 2-methyl-1-butanol, anti-2,3-hexanediol, prionic acid + ethanol (attractant 1); (ii) geranyl acetone, fuscumol acetate, fuscumol, monochamol, 3-hydroxyhexan-2-one, 2-methyl-1-butanol, anti-2,3-hexanediol, prionic acid + α-pinene + ethanol (attractant 2); and (iii) geranyl acetone, fuscumol acetate, fuscumol, monochamol, 3-hydroxyhexan-2-one, 2-methyl-1-butanol, anti-2,3-hexanediol, prionic acid + α-pinene + ipsenol (2-methyl-6-methylene-7-octen-4-ol) + ethanol (attractant 3). The three attractants had components in common of geranyl acetone, fuscumol acetate, fuscumol and monochamol, which are components of the pheromones of various species of the cerambycid subfamilies Spondylidinae and Lamiinae [27,28,29], 3-hydroxyhexan-2-one, 2-methyl-1-butanol, and anti-2,3-hexanediol, which are components of the pheromones of various species of the subfamily Cerambycinae [30,31,32], prionic acid, which is the sex pheromone of various species of the genus Prionus (Coleoptera: Cerambycidae: Prioninae) [33], and ethanol, which is released from infested trees [34,35,36], enhancing the attraction of wood-boring insects [37,38]. Attractant 3 additionally contained the components α-pinene, which is the kairomonic component released by host trees [39] and ipsenol, which is an aggregation pheromone of wood-boring insects of the subfamily Scolytinae (Curculionidae) [40,41]. Attractant 2 contained only α-pinene as an additional component. We assume that the blend of pheromones and ethanol was enhanced by α-pinene and ipsenol [42,43,44].
The preparation of the common part of the three attractants was carried out at the French National Institute of Agriculture, Food and the Environment (INRAE) by Dr Alain Roques. This blend consisted of 25 mg of geranyl acetone, 50 mg of fuscumol acetate, 50 mg of fuscumol, 50 mg of monochamol, 50 mg of 3-hydroxyhexan-2-one, 50 mg of 2-methyl-1-butanol, 50 mg of anti-2,3-hexanediol, and 1 mg of prionic acid dissolved in isopropanol (carrier) to a final volume of 1 mL. Aliquots of 1 mL of this mixture were poured into 1.5 mL glass vials with plastic lids and kept at 4 °C until the beginning of the experiment (Figure 2a). The extra components α-pinene (12 mL) (+ 16 mL ethanol) of attractant 2 or α-pinene (12 mL) and ipsenol (20 mg) (+ 16 mL ethanol) of attractant 3 are commercially available and contained in vials of 28 mL volume each with a constant evaporation rate of average 150 mg/day, lasting for 100 days, as measured by the manufacturer (NovAgrica, Athens, Greece). Ethanol alone as part of the first attractant is contained in the same type of vials (28 mL volume). The vials with ethanol alone were inspected frequently and filled if it was necessary. Therefore, per attractant two vials were used: attractant 1, a 1.5 mL vial containing the common part and a 28 mL vial containing ethanol (Figure 2b); attractant 2, a 1.5 mL vial containing the common part and a 28 mL vial containing α-pinene + ethanol (Figure 2c); and attractant 3, a 1.5 mL vial containing the common part and a 28 mL vial containing α-pinene + ipsenol + ethanol (Figure 2d). Upon installation of traps, the caps of the 28 mL vials were removed and the vials left open.

Figure 2.
(a) The common part of the three attractants in glass vials. (b) Attractant 1. (c) Attractant 2. (d) Attractant 3. (e) Multi-funnel trap. (f) The common part of the three attractants in a glass vial in a polyethylene bag with cotton wad. (g) Placement of attractant in the middle funnel of the multi-trap.
2.3. Experimental Design
The commercially available multi-funnel traps, which are suitable for catching species of cerambycids [45], were supplied by NovAgrica (Athens, Greece) and were used in the experiment. The multi-funnel trap consists of six black funnels with a removable white collection cup that facilitates the counting of the captures (dry trapping) (Figure 2e). A killing agent (transfluthrin 0.4% w/w strip) was applied in the collection cup to prevent captured insects from escaping. The traps were hung from trees with their bottoms 2.5 m above the ground. In the areas of Neos Kosmos and Kerameikos, 6 traps were placed for each attractant (i.e., 6 traps × 3 attractants × 2 areas = 36 traps), while in Sepolia, 3 traps were placed for each attractant (i.e., 3 traps × 3 attractants x 1 area = 9 traps). In each area, traps were suspended on main roads in blocks consisting of the three attractants and were 100 m apart (i.e., 6 blocks for Neos Kosmos and Kerameikos, 3 blocks in Sepolia). The distance between the blocks was 100 m. Before the suspension of the traps on mulberry trees, each vial of 1.5 mL containing the common part of the attractants was placed without the lid in a 65 mm × 95 mm, 100 µm thickness, resealable polyethylene bag (Minigrip bags, Avantor, The Metropolis, Singapore) with a cylindrical cotton wad (3.5 cm × 1 cm) [46] (Figure 2f). Attractants were placed in the middle funnel (Figure 2g) of the trap and replaced every three months. Trap inspections were conducted weekly from 19 July 2021 to 31 December 2022. At each trap check, traps were clockwise rotated to avoid the effect of a particular sampling location [47]. At each check, captures of X. chinensis adults were recorded. Then, all captured individuals were removed from the traps.
2.4. Statistical Analysis
Before the data of captures were analyzed, they were log (x + 1)-transformed to normalize variances and standardize means [10,48]. Data were analyzed using a three-way ANOVA [49]. The response variable was the number of captured adults. Main effects were attractant, date and experimental area. Their interactions (area x attractant, area x date, attractant × date, and area × attractant × date) were also considered in the analysis. Year was not considered in the analysis, since 2021 included fewer checks than 2022. Means were separated by the Tukey–Kramer HSD test, p = 0.05 [50]. All dates with zero captures in any treatment were dropped from the analysis. The analyses were carried out with the statistical package JMP 16.2 [51].
