The Effect of X-ray Irradiation on the Fitness and Field Adaptability of the Codling Moth: An Orchard Study in Northeast China
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
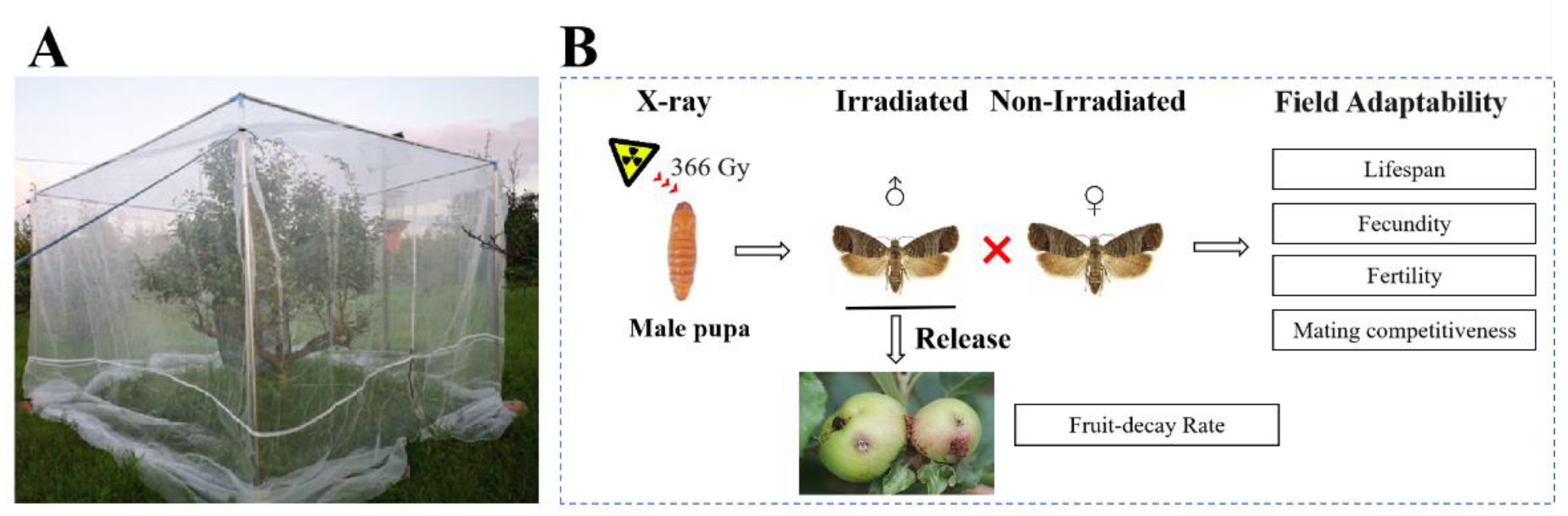
2.1. Insects
2.2. Irradiation
2.3. Orchard Cage Experiments
2.3.1. Effect of X-ray Irradiation on the