Parasitoid Wasp Acerophagus papayae: A Promising Solution for the Control of Papaya Mealybug Paracoccus marginatus in Cassava Fields in Vietnam
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites and Biological Materials
2.2. Experimental Designs
2.2.1. Biological Characteristics of A. papayae
Determining the Frequency of Parasitoids of the P. marginatus in Vietnam
| − | Very low | <5% |
| + | Low | 5–25% |
| ++ | Moderate | 25–50% |
| +++ | High | 50–75% |
| ++++ | Very high | >75% |
Determining the Immature Developmental Time of the Parasitoid Wasps A. papayae
Effect of Diets on the Longevity of Adult A. papayae
2.2.2. Parasitism of A. papayae on P. marginatus
Host Stage Susceptibility and Preference of A. papayae
Parasitoid Fecundity and Progeny of A. papayae
The Effect of Parasitism Activity on the Longevity of Female A. papaya
2.3. Data Analysis
3. Results
3.1. Diversity and Abundance of P. marginatus Parasitoids in Vietnam
3.2. Mating Activity and Egg-Laying Behavior of A. papayae under Laboratory Conditions
3.3. Life Cycle of the A. papayae and Developmental Time of Each Phase
3.4. The Potential of Using Honey Solution as a Diet for A. papayae in the Absence of a Host
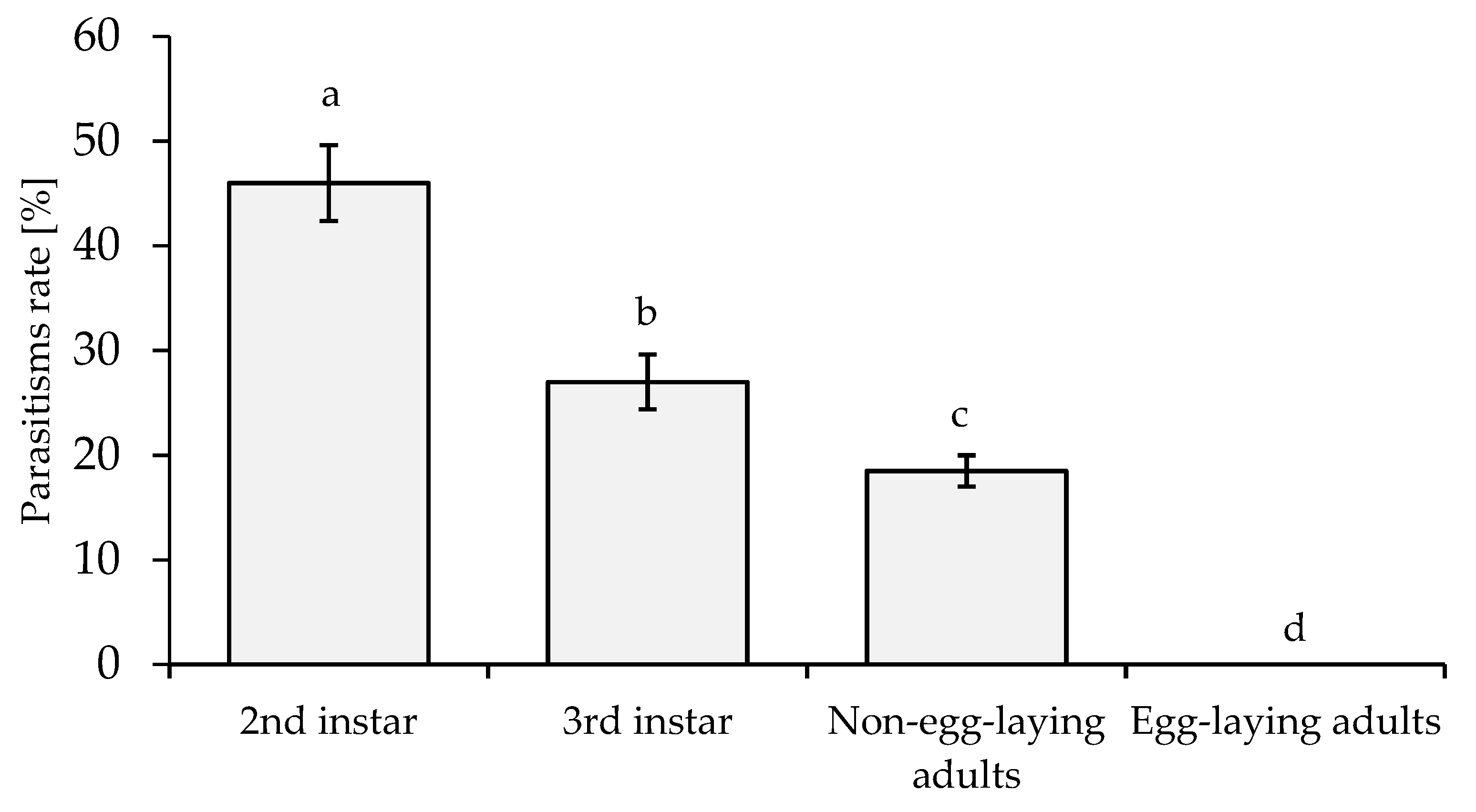
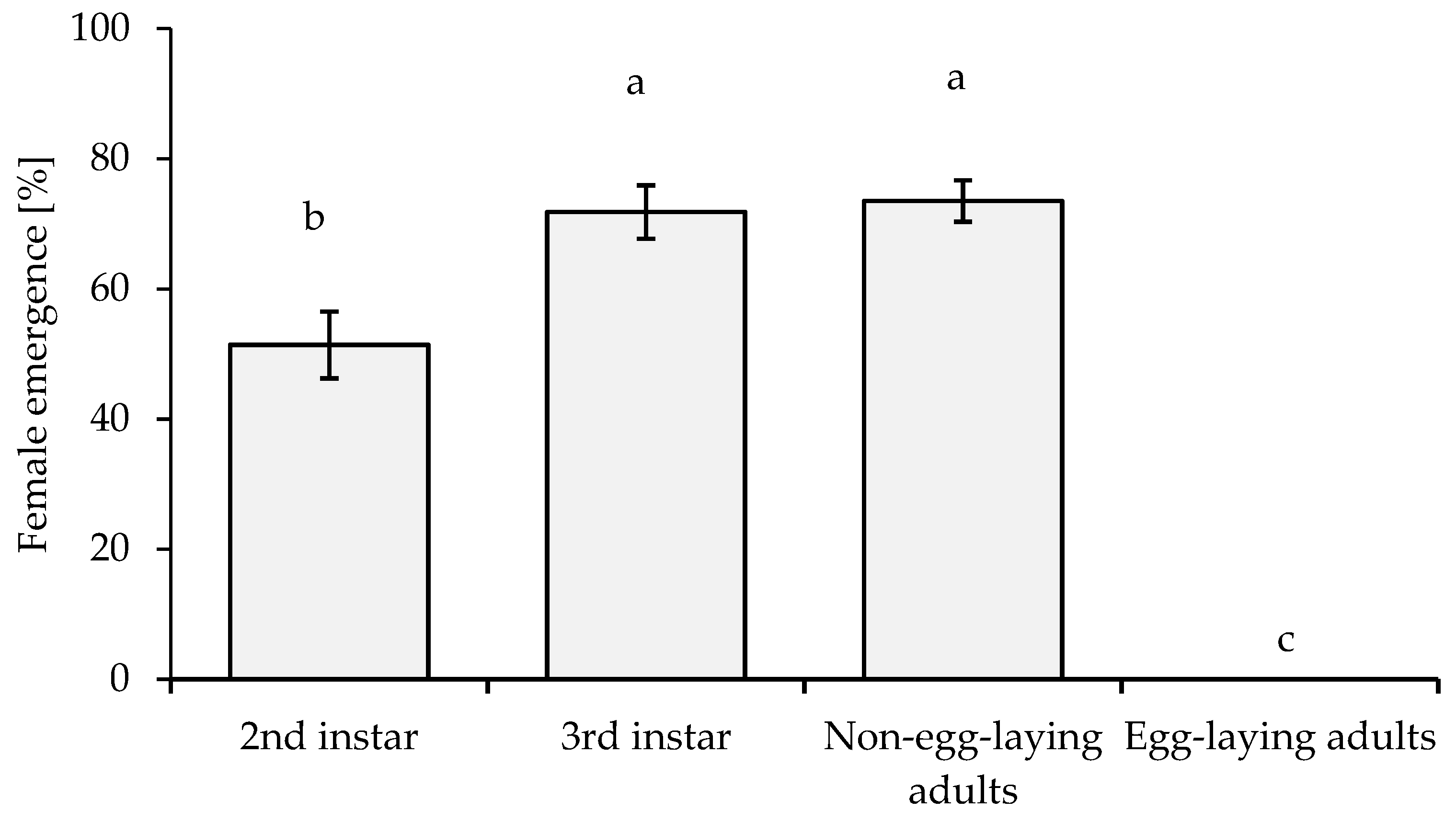
3.5. Susceptibility and Preference of Different Host Stages for A. papayae
3.6. Acerophagus papayae Reproduction on P. marginatus
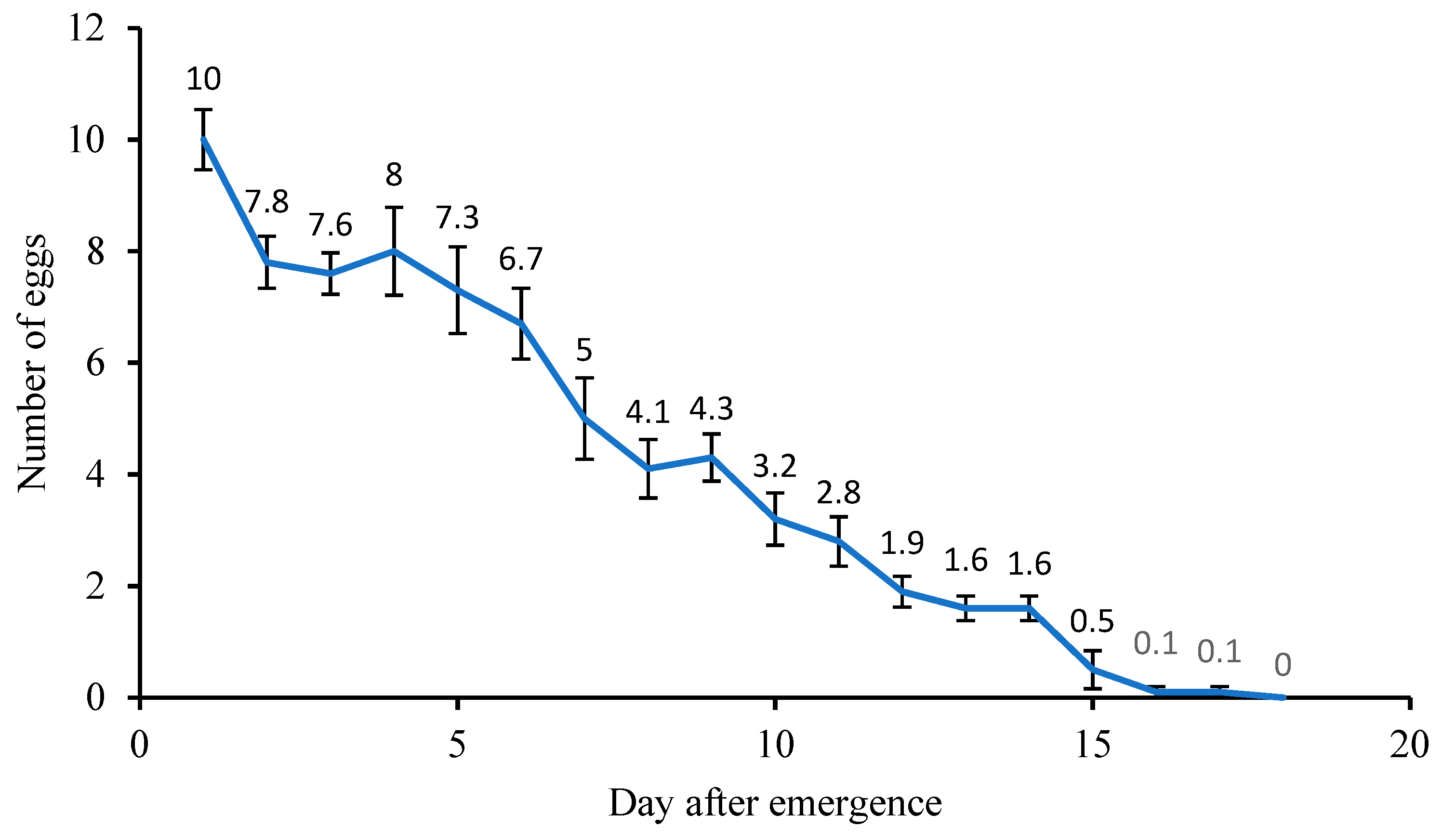
