Culicoides (Diptera: Ceratopogonidae) Abundance Is Influenced by Livestock Host Species and Distance to Hosts at the Micro Landscape Scale
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
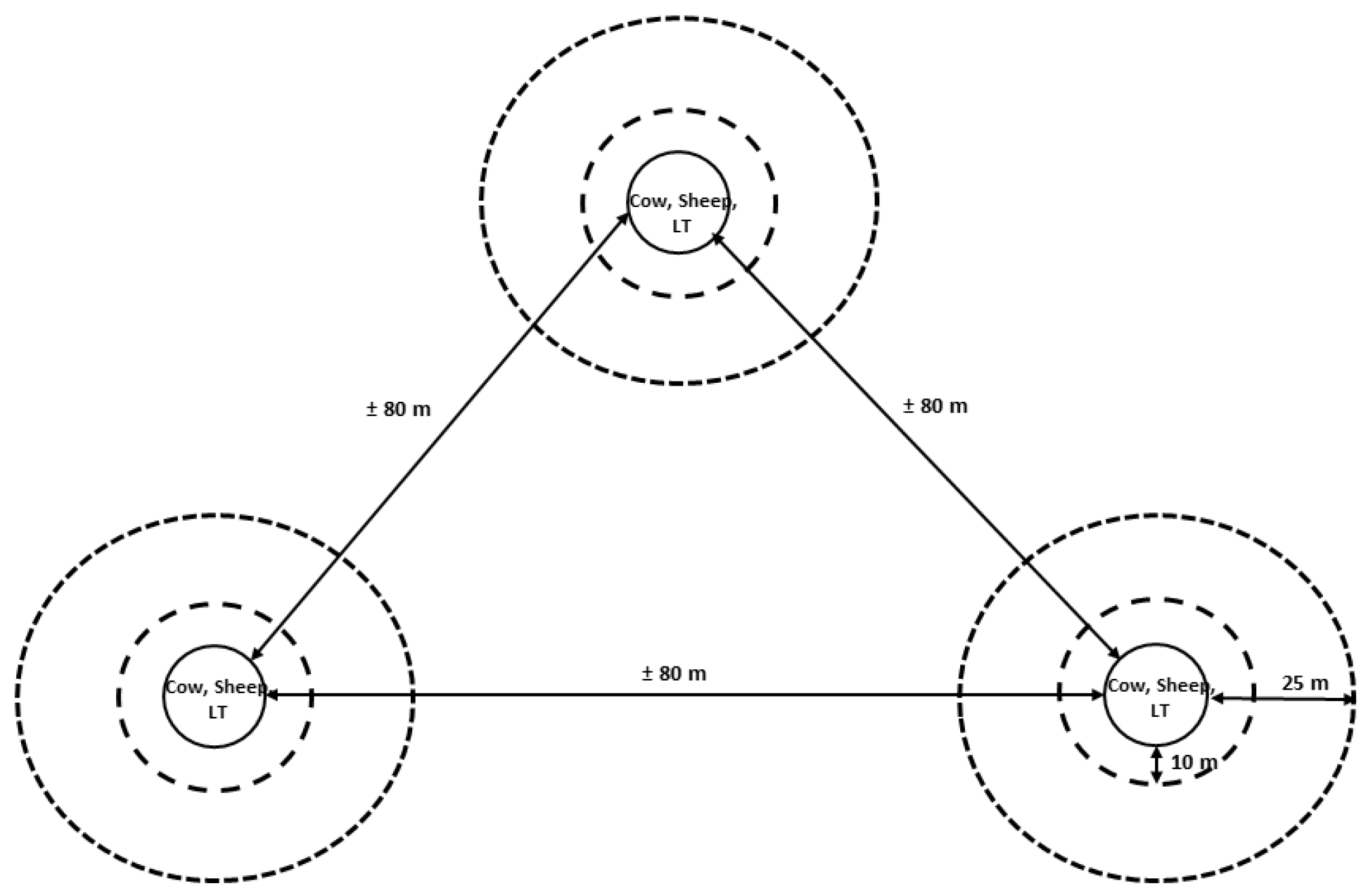
2.2. Study Design
2.3. Culicoides Identification
2.4. Statistical Analysis
2.4.1. Relationship between Baits, Number of Culicoides Sweep-Netted, and Distance
- (a)
- Day of sampling, which accounted for the influence of environmental variation (temperature and humidity) between sampling days and repeated measurements taken on the same hosts. Temperature and humidity were also assessed, but their influence was intrinsically explained by using day of sampling, leading to better model fits.