3. Results
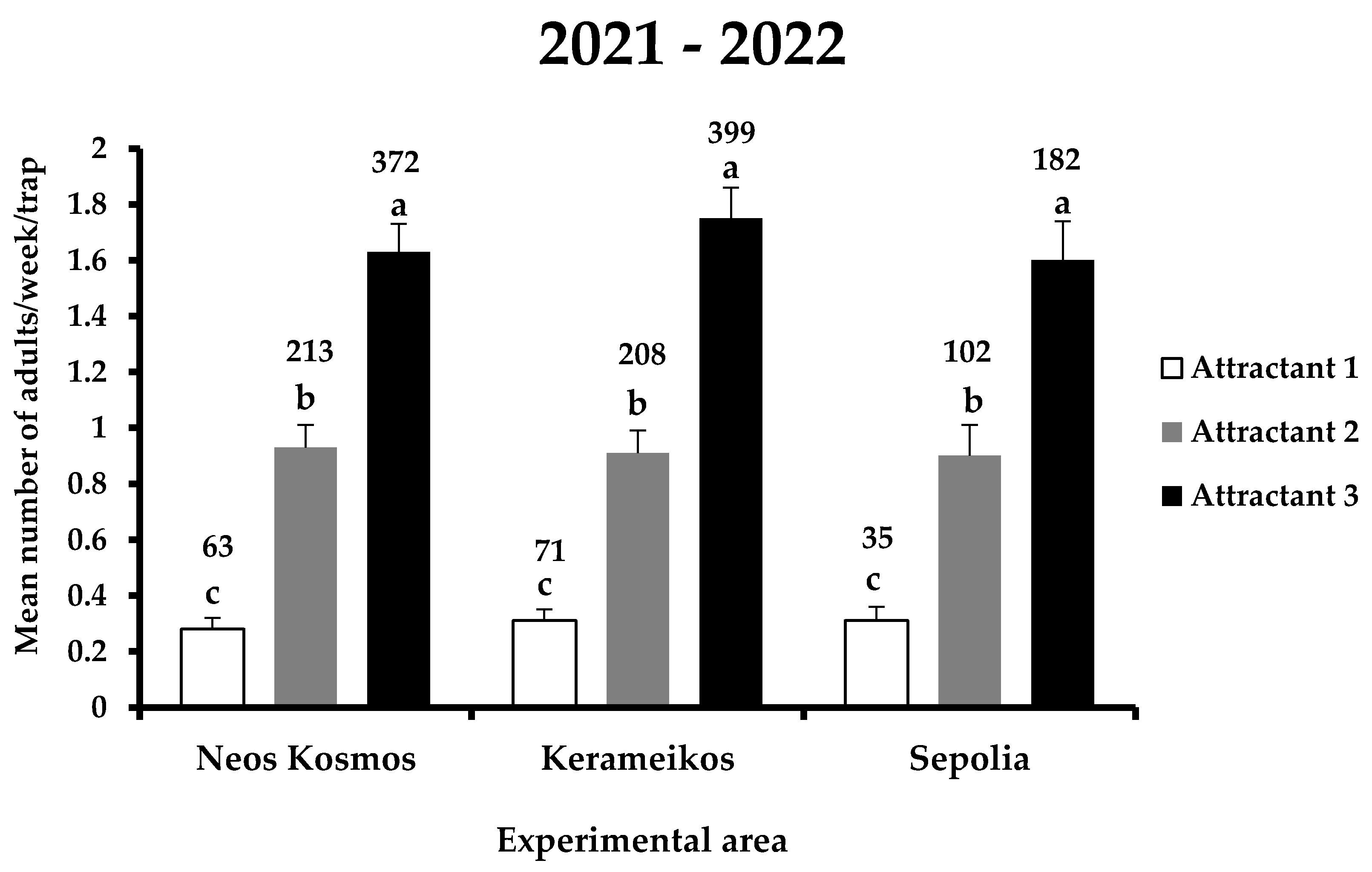
The main effects area and the interactions area x attractant and area x attractant x date were not significant (Table 1). The total number of X. chinensis adults captured in the traps for the entire experimental period was 1645, of which 169, 523, and 953 individuals were recorded in attractants 1, 2, and 3, respectively (Figure 3). Within each experimental area, significant differences were observed among the three attractants (F = 51.5; DF = 8, 1709; p < 0.01) (Figure 3). In Neos Kosmos, the most effective was attractant 3, catching a total of 372 adults, followed by attractant 2 and 1 with 213 and 63 individuals captured, respectively. The same trend was observed in Kerameikos and Sepolia, where the most catches were noted for attractant 3 (399 and 182 adults in Kerameikos and Sepolia, respectively) and the least for attractant 1 (71 and 35 adults in Kerameikos and Sepolia, respectively). No significant differences were recorded for each attractant among the three experimental areas, indicating that there was no influence of area on the performance of attractants.

Table 1.
ANOVA parameters for main effects and their interactions for X. chinensis adults captured weekly in each trap for attractants in three experimental areas during 2021–2022 (total DF = 1709).
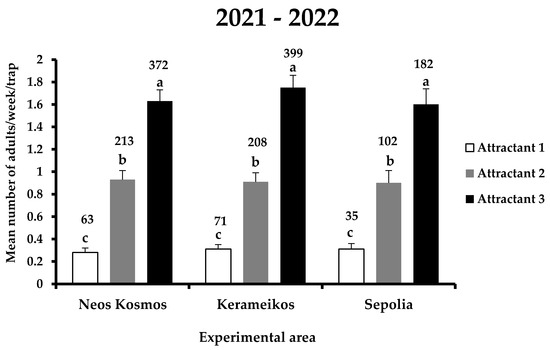
Figure 3.
Mean number (+ standard error) of X. chinensis adults captured weekly in traps baited with each attractant in the three experimental areas (i.e., Neos Kosmos, Kerameikos, and Sepolia) during the entire experimental period (2021–2022). Different letters indicate significant differences. Numbers above columns indicate the total number of captures. All dates when all treatments had zero catches were dropped.
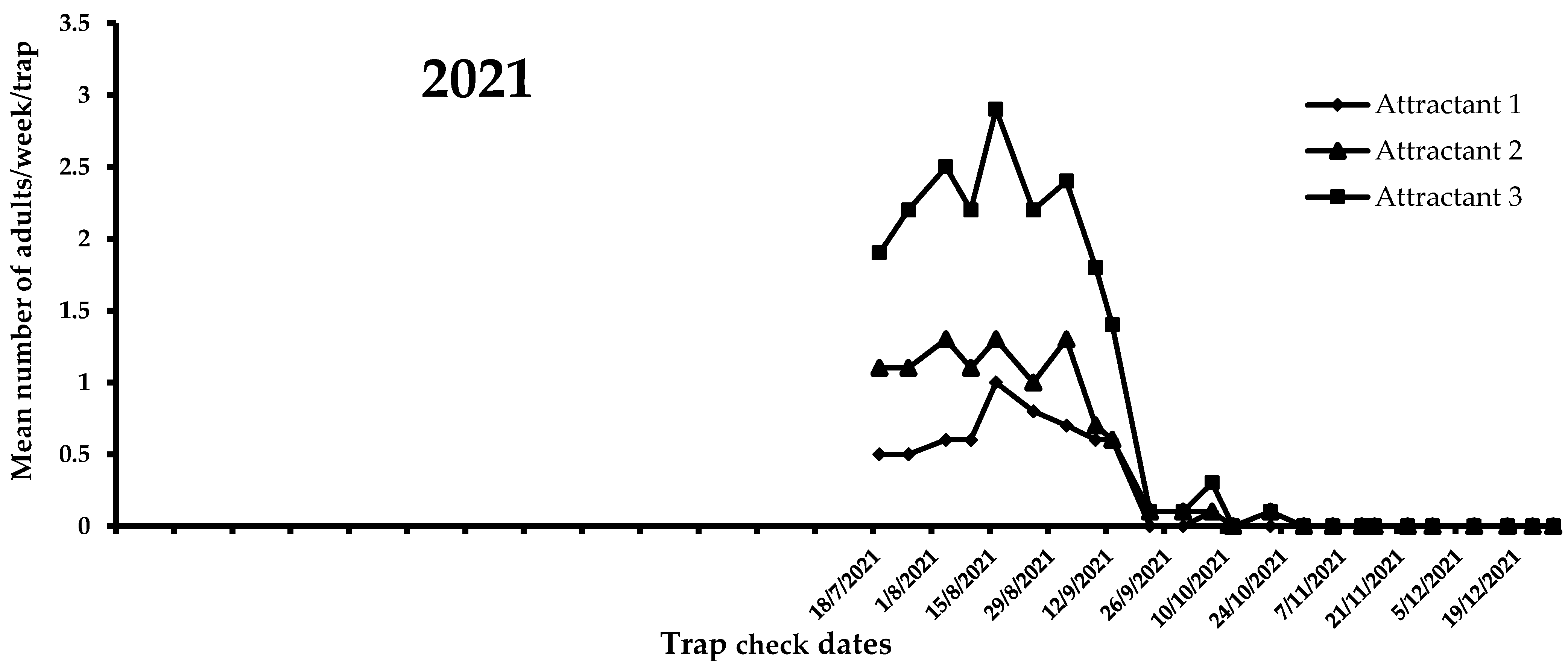
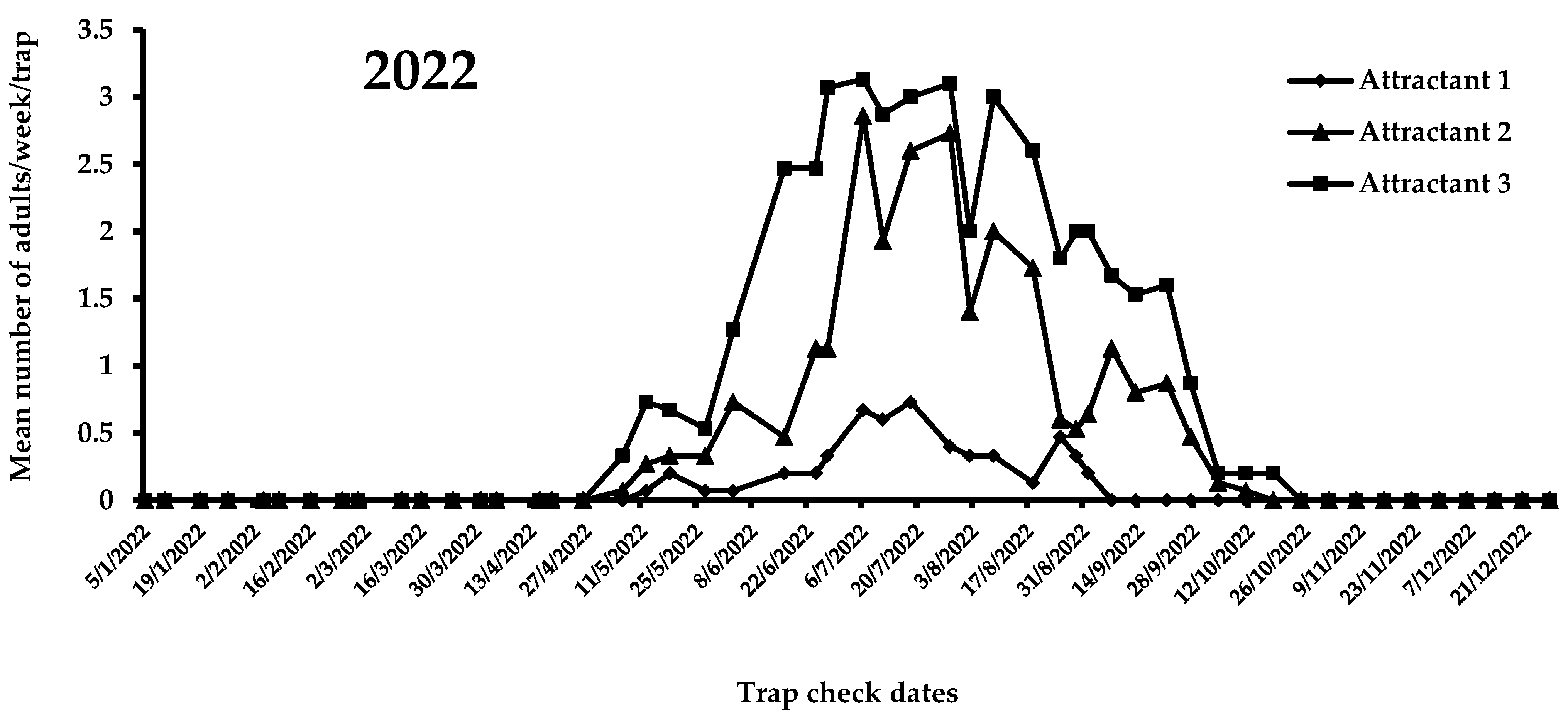
A view of the flight activity of X. chinensis recorded in 2021 and 2022 in Athens is shown in Figure 4 and Figure 5. The peaks of captures for 2021 and 2022 were observed in August and July, respectively. Specifically, the mean numbers of captures for August 2021 were 2.47, 1.15, and 0.75 for attractants 3, 2, and 1, respectively. In July 2022, the mean numbers of captures were 3.03, 