3.7. Parasitism and Longevity of Female A. papayae
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. Food and Agriculture Organization of the United Nations Statistics Division. Online Statistical Database: Food Balance. FAOSTAT. 2023. Available online: https://www.fao.org/faostat/en/#data/QCL/visualize (accessed on 20 January 2023).
- Prudencio, Y.C.; Al-Hassan, R. The food security stabilization roles of cassava in Africa. Food Policy 1994, 19, 57–64. [Google Scholar] [CrossRef]
- Onodu, B.C.; Culas, R.J. The role of Cassava production in improving food security in the delta state of Nigeria. In Food Security: Threat Factors, Policies and Challenges; Webb, J., Ed.; Food Science and Technology; Nova Science Publishers: Hauppauge, NY, USA, 2017; pp. 1–34. [Google Scholar]
- Tize, I.; Fotso, A.K.; Nukenine, E.N.; Masso, C.; Ngome, F.A.; Suh, C.; Lendzemo, V.W.; Nchoutnji, I.; Manga, G.; Parkes, E.; et al. New cassava germplasm for food and nutritional security in Central Africa. Sci. Rep. 2021, 11, 7394. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.H.; Nguyen, T.C.; Tong, Q.A. Cassava leaf production research in Vietnam. In Cassava Research and Development in Asia: Exploring New Opportunities for an Ancient Crop; Howeler, R.H., Ed.; Cali CIAT: Valle del Cauca, Colombia, 2007; pp. 494–495. Available online: https://cgspace.cgiar.org/bitstream/handle/10568/56568/cassava_research_development_asia.pdf?sequence=1 (accessed on 20 January 2023).