- (b)
- Sampler, i.e., the person performing the sweep-netting at different sampling locations (bait, distance); this accounts for variations introduced by different people performing the catches.
- (c)
- The observation effect, which accounts for daily varying overdispersion between individual Culicoides catches. This random effect was included only if the model needed correction for overdispersion.
- (d)
- Location, which assessed variation introduced by the samplings locations (which was evenly allocated to all hosts).
2.4.2. Relationship between the Rate of Blood-Fed Culicoides Species and Distance
3. Results
3.1. Distribution of Culicoides Midges
3.2. Culicoides Abundance and Bait and Distance Relationship
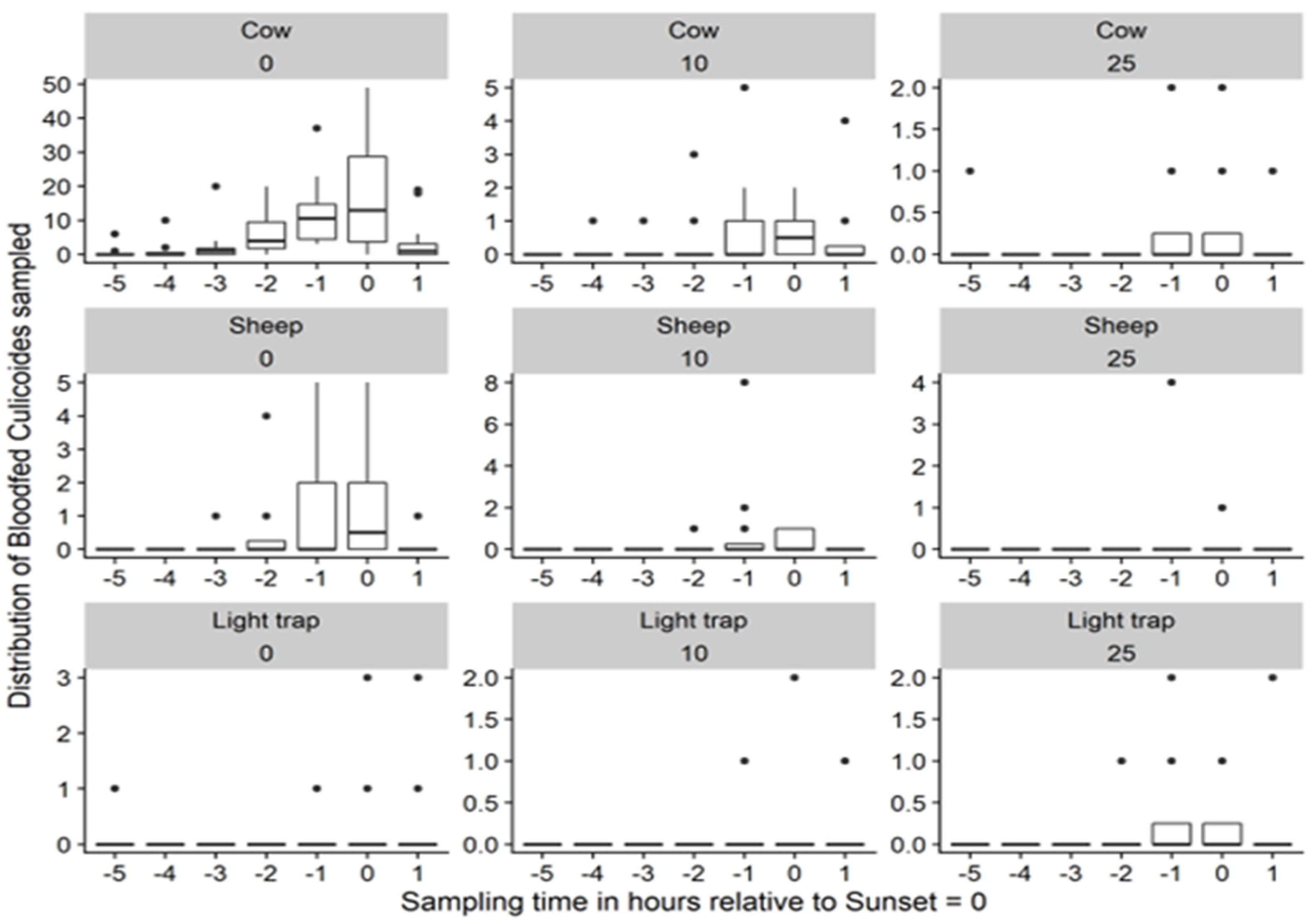
3.2.1. Distribution of Culicoides Midges by Sampling Hours
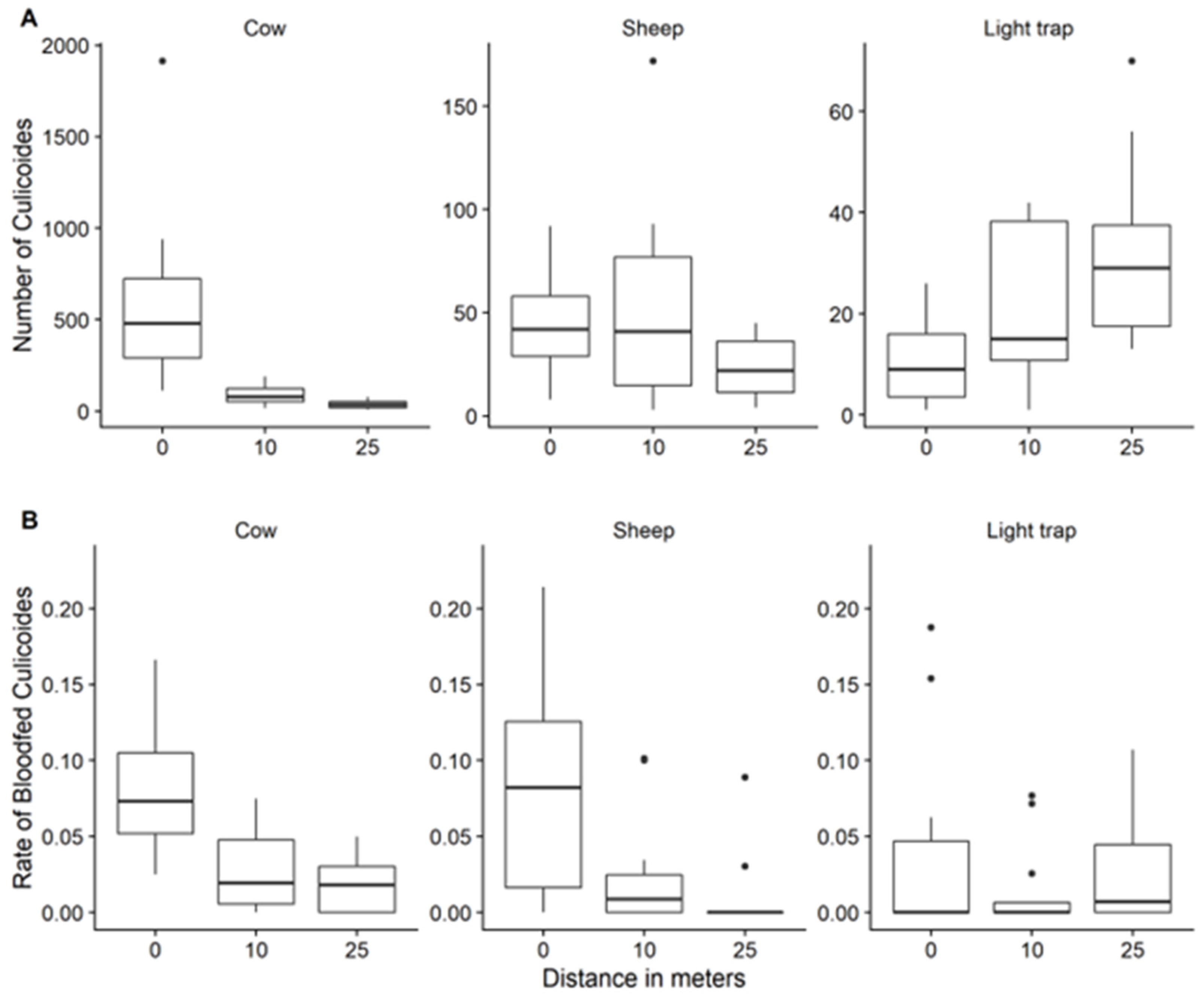
3.2.2. Distribution of Culicoides Midges by Distance to Bait
3.2.3. Distribution of Culicoides Midges by Distance between Baits
3.2.4. Rate of Blood-Fed Culicoides Species and Distance
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Backer, J.A.; Nodelijk, G. Transmission and Control of African Horse Sickness in The Netherlands: A Model Analysis. PLoS ONE 2011, 6, e23066. [Google Scholar] [CrossRef] [PubMed]
- Lo Iacono, G.; Robin, C.A.; Newton, J.R.; Gubbins, S.; Wood, J.L.N. Where are the horses? With the sheep or cows? Uncertain host location, vector-feeding preferences and the risk of African horse sickness transmission in Great Britain. J. R. Soc. Interface 2013, 10, 20130194. [Google Scholar] [CrossRef] [PubMed]
- Bessell, P.R.; Searle, K.R.; Auty, H.K.; Handel, I.G.; Purse, B.V.; de Bronsvoort, B.M. Epidemic potential of an emerging vector borne disease in a marginal environment: Schmallenberg in Scotland. Sci. Rep. 2014, 3, 1178. [Google Scholar] [CrossRef]