2.53, and 0.60 for attractants 3, 2, and 1, respectively. During 2021, significant differences were noted among captures in traps baited with the three tested attractants (F = 51.6; DF = 2, 584; p < 0.01). Traps baited with attractant 3 (i.e., 303 adults) caught significantly more adults than traps baited with attractant 1 (i.e., 89 adults) and 2 (i.e., 148 adults) (Figure 4). The highest mean number of X. chinensis adults captured from the beginning of observations until 13 September 2021 was recorded for attractant 3, followed by captures corresponding to attractant 2 and attractant 1 (Figure 4). The maximum mean number of catches was recorded on 16 August 2021, where 2.9 adults/week/trap were captured in traps baited with attractant 3. From 22 September 2021 onwards, a reduction in captures of X. chinensis was observed for all three tested attractants. From 29 October 2021 until 26 April 2022, there were zero captures (Figure 4 and Figure 5). A similar trend was recorded in 2022. There were significant differences in captures when traps were baited with attractants 1, 2, and 3 (F = 160.6; DF = 2, 1124; p < 0.01). Traps containing attractant 3 caught significantly more adults vs. traps containing attractant 2 vs. traps containing attractant 1 (Figure 5). The highest mean number of adults captured from the beginning to the end of X. chinensis flight activity was recorded for attractant 3, followed by captures corresponding to attractant 2 and attractant 1 (Figure 5). The first captures for attractants 2 and 3 occurred on 6 May 2022 (0.33 and 0.07 adults/week/trap for attractants 3 and 2, respectively), while traps baited with attractant 1 caught the first adults (0.07 adults/week/trap) one week later. The highest mean numbers of captured X. chinensis adults were noted on 6 July 2022 for attractants 2 and 3 (2.86 and 3.13 adults/week/trap for attractants 2 and 3, respectively), while for attractant 1 this was observed on 18 July (0.73 adults/week/trap). The last catches were observed on September 1, 2022 for attractant 1 (0.2 adults/week/trap), 11 October for attractant 2 (0.07 adults/week/trap), and 18 October for attractant 3 (0.2 adults/week/trap). No captures were recorded from 25 October to the end of December 2022.