- Nguyen, H.H.; Tran, C.K. Achievements in cassava research and development in Vietnam and orientation to 2020. In Report on Research on Cassava Development 2007–2012; Institute of Agricultural Science for Southern Vietnam: Ho Chi Minh, Vietnam, 2012; pp. 1–7. [Google Scholar]
- Shigaki, T. Cassava: The nature and uses. In Encyclopedia of Food and Health; Academic Press: Cambridge, MA, USA, 2016; pp. 687–693. [Google Scholar] [CrossRef]
- Hoang, L.; Nguyen, T.T.M.; Nguyen, B.M.; Hoang, K.; Ishitani, M.; Howeler, R. Cassava in Vietnam: Production and research; an overview. In Proceedings of the Paper Presented at Asia Cassava Research Workshop, Hosted by ILCMB-CIAT-VAAS/AGI, Hanoi, Vietnam, 3 November 2014; 15p. [Google Scholar]
- Hoang, K.; Nguyen, T.T.M.; Nguyen, B.M.; Howeler, R. Cassava Conservation And Sustainable Development in Vietnam. In Proceedings of the Sustainable Cassava Production in Asia for Multiple Uses and for Multiple Markets, the 9th Regional Cassava Workshop, Nanning, China, 27 November–3 December 2011. [Google Scholar]
- Safo-Kantanka, O.; Owusu-Nipah, J. Cassava varietal screening for cooking quality: Relationship between dry matter, starch content, mealiness and certain microscopic observations of the raw and cooked tuber. J. Sci. Food Agric. 1992, 60, 99–104. [Google Scholar] [CrossRef]
- Ebah-Djedji, B.C.; Dje, K.M.; Ue, B.N.; Zohouri, G.P.; Amani, N.G. Effect of harvest period on starch yield and dry matter content from the tuberous roots of improved cassava (Manihot esculenta Crantz) varieties. Pak. J. Nutr. 2012, 11, 414–418. [Google Scholar] [CrossRef]
- Bellotti, A.C. Cassava Pests and their Management. In Encyclopedia of Entomology; Capinera, J.L., Ed.; Springer: Dordrecht, The Netherlands, 2008. [Google Scholar] [CrossRef]
- Graziosi, I.; Minato, N.; Alvarez, E.; Ngo, D.T.; Hoat, T.X.; Aye, T.M.; Pardo, J.M.; Wongtiem, P.; Wyckhuys, K.A. Emerging pests and diseases of South-east Asian cassava: A comprehensive evaluation of geographic priorities, management options and research needs. Pest Manag. Sci. 2016, 72, 1071–1089. [Google Scholar] [CrossRef]
- Bellotti, A.C.; Herrera, C.J.; Hernández, M.P.; Ariasm, B.; Guerrero, J.M.; Melo, E.L. Three major cassava pests in Latin America, Africa and Asia. In A New Future for Cassava in Asia: Its Use as Food, Feed and Fuel to Benefit the Poor; Howeler, R.H., Ed.; The Nippon Foundation: Tokyo, Japan, 2010; pp. 544–577. [Google Scholar]
- Tokunaga, H.; Baba, T.; Ishitani, M.; Ito, K.; Kim, O.-K.; Ham, L.H.; Le, H.K.; Maejima, K.; Natsuaki, K.T.; Van Dong, N.; et al. Sustainable Management of Invasive Cassava Pests in Vietnam, Cambodia, and Thailand. In Crop Production under Stressful Conditions; Kokubun, M., Asanuma, S., Eds.; Springer: Singapore, 2018. [Google Scholar] [CrossRef]
- Le, T.T.N.; Truong, T.H.L.; La, V.H. Pest composition and natural enemies on cassava in Vietnam. In Proceedings of the 6th National Scientific Conference on Ecology and Biological Resources, Hanoi, Vietnam, 21 October 2015; Natural Science and Technology Publishing House: Hanoi, Vietnam, 2015; pp. 1551–1554. [Google Scholar]
- Williams, D.J.; de Willink, M.C.G. Mealybugs of Central and South America; CAB International: Wallingford, UK, 1992. [Google Scholar]
- Miller, D.R.; Miller, G.L.; Watson, G.W. Invasive species of mealybugs (Hemiptera: Pseudococcidae) and their threat to U.S. agriculture. Proc. Entomol. Soc. Wash. 2002, 104, 825–836. [Google Scholar]
- Walker, A.; Hoy, M.; Meyerdirk, D.E.; Papaya Mealybug. University of Florida: Featured Creatures. 2003. Available online: https://entnemdept.ufl.edu/creatures/fruit/mealybugs/papaya_mealybug.htm (accessed on 22 March 2023).