Lifespan, Fecundity, and Fertility of Male Adults of C. pomonella
2.3.2. Effect of X-ray Irradiation on the Mating Competitiveness of Male C. pomonella
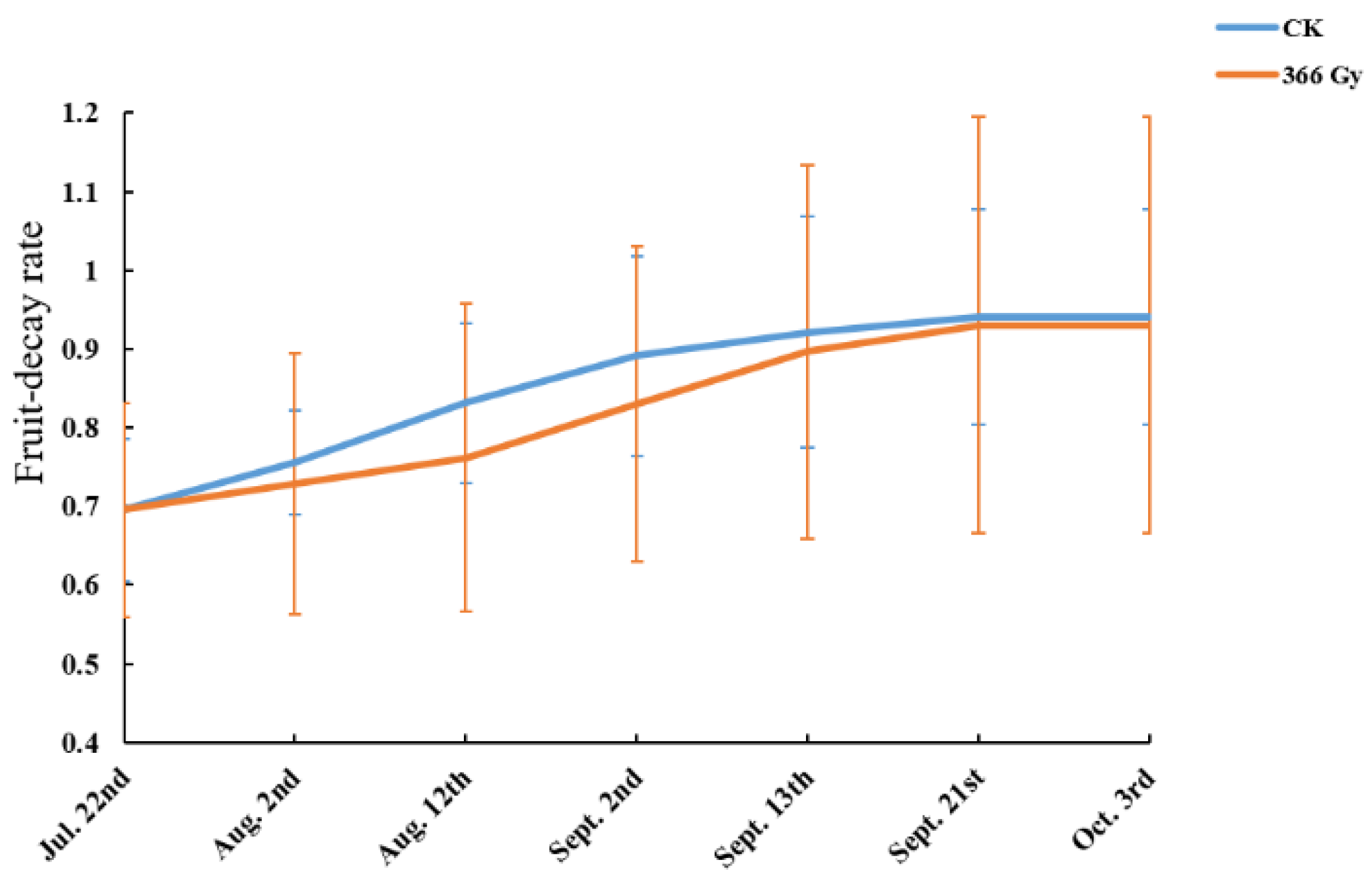
2.3.3. Effect of Pilot Release of Sterile Male Adults of C. pomonella on Fruit Infestation Rate of Nanguo Pear
2.4. Data Analysis
3. Results