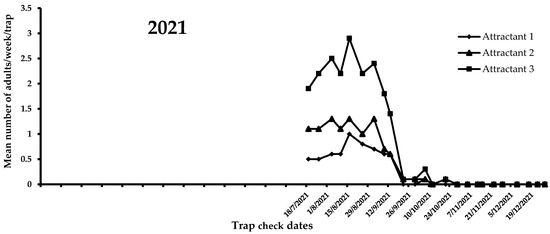
Figure 4.
Mean number of X. chinensis adults captured weekly in traps baited with attractants 1, 2, and 3 during 2021.
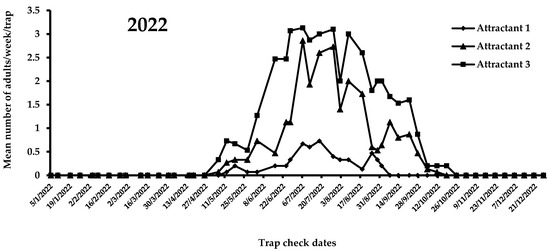
Figure 5.
Mean number of X. chinensis adults captured weekly in traps baited with attractants 1, 2, and 3 during 2022.
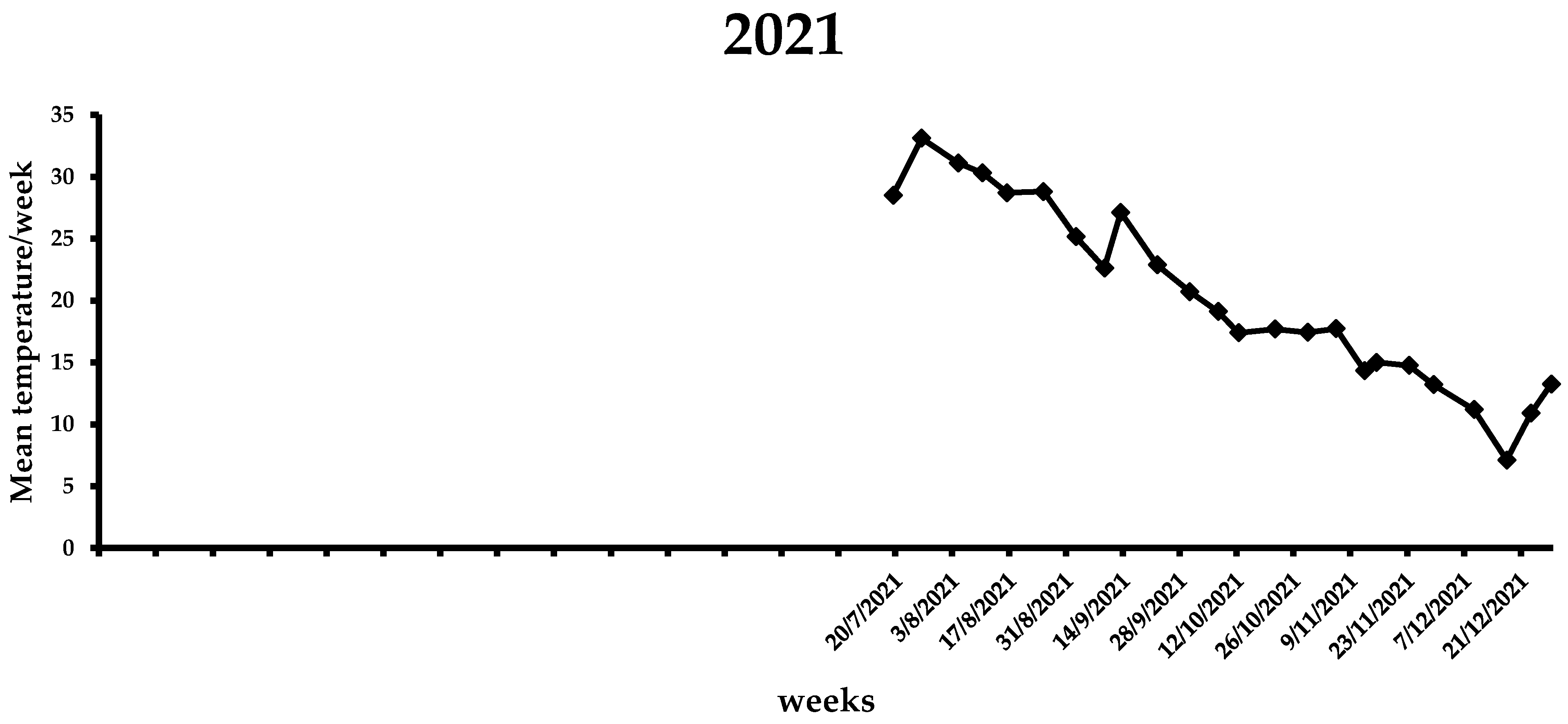
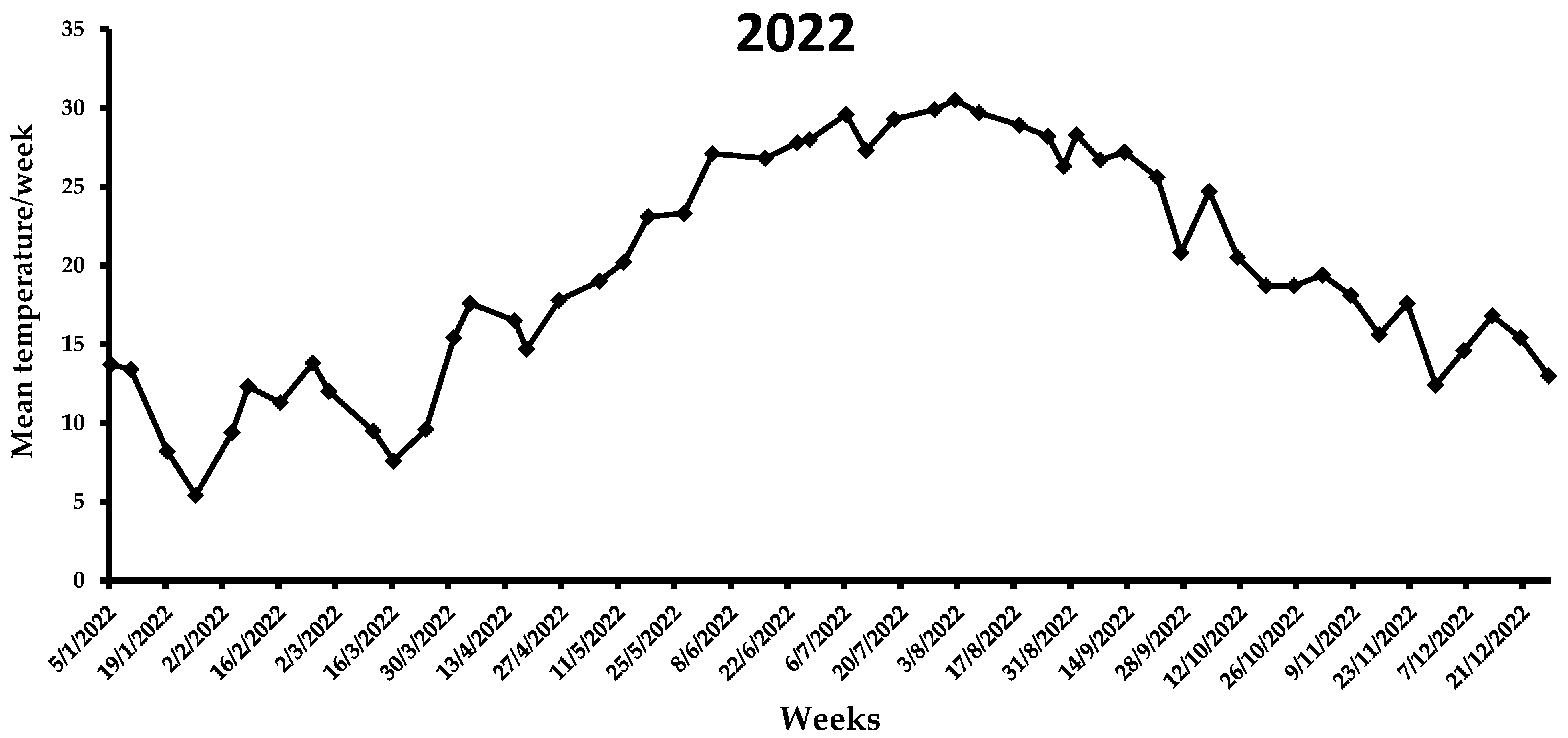
During the first week of the observations in 2021 (i.e., end of July), the mean weekly temperature was 28.5 °C (Figure 6), which increased in the following three weeks, ranging from 30.3 to 33.1 °C. A gradual reduction in the average weekly temperature was noted from middle August (i.e., 28.7 °C) to the end of 2021 (i.e., 7.1 °C). By the end of the flight period in 2021, the mean weekly temperature was <20 °C. In the first two weeks of 2022, the average weekly temperature increased, reaching ~13 °C, while for the following three weeks it remained <10 °C (Figure 7). The same trend was observed over the next 7 weeks, ranging from 7.6 to 13.8 °C. A gradual increase in the mean weekly temperature was observed between the end of March till 26 April, reaching 17.8 °C. During the first two weeks of May, where the first captures were recorded, the mean weekly temperature increased further, reaching 20.2 °C. In the following weeks until the beginning of August, the mean weekly temperature ranged from 23.1 to 30.5 °C, while it gradually decreased after the second week of August. Similarly to 2021, the mean weekly temperature dropped below 20 °C by the end of the flight period of X. chinensis.
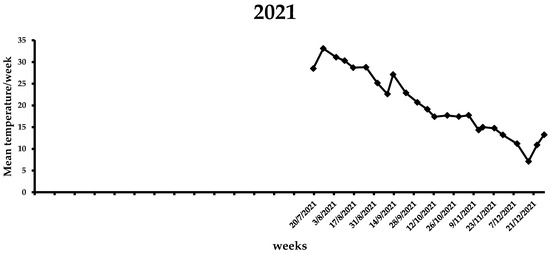
Figure 6.
Mean weekly temperature (°C) in Municipality of Athens during the 2021 experimental period. Data obtained from freemeteo.gr.
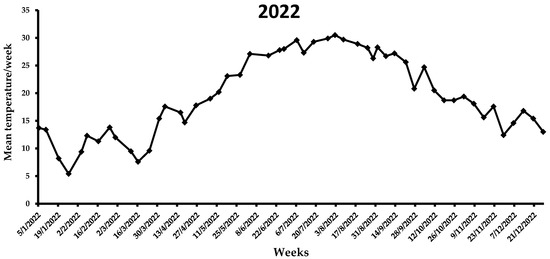
Figure 7.
Mean weekly temperature (°C) in Municipality of Athens during the 2022 experimental period. Data obtained from AccuWeather.com.
4. Discussion