- Heu, R.A.; Fukada, M.T.; Conant, P. Papaya mealybug, Paracoccus marginatus Williams and Granara de Willink (Hemiptera: Pseudococcidae). New Pest Advisory No. 04-03. Department of Agriculture, Hawaii. Mol. Evol. 2007, 16, 11–120. [Google Scholar]
- Mani, M.; Shylesha, A.N.; Shivaraju, C. First report of the invasive papaya mealybug, Paracoccus marginatus Williams & Granara de Willink (Homoptera: Pseudococcidae) in Rajasthan. Pest Manag. Hortic. Ecosyst. 2012, 18, 234. [Google Scholar]
- Finch, E.A.; Beale, T.; Chellappan, M.; Goergen, G.; Gadratagi, B.G.; Khan, M.A.M.; Rehman, A.; Rwomushana, I.; Sarma, A.K.; Wyckhuys, K.A.; et al. The potential global distribution of the papaya mealybug, Paracoccus marginatus, a polyphagous pest. Pest Manag. Sci. 2021, 77, 1361–1370. [Google Scholar] [CrossRef]
- Tanwar, R.; Jeyakumar, P.; Vennila, S. Papaya Mealybug and Its Management Strategies; NCIPM Technical Bulletin 22; National Centre for Integrated Pest Management: New Delhi, India, 2010.
- Muniappan, R. Invasion of Papaya Mealybug in Asia; Virginia Tech: Blacksburg, VA, USA, 2008. [Google Scholar]
- Kansiime, M.K.; Rwomushana, I.; Idah Mugambi, I.; Makale, F.; Julien Lamontagne-Godwin, J.; Chacha, D.; Kibwage, P.; Oluyali, J.; Day, R. Crop losses and economic impact associated with papaya mealybug (Paracoccus marginatus) infestation in Kenya. Int. J. Pest Manag. 2020, 69, 150–163. [Google Scholar] [CrossRef]
- Mwanauta, R.W.; Ndakidemi, R.A.; Venkataramana, P.B. Biopesticide efficacy of four plant essential oils against papaya mealybug, Paracoccus marginatus Williams and Granara de Willink (Hemiptera: Pseudococcidae). Heliyon 2023, 9, e14162. [Google Scholar] [CrossRef]
- Le, T.T.N.; Graziosi, I.; Cira, T.M.; Gates, M.W.; Parker, L.; Wyckhuys, K.A.G. Landscape context does not constrain biological control of Phenacoccus manihoti in intensified cassava systems of southern Vietnam. Biol. Control 2018, 121, 129–139. [Google Scholar] [CrossRef]
- Muniappan, R.; Shepard, B.M.; Watson, G.W.; Carner, G.R.; Sartiami, D.; Rauf, A.; Hammig, M.D. First report of the papaya mealybug, Paracoccus marginatus (Hemiptera: Pseudococcidae), in Indonesia and India. J. Agric. Urban Entomol. 2008, 25, 37–40. [Google Scholar] [CrossRef]
- Muniappan, R. Invasion of papaya mealybug, Paracoccus marginatus in Asia. In Proceedings of the 57th Annual Meeting of the Entomological Society of America, Indianapolis, IN, USA, 13–16 December 2009. [Google Scholar]
- Pham, V.L. Insect and small spiders damage crop plants in Vietnam. Nong Nghiep J. 2002, 1, 266–267. [Google Scholar]
- Nguyen, T.C. Some enemies of scale insects on fruit, industrial and ornamental crops at Ho Chi Minh area and surrounding provinces. In Proceedings of the 6th Vietnam National Conference on Entomology, Hanoi, Vietnam, 9–10 May 2008. [Google Scholar]
- Muniappan, R.; Meyerdirk, D.E.; Sengebau, F.M.; Berringer, D.D.; Reddy, G.V.P. Classical biological control of the papaya mealybug, Paracoccus marginatus (Hemiptera: Pseudococcidae) in the republic of Palau. Fla. Entomol. 2006, 89, 212–217. [Google Scholar] [CrossRef]
- Muniappan, R. Papaya Mealybug Parasitoid in Bangladesh; IAPPS Newsletter; Tamò, M., Ed.; International Association for the Plant Protection Sciences: Cotonou, Republic of Benin, 2014; Available online: https://www.plantprotection.org/iapps-newsletter-3/ (accessed on 22 March 2023).