- Hartemink, N.A.; Purse, B.V.; Meiswinkel, R.; Brown, H.E.; de Koeijer, A.; Elbers, A.R.W.; Boender, G.J.; Rogers, D.J.; Heesterbeek, H.A.P. Mapping the basic reproduction number (R0) of vector-borne diseases; a case study on bluetongue virus in the Netherlands. Epidemics 2009, 1, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Goffredo, M.; Meiswinkel, R. Entomological surveillance of bluetongue in Italy: Methods of capture, catch analysis and identification of Culicoides biting midges. Vet. Ital. 2004, 40, 260–265. [Google Scholar]
- Venter, G.J.; Majatladi, D.M.; Labuschagne, K.; Boikanyo, S.N.B.; Morley, L. The attraction range of the Onderstepoort 220v light trap for Culicoides biting midges as determined under South African field conditions. Vet. Parasitol. 2012, 190, 222–229. [Google Scholar] [CrossRef]
- Steyskal, G.C.; Murphy, W.L.; Hoover, E.M. Insects and Mites: Techniques for Collection and Preservation; US Department of Agriculture, Agricultural Research Service: Beltsville, MA, USA, 1986; Volume 1443, p. 103.
- Elbers, A.R.W.; Meiswinkel, R. Culicoides (Diptera:Ceratopogonidae) host preferences and biting rates in the Netherlands: Comparing cattle, sheep and the black-light trap. Vet. Parasitol. 2014, 205, 330–337. [Google Scholar] [CrossRef]
- Meiswinkel, R.; Elbers, A.R.W. The dying of the light: Diel periodicity in Culicoides and light trap efficacy at temperate latitudes. Med. Vet. Entomol. 2016, 30, 53–63. [Google Scholar] [CrossRef]
- Elbers, A.R.W.; Meiswinkel, R. Limited attractant range of the black-light suction trap for the capture of Culicoides biting midges (Diptera: Ceratopogonidae). J. Appl. Entomol. 2016, 140, 386–394. [Google Scholar] [CrossRef]