The findings of the current study revealed that the most effective attractant mixture was attractant 3, which contained the two additional components α-pinene and ipsenol (+ethanol). When ipsenol was absent (attractant 2) or when α-pinene and ipsenol were absent (attractant 1), captures were significantly fewer than those corresponding to attractant 2. Captures corresponding to attractant 2, which contained α-pinene (+ethanol), were significantly higher than the captures corresponding to attractant 1 (+ethanol). Therefore, the combination of α-pinene and ipsenol with the common part of all attractants and ethanol resulted in the increased attraction of X. chinensis adults compared with the presence of α-pinene with the common part of the attractants + ethanol. Ethanol and α-pinene perhaps act synergistically to attract many species of wood-boring and bark beetles, including species of the family Cerambycidae, issues that have classified them as broad-spectrum combinational attractants [43,52,53]. According to Rodríguez-González et al. [42], the addition of ethanol to the compound 3-hydroxy-2-hexanone, which is produced by the males of Xylotrechus arvicola (Olivier) (Coleoptera: Cerambycidae: Cerambycinae), increased the attractiveness of this compound to both sexes of this species. In previous studies, it has been noted that the kairomones ethanol and α-pinene increased the attractiveness of monochamol, which is a component of Monochamus galloprovincialis Olivier (Coleoptera: Cerambycidae: Lamiinae) pheromone [54,55]. Hoch et al. [46] reported that the inclusion of α-pinene and ethanol in a mixture of geranyl acetone, fuscumol acetate, fuscumol, monochamol, 3-hydroxyhexan-2-one, 2-methyl-1-butanol, anti-2,3-hexanediol, and prionic acid significantly increased the number of captured individuals of species of the family Cerambycidae compared to the captures recorded in the mixture without the addition of kairomones. In our study, the addition of ipsenol to attractant 3 further enhanced its effectiveness. Similarly, Miller et al. [44] reported that the number of captured individuals of Monochamus scutellatus (Say) (Coleoptera: Cerambycidae: Lamiinae) in traps that had a mixture of ipsenol and α-pinene was higher than the number of captures in traps that had α-pinene alone. The authors also found that ipsenol significantly increased the number of captures of Acanthocinus princeps (Walker), Acanthocinus obliquus (LeConte), Acanthocinus nodosus (F.), Acanthocinus obsoletus (Olivier) (Coleoptera: Cerambycidae: Lamiinae), and Rhagium inquisitor (L.) (Coleoptera: Cerambycidae: Lepturinae) when α-pinene was added to the traps.