- Mastoi, M.I.; Azura, N.; Muhamad, R.; Idris, B.; Solangi, K.; Arfan, G.; Bhatti, I.; Khoso, N. A Report of natural enemies of papaya mealybug, Paracoccus Marginatus (Hemiptera: Pseudococcidae) in Peninsular Malaysia. Sci. Int. 2016, 28, 371–374. [Google Scholar]
- Amarasekare, K.G.; Mannion, C.M.; Epsky, N.D. Efficiency and establishment of three introduced parasitoids of the mealybug, Paracoccus marginatus (Hemiptera: Pseudococcidae). Biol. Control 2009, 51, 91–95. [Google Scholar] [CrossRef]
- Meyerdirk, D.E.; Muniappan, R.; Warkentin, R.; Bamba, R.; Reddy, G.V.P. Biological control of the papaya mealybug, Paracoccus marginatus (Hemiptera: Pseudococcidae) in Guam. Plant Prot. Q. 2004, 19, 110–114. [Google Scholar]
- Sakthivel, N. Effectiveness of three introduced encyrtid parasitic wasps (Acerophagus papayae, Anagyrus loecki and Pseudleptomastix mexicana) against papaya mealybug, Paracoccus marginatus, infesting mulberry in Tamil Nadu. J. Biopestic. 2013, 6, 71–76. [Google Scholar]
- Noyes, J.S.; Schauff, M.E. New Encyrtidae (Hymenoptera) from papaya mealybug (Paracoccus marginatus|Williams and Granara de Willink) (Hemiptera: Sternorhyncha: Pseudococcidae). Proc. Entomol. Soc. Wash. 2003, 105, 180–185. [Google Scholar]
- Amarasekare, K.G.; Mannion, C.M.; Epsky, N.D. Host instar susceptibility and selection and interspecific competition of three introduced parasitoids of the mealybug Paracoccus marginatus (Hemiptera: Pseudococcidae). Environ. Entomol. 2010, 39, 1506–1512. [Google Scholar] [CrossRef] [PubMed]
- Gomez, K.A.; Gomez, A.A. Statistical Procedures for Agricultural Research, 2nd ed.; John Wiley and Sons: New York, NY, USA, 1984; p. 680. ISBN 978-0-471-87092-0. [Google Scholar]
- Sokal, R.R.; Rohlf, F.J. Biometry: The Principles and Practice of Statistics in Biological Research, 3rd ed.; W. H. Freeman and Company: New York, NY, USA, 1995; p. 887. [Google Scholar]
- Warton, D.I.; Hui, F.K.C. The arcsine is asinine: The analysis of proportions in ecology. Ecology 2011, 92, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Muniappan, R. Ecology and Management of Paracoccus marginatus (Papaya Mealybug) (Hemiptera: Pseudococcidae) in the Indian Subcontinent—Achievements, and Lessons. Indian J. Entomol. 2021, 84, 475–482. [Google Scholar] [CrossRef]
- Available online: https://www.cabi.org/news-article/natural-enemy-fight-increased-against-papaya-mealybug-in-kenya/ (accessed on 23 March 2023).