- Wolda, H.; Wong, M. Tropical insect diversity and seasonality. Sweep-samples vs. Light-traps. Proc. R. Dutch Acad Sci. Ser. C Biol. Med. Sci. 1988, 91, 203–216. [Google Scholar]
- Parker, A.H. Observations on the seasonal and daily incidence of certain biting midges (Culicoides Latreille-Diptera, Ceratopogonidae) in Scotland. Trans. R. Entomol. Soc. Lond. 1949, 100, 179–190. [Google Scholar] [CrossRef]
- Lühken, R.; Kiel, E. Distance from the stable affects trapping of biting midges (Diptera, Ceratopogonidae). J. Vect. Ecol. 2012, 37, 453–457. [Google Scholar] [CrossRef]
- Rigot, T.; Vercauteren Drubbel, M.; Delecolle, J.-C.; Gilbert, M. Farms, pastures and woodlands: The fine-scale distribution of Palearctic Culicoides spp. biting midges along an agro-ecological gradient. Med. Vet. Entomol. 2013, 27, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Rigot, T.; Gilbert, M. Quantifying the spatial dependence of Culicoides midge samples collected by Onderstepoort-type black-light traps: An experimental approach to infer the range of attraction of light traps. Med. Vet. Entomol. 2012, 26, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Schmidtmann, E.T.; Jones, C.J.; Gollands, B. Comparative host-seeking activity of Culicoides (Diptera: Ceratopogonidae) attracted to pastered livestock in central New York state, USA. J. Med. Entomol. 1980, 17, 221–230. [Google Scholar] [CrossRef]
- Viennet, E.; Garros, C.; Gardès, L.; Rakotoarivony, I.; Allène, X.; Lancelot, R.; Crochet, D.; Moulia, C.; Baldet, T.; Balenghien, T. Host preferences of Palaearctic Culicoides biting midges: Implications for transmission of orbiviruses. Med. Vet. Entomol. 2013, 27, 255–266. [Google Scholar] [CrossRef]