One other important finding of the present study was the depiction of the flight activity of X. chinensis, revealing valuable data on the ecology of this pest that could be useful for its successful management. Specifically, during the monitoring of the traps, it was found that the flight of X. chinensis begins around the end of April and terminates around the end of October. This stands in agreement with the suggestion of Leivadara et al. [1] for the emergence of X. chinensis in Crete (Greece), which takes place between May and June. In Spain (Catalonia), Sarto i Monteys and Torras i Tutusaus [2], after two years of observations of specimens kept in cages under semi-field environment conditions, reported that adults emerge later, in July and August, exactly the same period as adults of X. chinensis emerge in Japan, which is one of the countries of origin of this species [22]. The authors also found that in both years of their observations, the emergence of adults began after ten or seven consecutive days with mean daily temperature >20 °C, for 2016 and 2017, respectively [2]. In Athens, the first captures of traps were recorded at the beginning of May 2022. In the time between the previous check (late April) of the traps and the check of the first captures, it was observed that the average daily temperature in Athens was ~20 °C. New adults of X. chinensis after eclosion have been reported to remain inside the trees for a period between 12 and 40 days before leaving the trees [17,18], probably awaiting stabilization of the temperature above 20 °C. Hence, considering our results and those obtained from Spain, we assume that emergence of X. chinensis adults from infested trees requires certain temperature conditions. Additional research is required to verify this hypothesis.
Concerning the termination of the flight period of X. chinensis, in both years of the study, the last captures were recorded between 18 and 21 October. Although the life span of X. chinensis adults is not known, considering the period of adults’ emergence and the flight activity of X. chinensis obtained in our experiments, we assume that the last adults that emerged from the trees correspond to the last individuals captured in the traps. This issue should be further investigated. Regarding the performance of each attractant, attractant 1 ceased to be active in early September. The early reduction in the captures of attractant 1 compared to attractants 2 and 3 is probably due to the low performance exhibited since the beginning of X. chinensis flight activity. It should be noted that the last captures in 2022 were recorded in traps containing attractant 3, although in 2021 the last captures were recorded in traps containing either attractant 2 or attractant 3. Having expanded 334 km2 in Catalonia in two years, X. chinensis is expected to spread rapidly to nearby areas [8,11,55] and through wooden boxes that are used for the transportation of goods [16]. Therefore, attractant 3 could be used to detect early arrivals of the pest. Precise records of X. chinensis in new areas could trigger management measurements to restrict further dispersal of this species.
5. Conclusions
To conclude, the most effective attractant was found to be attractant 3, which can help the successful monitoring of X. chinensis. The combination of α-pinene and ipsenol enhanced the activity of the basic part of the blend (i.e., pheromones + ethanol), while the role of ethanol is rather uncertain. It does not seem likely that X. chinensis was attracted to the basic part of the blend, because it was the least active attractant. Thus, it seems more likely that the pest was attracted to the blend of kairomones, with the data showing that α-pinene and ipsenol were attractive. Further investigation is needed by testing other blends of attractants. In addition, the role of α-pinene, ipsenol, and ethanol should be evaluated for the attraction of X. chinensis to make a direct comparison to attractant 3. The precise monitoring of populations of noxious insect populations is crucial, since it provides timely and detailed information on pest densities, resulting in a reduction in synthetic insecticides and the prompt application of ecologically friendly methods, such as mass trapping. The next step of investigation deals with the assessment of new trap devices to optimize the attractant-based trapping protocol of this noxious species.
Author Contributions
Conceptualization, N.G.K., M.C.B., P.V.P., D.P.P. and G.T.P.; methodology, N.G.K., M.C.B., S.A., A.S., P.V.P., D.P.P. and G.T.P.; validation, N.G.K., M.C.B., A.S., S.A., P.V.P., D.P.P. and G.T.P.; formal analysis, N.G.K. and M.C.B.; investigation, N.G.K., M.C.B., A.S., S.A., P.V.P., D.P.P. and G.T.P.; data curation, N.G.K., M.C.B., A.S., S.A., P.V.P., D.P.P. and G.T.P.; writing—original draft preparation, N.G.K., M.C.B. and A.S.; writing—review and editing, N.G.K., M.C.B., A.S., S.A., P.V.P., D.P.P. and G.T.P.; visualization, N.G.K., M.C.B., A.S., S.A., P.V.P., D.P.P. and G.T.P.; supervision, N.G.K., P.V.P., D.P.P. and G.T.P.; project administration; N.G.K., M.C.B., P.V.P., D.P.P. and G.T.P.; resources, N.G.K., P.V.P., D.P.P. and G.T.P.; funding acquisition, N.G.K., P.V.P., D.P.P. and G.T.P. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the project 33.0256 “Management of Xyloterchus chinensis (Coleoptera: Cerambycidae) with pheromones and insecticides in Municipality of Athens” (Municipality of Athens).
Data Availability Statement
Data are contained within the article.
Acknowledgments
We thank E.P. Nika for technical assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Leivadara, E.; Leivadaras, I.; Vontas, I.; Trichas, A.; Simoglou, K.; Roditakis, E.; Avtzis, D.N. First record of Xylotrechus chinensis (Coleoptera, Cerambycidae) in Greece and in the EPPO region. EPPO Bull. 2018, 48, 277–280. [Google Scholar] [CrossRef]
- Sarto i Monteys, V.; Torras i Tutusaus, G. A new alien invasive longhorn beetle, Xylotrechus chinensis (Cerambycidae), is infesting mulberries in Catalonia (Spain). Insects 2018, 9, 52. [Google Scholar] [CrossRef]
- Korakaki, E.; Legakis, A.; Katsanevakis, S.; Koulelis, P.P.; Avramidou, E.V.; Soulioti, N.; Petrakis, P.V. Invasive Alien Species of Greece. In Invasive Alien Species; Pullaiah, T., Ielmini, M.R., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2021; Volume 3, pp. 124–189. [Google Scholar]
- Han, Y.; Lyu, D. Taxonomic review of the genus Xylotrechus (Coleoptera: Cerambycidae: Cerambycinae) in Korea with a newly recorded species. Korean J. Appl. Entomol. 2010, 49, 69–82. [Google Scholar] [CrossRef]
- Sama, G.; Löbl, I. Cerambycinae. In Catalogue of Palaearctic Coleoptera. Chrysomeloidea; Löbl, I., Smetana, A., Eds.; Apollo Books: Stenstrup, Denmark, 2010; pp. 143–207. [Google Scholar]
- Schrader, G. Express—PRA zu Xylotrechus chinensis. Available online: https://pflanzengesundheit.julius-uehn.de/dokumente/upload/b75e3_xylotrechus-chinensis_express-pra.pdf (accessed on 2 July 2023).
- Valladares, L.; Brustel, H.; Cocquempot, C. Xylotrechus chinensis: Nouvelle Espèce Invasive Arrivée d’Asie. Available online: https://passion-entomologie.fr/xylotrechus-chinensisnouvelle-especeinvasive/ (accessed on 2 July 2023).