- Amarasekare, K.G.; Mannion, C.M.; Epsky, N.D. Developmental Time, Longevity, and Lifetime Fertility of Three Introduced Parasitoids of the Mealybug Paracoccus marginatus (Hemiptera: Pseudococcidae). Environ. Entomol. 2012, 41, 1184–1189. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.H.; Oetting, R.D. Host stage selection of the mealybug parasitoid Anagyrus spec. nov near sinope. Entomol. Exp. Appl. 2006, 121, 39–50. [Google Scholar] [CrossRef]
- Mastoi, A.; Nur Azura, R.; Muhamad, I.M.; Ibrahim, Y. Survey of Papaya Mealybug, Paracoccus marginatus (Hemiptera: Pseudococcidae) and its natural enemies in Penninsular Malaysia. Pak. J. Agric. Agril. Eng. Vet. Sci. 2014, 30, 172–186. [Google Scholar]
- Mastoi, M.I.; Nur Azura Adam, N.A.; Muhamad, R.; Idris Abd Ghani, I.A.; Gilal, A.A.; Khan, J.; Bhatti, A.R.; Ahmed Zia, A.; Sahito, J.G.M. Efficiency of Acerophagus Papayae on different host stage combinations of Papaya Mealybug, Paracoccus Marginatus. Pak. J. Agri. Sci. 2018, 55, 375–379. [Google Scholar] [CrossRef]
- Divya, S.; Kalyanasundaram, M.; Karuppuchamy, P. Effect of Adult nutrition on longevity and parasitisation efficiency of Acerophagus papayae Noyes and Schauff (Hymenoptera: Encyrtidae). J. Biol. Control 2011, 25, 316–319. [Google Scholar]
- Wati, M.; Rauf, A.; Pudjianto, P. Aspects of Biology of Acerophagus Papayae Noyes & Schauff (Hymenoptera: Encyrtidae), Parasitoid of the Papaya Mealybug. J. Trop. Plant Pests Dis. 2019, 19, 52–63. [Google Scholar]
- Nisha, R.; Kennedy, J.S. Life cycle of Papaya mealybug Paracoccus marginatus Williams and Granara de Willink on different host plants vis-à-vis divergent natural selection. J. Entomol. Zool. Stud. 2017, 5, 91–102. [Google Scholar]

| Scientific Name | Occurrence Frequency |
|---|---|
| A. papayae | +++ |
| A. loecki | ++ |
| Treatments | Longevity of A. papayae (Day) | |
|---|---|---|
| Female | Male | |
| (AVG ± SE) | (AVG ± SE) | |
| Distilled water | 3.2 ± 0.13 d | 2.2 ± 0.25 c |
| Honey 10% * | 6.5 ± 0.45 c | 4.3 ± 0.40 b |
| Honey 30% * | 7.6 ± 0.27 c | 5.4 ± 0.43 b |
| Honey 50% * | 12.5 ± 0.40 a | 7.3 ± 0.34 a |
| Honey 70% * | 9.6 ± 0.56 b | 5.6 ± 0.34 b |
| p-value | <0.01 | <0.01 |
| Treatment + 50% Honey Solution | Longevity of Female A. papayae (Days) | |
|---|---|---|
| Min–Max | AVG ± SE | |
| Female without parasitism | 11–14 | 12.5 ± 0.40 |
| Female with parasitism | 12–18 | 14.2 ± 0.59 |
| t-test (p value) | 0.0288 | |
| df (within groups) | 18 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, K.H.; Tran, T.H.D.; Tran, D.H.; Nguyen, T.D.; Van Doan, C. Parasitoid Wasp Acerophagus papayae: A Promising Solution for the Control of Papaya Mealybug Paracoccus marginatus in Cassava Fields in Vietnam. Insects 2023, 14, 528. https://doi.org/10.3390/insects14060528
Le KH, Tran THD, Tran DH, Nguyen TD, Van Doan C. Parasitoid Wasp Acerophagus papayae: A Promising Solution for the Control of Papaya Mealybug Paracoccus marginatus in Cassava Fields in Vietnam. Insects. 2023; 14(6):528. https://doi.org/10.3390/insects14060528
Chicago/Turabian StyleLe, Khac Hoang, Thi Hoang Dong Tran, Dang Hoa Tran, Tuan Dat Nguyen, and Cong Van Doan. 2023. "Parasitoid Wasp Acerophagus papayae: A Promising Solution for the Control of Papaya Mealybug Paracoccus marginatus in Cassava Fields in Vietnam" Insects 14, no. 6: 528. https://doi.org/10.3390/insects14060528
APA StyleLe, K. H., Tran, T. H. D., Tran, D. H., Nguyen, T. D., & Van Doan, C. (2023). Parasitoid Wasp Acerophagus papayae: A Promising Solution for the Control of Papaya Mealybug Paracoccus marginatus in Cassava Fields in Vietnam. Insects, 14(6), 528. https://doi.org/10.3390/insects14060528