- Ayllón, T.; Nijhof, A.M.; Weiher, W.; Bauer, B.; Allène, X.; Clausen, P.-H. Feeding behaviour of Culicoides spp. (Diptera: Ceratopogonidae) on cattle and sheep in northeast Germany. Parasites Vectors 2014, 7, 34. [Google Scholar] [CrossRef] [PubMed]
- Fall, M.; Fall, A.G.; Seck, M.T.; Bouyer, J.; Diarra, M.; Lancelot, R.; Gimonneau, G.; Garros, C.; Bakhoum, M.T.; Faye, O.; et al. Host preferences and circadian rhythm of Culicoides (Diptera: Ceratopogonidae), vectors of African horse sickness and bluetongue viruses in Senegal. Acta Trop. 2015, 149, 239–245. [Google Scholar] [CrossRef]
- Campbell, J.A.; Pelham-Clinton, E.C. A taxonomic review of the British species of “Culicoides” Latreille (Diptera, Ceratopogonidae). Proc. R. Soc. Edinb. 1960, 67, 181–302. [Google Scholar] [CrossRef]
- Glukhova, V.M. Blood-sucking midges of the genera Culicoides and Forcipomyia (Ceratopogonidae). Fauna SSSR 1989, 139, 1–408. [Google Scholar]
- Delécolle, J.-C. Nouvelle Contribution a L’etude Systématique et Iconographique des Espèces du Genre Culicoides (Diptera: Ceratopogonidae) du Nord-Est de la France. Master’s Thesis, Université Louis Pasteur de Strasbourg, UFR Sciences de la Vie et de la Terre, Strasbourg, France, 1985. [Google Scholar]
- Gomulski, L.M.; Meiswinkel, R.; Delécolle, J.-C.; Goffredo, M.; Gasperi, G. Phylogenetic relationships of the subgenus Avaritia Fox, 1955 including Culicoides obsoletus (Diptera, Ceratopogonidae) in Italy based on internal transcribed spacer 2 ribosomal DNA sequences. Syst. Entomol. 2005, 30, 619–631. [Google Scholar] [CrossRef]
- Dyce, A.L. The recognition of nulliparous and parous Culicoides (Diptera: Ceratopogonidae) without dissection. J. Aust. Entomol. Soc. 1969, 8, 11–15. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: http://www.r-project.org (accessed on 22 November 2021).