- Bragard, C.; Baptista, P.; Chatzivassiliou, E.; Di Serio, F.; Gonthier, P.; Jaques Miret, J.A.; Justesen, A.F.; Magnusson, C.S.; Milonas, P.; Navas-Cortes, J.A. Pest categorisation of Xylotrechus chinensis. EFSA J. 2021, 19, e07022. [Google Scholar]
- Demetriou, J.; Kalaentzis, K.; Kazilas, C.; Koutsoukos, E.; Avtzis, D.; Georgiadis, C. Revisiting the non-native insect fauna of Greece: Current trends and an updated checklist. NeoBiota 2021, 65, 93–108. [Google Scholar] [CrossRef]
- Kavallieratos, N.G.; Boukouvala, M.C.; Skourti, A.; Nika, E.P.; Papadoulis, G.T. Trunk injection with insecticides manages Xylotrechus chinensis (Chevrolat) (Coleoptera: Cerambycidae). Insects 2022, 13, 1106. [Google Scholar] [CrossRef]
- Sarto i Monteys, V.; Ribes, A.C.; Savin, I. The invasive longhorn beetle Xylotrechus chinensis, pest of mulberries, in Europe: Study on its local spread and efficacy of abamectin control. PLoS ONE 2021, 16, e0245527. [Google Scholar] [CrossRef]
- Cocquempot, C.; Desbles, F.; Mouttet, R.; Valladares, L. Xylotrechus chinensis (Chevrolat, 1852), nouvelle espèce invasive pour la France métropolitaine (Coleoptera, Cerambycidae, Clytini). Bull. Soc. Entomol. Fr. 2019, 124, 27–32. [Google Scholar] [CrossRef]
- EPPO (European and Mediterranean Plant Protection Organization). New data on quarantine pests and pests of the EPPO alert list. EPPO Rep. Serv. 2020, 91, 5. [Google Scholar]
- EPPO (European and Mediterranean Plant Protection Organization). Invasive alien wood borers: Trapping studies in France. EPPO Rep. Serv. 2021, 157, 7. [Google Scholar]
- Philadelphia, U.S.; Customs and Border Protection. Philly CBP Intercepts Nation’s First Xylotrechus chinensis Beetle. Available online: https://www.cbp.gov/newsroom/local-media-release/philly-cbpintercepts-nations-first-xylotrechus-chinensis-beetle (accessed on 2 July 2023).
- EPPO (European and Mediterranean Plant Protection Organization). Alert list—Xylotrechus chinensis (Coleoptera: Cerambycidae). Available online: https://www.eppo.int/ACTIVITIES/plant_quarantine/alert_list_insects/xylotrechus_chinensis (accessed on 2 July 2023).
- Murakami, M. Study on biology and control of Xylotrechus chinensis Chevrolat. Tech. Bull. Seric. Exp. Stat. 1960, 77, 1–23. [Google Scholar]
- Togashi, K.; Sasaki, M.; Fujii, T. Relationship between body size and ovariole number in three cerambycids inhabiting mulberry orchards. Appl. Entomol. Zool. 2020, 55, 9–16. [Google Scholar] [CrossRef]
- Fettig, C.J.; Grosman, D.M.; Munson, A.S. Efficacy of abamectin and tebuconazole injections to protect lodgepole pine from mortality attributed to mountain pine beetle (Coleoptera: Curculionidae) attack and progression of blue stain fungi. J. Entomol. Sci. 2013, 48, 270–278. [Google Scholar] [CrossRef]
- Nicolopoulou-Stamati, P.; Maipas, S.; Kotampasi, C.; Stamatis, P.; Hens, L. Chemical pesticides and human health: The urgent need for a new concept in agriculture. Front. Public Health 2016, 4, 148. [Google Scholar] [CrossRef]
- Potamitis, I.; Rigakis, I.; Tatlas, N.A.; Potirakis, S. In-vivo vibroacoustic surveillance of trees in the context of the IoT. Sensors 2019, 19, 1366. [Google Scholar] [CrossRef]
- Kuwahara, Y.; Matsuyama, S.; Suzuki, T. Identification of 2,3-octanediol, 2-hydroxy-3-octanone, and 3-hydroxy-2-octanone from male Xylotrechus chinensis Chevrolat as possible sex pheromones (Coleoptera: Cerambycidae). Appl. Entomol. Zool. 1987, 22, 25–28. [Google Scholar] [CrossRef]
- Iwabuchi, K.; Takahashi, J.; Sakai, T. Occurrence of 2, 3-octanediol and 2-hydroxy-3-octanone, possible male sex pheromone in Xylotrechus chinensis Chevrolat (Coleoptera: Cerambycidae). Appl. Entomol. Zool. 1987, 22, 110–111. [Google Scholar] [CrossRef]
- EPPO (European and Mediterranean Plant Protection Organization). Xylotrechus chinensis (Coleoptera: Cerambycidae): Addition to the EPPO alert list. EPPO Rep. Serv. 2018, 157, 7–9. [Google Scholar]
- Fan, J.T.; Denux, O.; Courtin, C.; Bernard, A.; Javal, M.; Millar, J.G.; Hanks, L.M.; Roques, A. Multi-component blends for trapping native and exotic longhorn beetles at potential points-of-entry and in forests. J. Pest Sci. 2019, 92, 281–297. [Google Scholar] [CrossRef]
- Miller, D.R.; Crowe, C.M.; Mayo, P.D.; Reid, L.S.; Silk, P.J.; Sweeney, J.D. Interactions between ethanol, syn-2,3-hexanediol, 3-hydroxyhexan-2-one, and 3-hydroxyoctan-2-one lures on trap catches of hardwood longhorn beetles in southeastern United States. J. Econ. Entomol. 2017, 110, 2119–2128. [Google Scholar] [CrossRef]
- Sweeney, J.D.; Silk, P.J.; Gutowski, J.M.; Wu, J.; Lemay, M.A.; Mayo, P.D.; Magee, D.I. Effect of chirality, release rate, and host volatiles on response of Tetropium fuscum (F.), Tetropium cinnamopterum Kirby, and Tetropium castaneum (L.) to the aggregation pheromone, fuscumol. J. Chem. Ecol. 2010, 36, 1309–1321. [Google Scholar] [CrossRef]
- Pajares, J.A.; Alvarez, G.; Ibeas, F.; Gallego, D.; Hall, D.R.; Fahman, D.I. Identification and field activity of a male-produced aggregation pheromone in the pine sawyer beetle, Monochamus galloprovincialis. J. Chem. Ecol. 2010, 36, 570–583. [Google Scholar] [CrossRef]
- Mitchell, R.F.; Graham, E.E.; Wong, J.C.H.; Reagel, P.F.; Striman, B.L.; Hughes, G.P.; Paschen, M.A.; Ginzel, M.D.; Millar, J.G.; Hanks, L.M. Fuscumol and fuscumol acetate are general attractants for many species of cerambycid beetles in the subfamily Lamiinae. Entomol. Exp. Appl. 2011, 141, 71–77. [Google Scholar] [CrossRef]
- Hanks, L.M.; Millar, J.G.; Moreira, J.A.; Barbour, J.D.; Lacey, E.S.; McElfresh, J.S.; Reuter, F.R.; Ray, A.M. Using generic pheromone lures to expedite identification of aggregation pheromones for the cerambycid beetles Xylotrechus nauticus, Phymatodes lecontei, and Neoclytus modestus modestus. J. Chem. Ecol. 2007, 33, 889–907. [Google Scholar] [CrossRef]