- Elbers, A.R.W.; Meiswinkel, R. Culicoides (Diptera: Ceratopogonidae) and livestock in the Netherlands: Comparing host preference and attack rates on a Shetland pony, a dairy cow and a sheep. J. Vect. Ecol. 2015, 40, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Baylis, M. Research gaps in understanding how climate change will affect arboviral diseases. Anim. Health Res. Rev. 2013, 14, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, S.; Szmaragd, C.; Barber, J.; Labuschagne, K.; Gubbins, S.; Mellor, P.S. An assessment of Culicoides surveillance techniques in northern Europe: Have we underestimated a potential bluetongue virus vector? J. Appl. Ecol. 2008, 45, 1237–1245. [Google Scholar]
- Lassen, S.B.; Nielsen, S.A.; Kristensen, M. Identity and diversity of blood meal hosts of biting midges (Diptera: Ceratopogonidae: Culicoides Latreille) in Denmark. Parasites Vectors 2012, 5, 143. [Google Scholar] [CrossRef]
- Bobeva, A.; Zehtindjiev, P.; Ilieva, M.; Dimitrov, D.; Mathis, A.; Bensch, S. Host preferences of ornithophilic biting midges of the genus Culicoides in the Eastern Balkans. Med. Vet. Entomol. 2015, 29, 291–296. [Google Scholar] [CrossRef]
- Barnard, D.R.; Jones, R.H. Diel and seasonal patterns of flight activity of Ceratopogonidae in Northeastern Colorado: Culicoides. Environ. Entomol. 1980, 9, 446–451. [Google Scholar] [CrossRef]
- Lillie, T.H.; Kline, D.L.; Hall, D.W. Diel and seasonal activity of Culicoides spp. (Diptera: Ceratopogonidae) near Yankeetown, Florida, monitored with a vehicle-mounted insect trap. J. Med. Entomol. 1987, 24, 503–511. [Google Scholar] [CrossRef]
- Kettle, D.S.; Edwards, P.B.; Barnes, A. Factors affecting number of Culicoides in truck traps in coastal Queensland. Med. Vet. Entomol. 1998, 12, 367–377. [Google Scholar] [CrossRef]
- Sanders, C.J.; Gubbins, S.; Mellor, P.S.; Barber, J.; Golding, N.; Harrup, L.E.; Carpenter, S. Investigation of diel activity of Culicoides biting midges (Diptera: Ceratopogonidae) in the United Kingdom by using a vehicle-mounted Trap. J. Med. Entomol. 2012, 49, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Elbers, A.R.W.; Gonzales, J.L.; Meiswinkel, R. Comparing Culicoides biting rates in horses and cattle in The Netherlands: Potential relevance to African horse sickness epidemiology. Entomol. Exp. Appl. 2018, 66, 535–544. [Google Scholar] [CrossRef]
- Gerry, A.C.; Monteys, V.S.I.; Moreno Vidal, J.-O.; Fancino, O.; Mullens, B.A. Biting rates of Culicoides midges (Diptera: Ceratopogonidae) on sheep in Northeastern Spain in relation to midge capture using UV light and carbon dioxide-baited traps. J. Med. Entomol. 2009, 46, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Szmaragd, C.; Wilson, A.J.; Carpenter, S.; Wood, J.L.N.; Mellor, P.S.; Gubbins, S. A modeling framework to describe the transmission of bluetongue virus within and between farms in Great Britain. PLoS ONE 2009, 4, e7741. [Google Scholar] [CrossRef]
- Ducheyne, E.; Miranda Chueca, M.A.; Lucientes, J.; Calvete, C.; Estrada, R.; Boender, G.J.; Goossens, E.; De Clercq, E.M.; Hendrickx, G. Abundance modelling of invasive and indigenous Culicoides species in Spain. Geospat. Health 2013, 8, 241–254. [Google Scholar] [CrossRef]


| Host Distance to Host Species | Cow | Sheep | Black-Light Suction Trap | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 m | 10 m | 25 m | 0 m | 10 m | 25 m | 0 m | 10 m | 25 m | |