- Hanks, L.M.; Millar, J.G.; Mongold-Diers, J.A.; Wong, J.C.H.; Meier, L.R.; Reagel, P.F.; Mitchell, R.F. Using blends of cerambycid beetle pheromones and host volatiles to simultaneously attract a diversity of cerambycid species. Can. J. For. Res. 2012, 42, 1050–1059. [Google Scholar] [CrossRef]
- Millar, J.G.; Hanks, L.M. Chemical Ecology of Cerambycids. In Cerambycidae of the World: Biology and Pest Management; Wang, Q., Ed.; CRC Press/Taylor and Francis Group: Boca Raton, FL, USA, 2017; pp. 161–208. [Google Scholar]
- Barbour, J.D.; Millar, J.G.; Rodstein, J.; Ray, A.M.; Alston, D.G.; Rejzek, M.; Dutcher, J.D.; Hanks, L.M. Synthetic 3,5-dimethyldodecanoic acid serves as a general attractant for multiple species of Prionus (Coleoptera: Cerambycidae). Ann. Entomol. Soc. Am. 2011, 104, 588–593. [Google Scholar] [CrossRef]
- Kelsey, R.G.; Joseph, G. Ethanol in ponderosa pine as an indicator of physiological injury from fire and its relationship to secondary beetles. Can. J. For. Res. 2003, 33, 870–884. [Google Scholar] [CrossRef]
- Ranger, C.M.; Reding, M.E.; Persad, A.B.; Herms, D.A. Ability of stress-related volatiles to attract and induce attacks by Xylosandrus germanus and other ambrosia beetles. Agric. For. Entomol. 2010, 12, 177–185. [Google Scholar] [CrossRef]
- Kelsey, R.G.; Gallego, D.; Sánchez-García, F.J.; Pajares, J.A. Ethanol accumulation during severe drought may signal tree vulnerability to detection and attack by bark beetles. Can. J. For. Res. 2014, 44, 554–561. [Google Scholar] [CrossRef]
- Hanks, L.M.; Millar, J.G. Field bioassays of cerambycid pheromones reveal widespread parsimony of pheromone structures, enhancement by host plant volatiles, and antagonism by components from heterospecifics. Chemoecology 2013, 23, 21–44. [Google Scholar] [CrossRef]
- Rodríguez-González, A.; Sánchez-Maíllo, E.; Peláez, H.J.; Mayo, S.; González-López, O.; Carro-Huerga, G.; Casquero, P.A. Evaluation of commercial and prototype traps for Xylotrechus arvicola (Coleoptera: Cerambycidae), an insect pest in Spanish vineyards. Aust. J. Grape Wine Res. 2018, 24, 190–196. [Google Scholar] [CrossRef]
- Sukovata, L.; Dziuk, A.; Plewa, R.; Jaworski, T. The effect of trap color on catches of Monochamus galloprovincialis and three most numerous non-target insect species. Insects 2022, 13, 220. [Google Scholar] [CrossRef]
- Costello, S.L.; Negrón, J.F.; Jacobi, W.R. Traps and attractants for wood-boring insects in ponderosa pine stands in the Black Hills, South Dakota. J. Econ. Entomol. 2008, 101, 409–420. [Google Scholar] [CrossRef]
- Blomquist, G.J.; Figueroa-Teran, R.; Aw, M.; Song, M.; Gorzalski, A.; Abbott, N.L.; Chang, E.; Tittiger, C. Pheromone production in bark beetles. Insect Biochem. Mol. Biol. 2010, 40, 699–712. [Google Scholar] [CrossRef]
- Rodríguez-González, A.; Sánchez-Maillo, E.; Peláez, H.J.; González-Núñez, M.; Hall, D.R.; Casquero, P.A. Field evaluation of 3-hydroxy-2-hexanone and ethanol as attractants for the cerambycid beetle pest of vineyards, Xylotrechus arvicola. Pest Manag. Sci. 2017, 73, 1598–1603. [Google Scholar] [CrossRef]
- Miller, D.R.; Rabaglia, R.J. Ethanol and (−)-α-pinene: Attractant kairomones for bark and ambrosia beetles in the southeastern U.S. J. Chem. Ecol. 2009, 35, 435–448. [Google Scholar] [CrossRef]
- Miller, D.R.; Allison, J.D.; Crowe, C.M.; Dickinson, D.M.; Eglitis, A.; Hofstetter, R.W.; Munson, A.S.; Poland, T.M.; Reid, L.S.; Steed, B.E.; et al. Pine sawyers (Coleoptera: Cerambycidae) attracted to α-pinene, monochamol, and ipsenol in North America. J. Econ. Entomol. 2016, 109, 1205–1214. [Google Scholar] [CrossRef]
- Dodds, K.J.; DiGirolomo, M.F. Effect of cleaning multiple-funnel traps on captures of bark and woodboring beetles in northeastern United States. Insects 2020, 11, 702. [Google Scholar] [CrossRef]
- Hoch, G.; Connell, J.; Roques, A. Testing multi-lure traps for surveillance of native and alien longhorn beetles (Coleoptera, Cerambycidae) at ports of entry and in forests in Austria. Manag. Biol. Invasions 2020, 11, 677–688. [Google Scholar] [CrossRef]
- Kavallieratos, N.G.; Athanassiou, C.G.; Balotis, G.N.; Tatsi, G.T.; Mazomenos, B.E. Factors affecting male Prays oleae (Lepidoptera: Yponomeutidae) captures in pheromone-baited traps in olive orchards. J. Econ. Entomol. 2005, 98, 1499–1505. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis; Pearson: Harlow, UK, 2014. [Google Scholar]
- Sall, J.; Lehman, A.; Creighton, L. JMP start statistics. In A Guide to Statistics and Data Analysis Using JMP and JMP in Software; Duxbury Press: Belmont, ON, Canada, 2001. [Google Scholar]
- Sokal, R.R.; Rohlf, F.J. Biometry; Freeman & Company: New York, NY, USA, 1995. [Google Scholar]
- SAS Institute Inc. Using JMP 16.2; SAS Institute Inc.: Cary, NC, USA, 2021. [Google Scholar]
- Brockerhoff, E.G.; Jones, D.C.; Kimberley, M.O.; Suckling, D.M.; Donaldson, T. Nationwide survey for invasive wood-boring and bark beetles (Coleoptera) using traps baited with pheromones and kairomones. For. Ecol. Manag. 2006, 228, 234–240. [Google Scholar] [CrossRef]
- Dodds, K.J.; Dubois, G.D.; Hoebeke, R.R. Trap type, lure placement, and habitat effects on Cerambycidae and Scolytinae (Coleoptera) catches in the Northeastern United States. J. Econ. Entomol. 2010, 103, 698–707. [Google Scholar] [CrossRef] [PubMed]
- Pajares, J.; Álvarez, G.; Hall, D.; Ibarra, N.; Hoch, G.; Halbig, P.; Cocoş, D.; Johansson, H.; Schroeder, M. Attractants for management of the pine sawyer beetle Monochamus sutor, a potential vector of Bursaphelenchus xylophilus. J. Appl. Entomol. 2017, 141, 97–111. [Google Scholar] [CrossRef]
- EPPO (European and Mediterranean Plant Protection Organization). Available online: https://gd.eppo.int/taxon/XYLOCH (accessed on 19 July 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).