| Total (%) | Total (%) | Total (%) | Total (%) | Total (%) | Total (%) | Total (%) # | Total (%) | Total (%) | |
| C. obsoletus/scoticus | 2525 (32.8) | 384 (35.3) | 141 (28.7) | 221 (41.5) | 205 (32.1) | 74 (25.0) | 31.3 (21.5) | 81 (26.3) | 89 (23.0) |
| C. dewulfi | 2813 (36.6) | 226 (20.8) | 88 (17.9) | 206 (38.6) | 160 (25.1) | 66 (22.3) | 31 (21.3) | 70 (22.7) | 91 (23.5) |
| C. chiopterus | 1625 (21.1) | 351 (32.2) | 202 (41.1) | 77 (14.5) | 219 (34.3) | 116 (39.2) | 58.9 (40.5) | 131 (42.5) | 159 (41.1) |
| C. punctatus | 327 (4.2) | 40 (3.7) | 24 (4.9) | 15 (2.8) | 19 (3.0) | 7 (2.4) | 12.8 (8.8) | 16 (5.2) | 20 (5.2) |
| C. achrayi | 201 (2.6) | 36 (3.3) | 6 (1.2) | 2 0.4) | 3 (0.5) | 10 (3.4) | 2.4 (1.7) | 2 (0.6) | 9 (2.3) |
| C. pulicaris | 90 (1.2) | 15 (1.4) | 7 (1.4) | 4 (0.8) | 10 (1.6) | 2 (0.7) | 3.4 (2.3) | 2 (0.6) | 8 (2.1) |
| C. pallidicornis | 50 (0.6) | 17 (1.6) | 2 (0.04) | 0 | 2 (0.3) | 2 (0.7) | 1.2 (0.8) | 0 | 3 (0.8) |
| C. impunctatus | 30 (0.4) | 7 (0.6) | 7 (1.4) | 8 (1.5) | 2 (0.3) | 2 (0.7) | 1.3 (0.9) | 1 (0.3) | 1 (0.3) |
| C. heliophilus | 11 (0.1) | 0 | 0 | 0 | 0 | 2 (0.7) | 0 | 0 | 0 |
| C. stigma | 9 (0.1) | 2 (0.2) | 1 (0.02) | 0 | 1 (0.2) | 2 (0.7) | 0.1 (0.03) | 1 (0.3) | 0 |
| C. pictipennis | 4 (0.05) | 2 (0.2) | 8 (1.6) | 0 | 6 (0.9) | 2 (0.7) | 0.8 (0.6) | 1 (0.3) | 4 (1.0) |
| C. albicans | 4 (0.05) | 2 (0.2) | 2 (0.04) | 0 | 2 (0.3) | 2 (0.7) | 0.2 (0.1) | 1 (0.3) | 0 |
| C. sp.nr.newsteadi | 2 (0.03) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| C. grisescens | 2 (0.03) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| C. furcillatus | 2 (0.03) | 4 (0.4) | 2 (0.04) | 0 | 0 | 6 (2.0) | 0 | 0 | 2 (0.5) |
| C. subfascipennis | 0 | 2 (0.2) | 1 (0.02) | 0 | 1 (0.2) | 2 (0.7) | 0.4 (0.3) | 1 (0.3) | 0 |
| C. festivipennis | 0 | 0 | 0 | 0 | 4 (0.6) | 0 | 1.2 (0.8) | 0 | 0 |
| C. lupicaris | 0 | 0 | 0 | 0 | 2 (0.3) | 0 | 0.05 (0.03) | 1 (0.3) | 0 |
| C. segnis | 0 | 1 (0.1) | 1 (0.02) | 0 | 2 (0.3) | 1 (0.3) | 0 | 0 | 0 |
| C. kubenensis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (0.3) |
| C. riethi | 0 | 0 | 0 | 0 | 0 | 0 | 0.15 (0.1) | 0 | 0 |
| C. fascipennis | 0 | 0 | 0 | 0 | 0 | 0 | 0.05 (0.03) | 0 | 0 |
| C. circumscriptus | 0 | 0 | 0 | 0 | 0 | 0 | 0.2 (0.1) | 0 | 0 |
| Total (%) | 7695 (100) | 1089 (100) | 492 (100) | 533 (100) | 638 (100) | 296 (100) | 145.4 (100) | 308 (100) | 387 (100) |
| Species richness | 15 | 14 | 14 | 7 | 15 | 15 | 17 | 12 | 11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elbers, A.R.W.; Gonzales, J.L. Culicoides (Diptera: Ceratopogonidae) Abundance Is Influenced by Livestock Host Species and Distance to Hosts at the Micro Landscape Scale. Insects 2023, 14, 637. https://doi.org/10.3390/insects14070637
Elbers ARW, Gonzales JL. Culicoides (Diptera: Ceratopogonidae) Abundance Is Influenced by Livestock Host Species and Distance to Hosts at the Micro Landscape Scale. Insects. 2023; 14(7):637. https://doi.org/10.3390/insects14070637
Chicago/Turabian StyleElbers, Armin R. W., and José L. Gonzales. 2023. "Culicoides (Diptera: Ceratopogonidae) Abundance Is Influenced by Livestock Host Species and Distance to Hosts at the Micro Landscape Scale" Insects 14, no. 7: 637. https://doi.org/10.3390/insects14070637
APA StyleElbers, A. R. W., & Gonzales, J. L. (2023). Culicoides (Diptera: Ceratopogonidae) Abundance Is Influenced by Livestock Host Species and Distance to Hosts at the Micro Landscape Scale. Insects, 14(7), 637. https://doi.org/10.3390/insects14070637