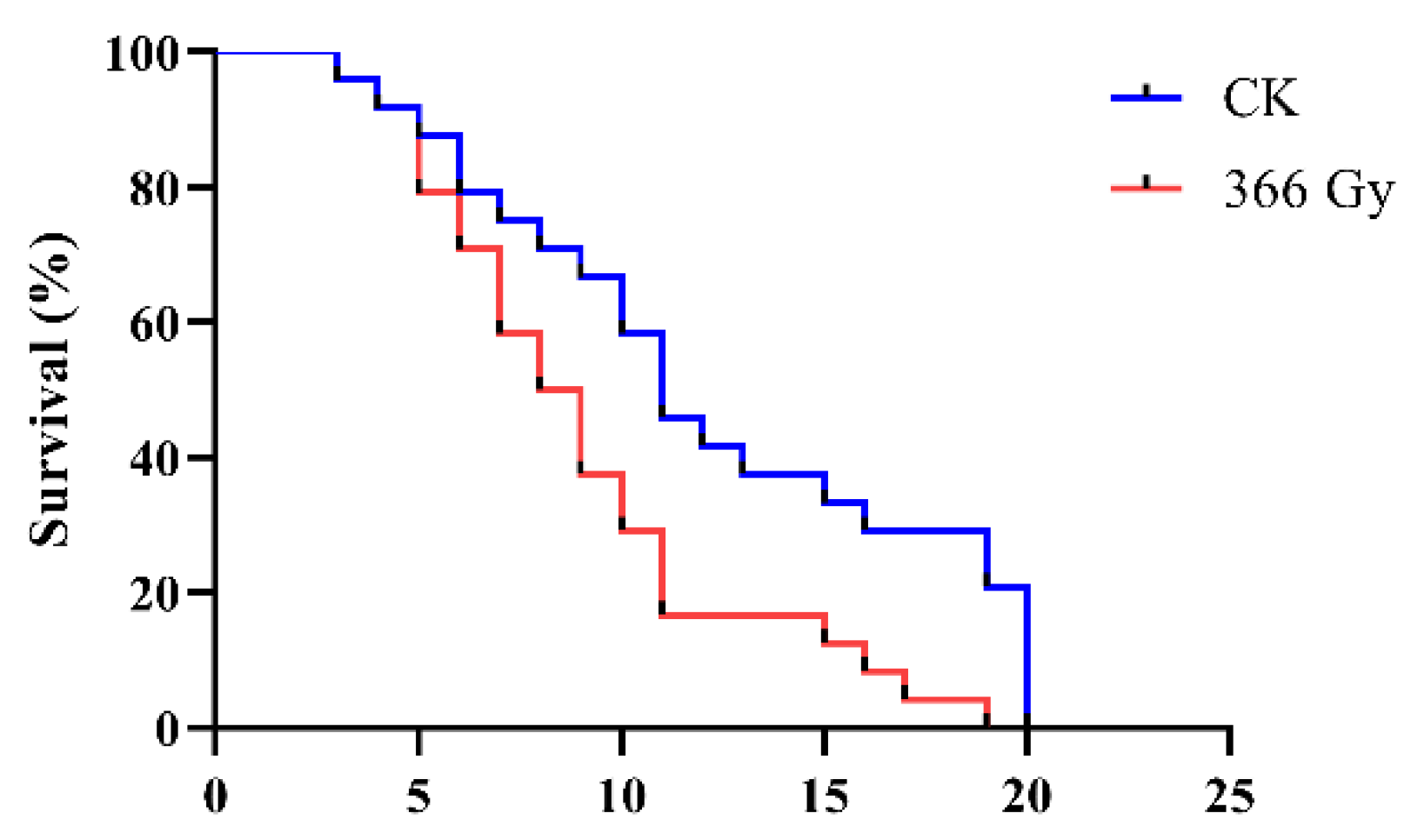
3.1. Effect of X-ray Irradiation on the Lifespan of Male Adults of C. pomonella
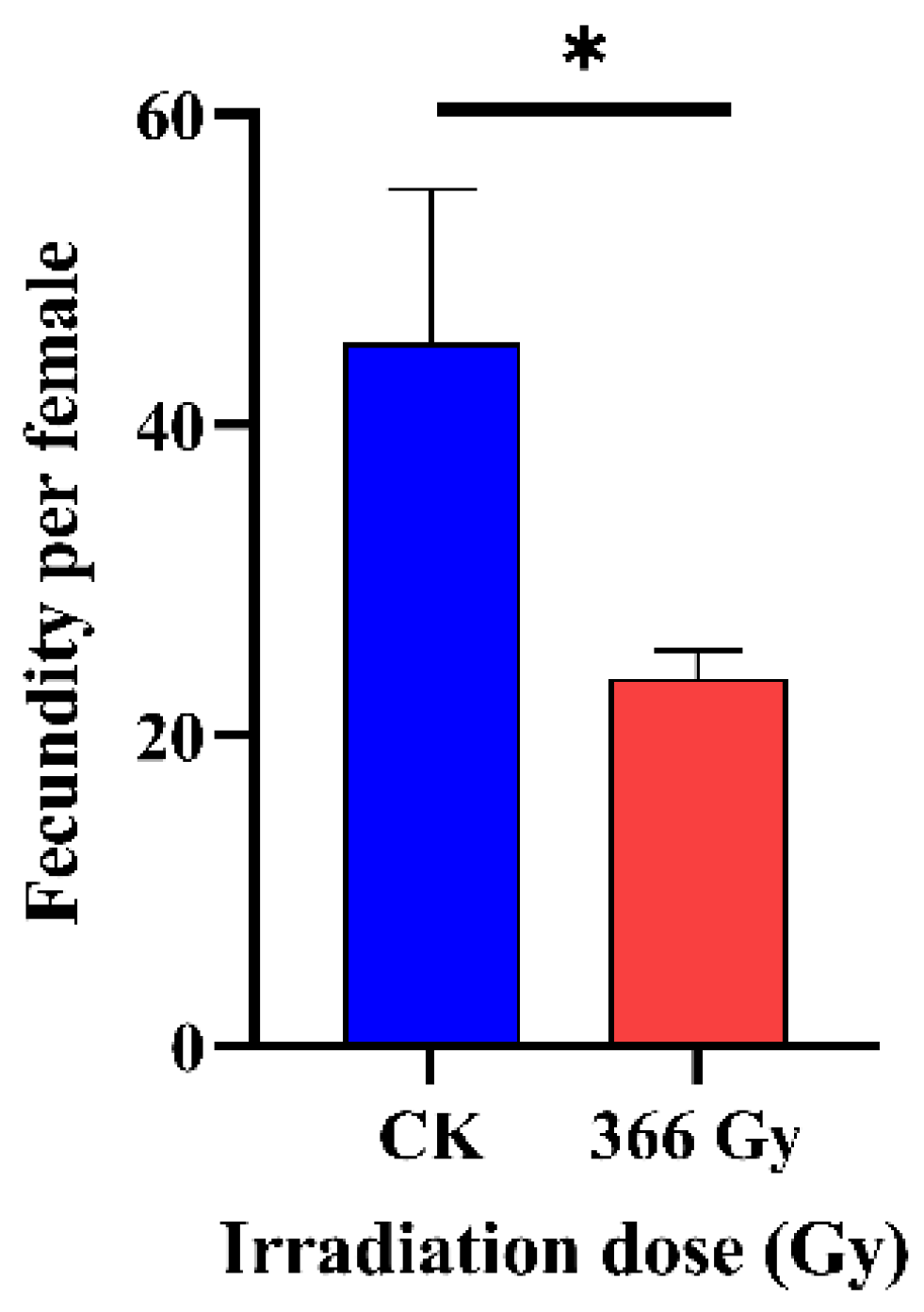
3.2. Effect of X-ray Irradiation on the Fecundity of C. pomonella
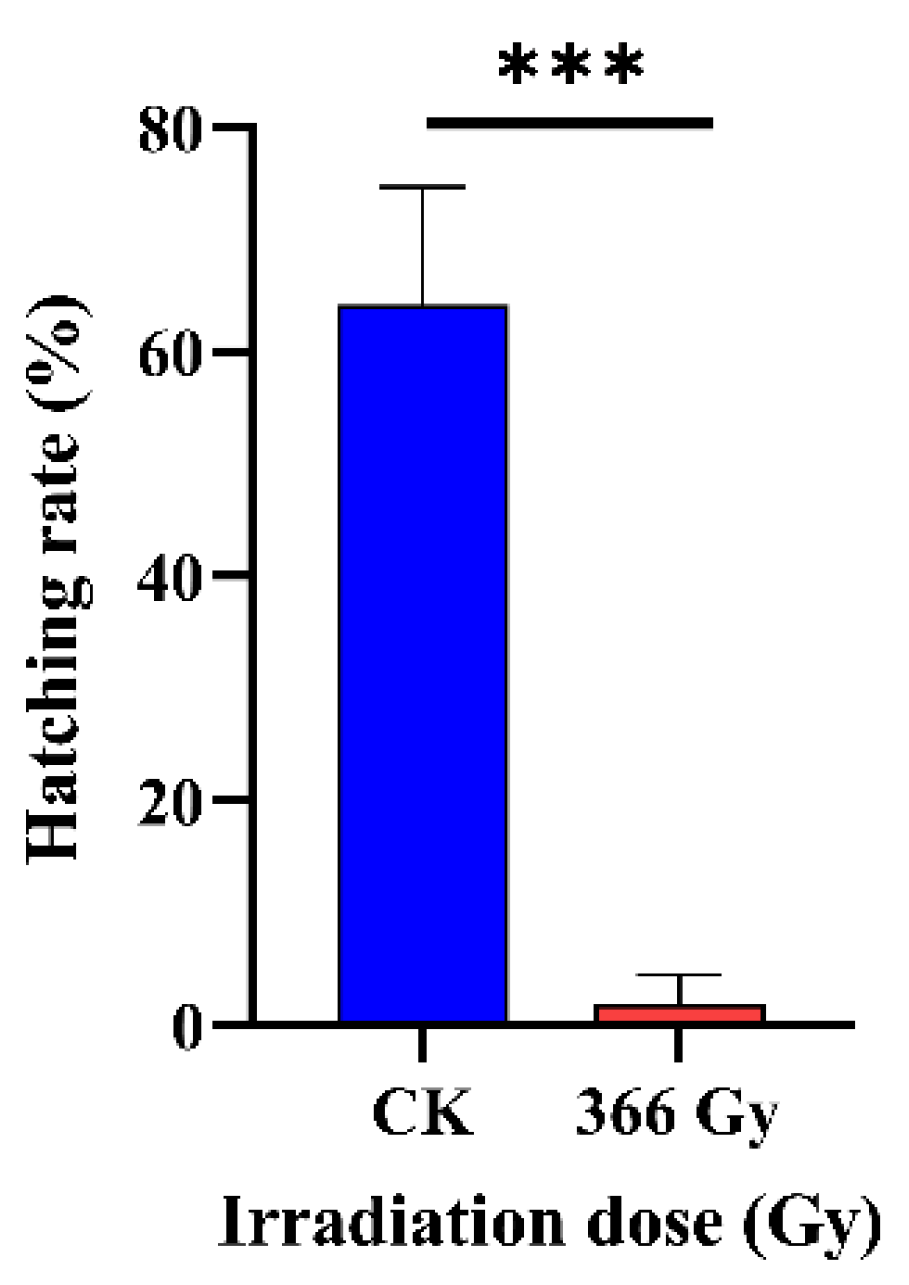
3.3. Effect of X-ray Irradiation on the Fertility of C. pomonella
3.4. Effect of X-ray Irradiation on the Male Mating Competitiveness of C. pomonella
3.5. Effect of Pilot Release of Sterile Male Adults of C. pomonella on Field Fruit Infestation Rate
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Lawrence, A.; Lacey, D.T.; Charles, V.; Steven, P.A. Codling moth granulovirus: A comprehensive review. Biocontrol Sci. Technol. 2008, 18, 639–663. [Google Scholar] [CrossRef]
- Martina, K.B.; Renata, B.; Katarina, M.; Mikac, D.L.; Ivana, P.Ž. Pest management challenges and control practices in codling moth: A review. Insects 2020, 11, 38. [Google Scholar] [CrossRef]
- Yang, A.J.; Li, P.R.; Ju, D.; Hu, C.; Wang, X.Q.; Cai, M.; Lu, X.P.; Yang, X.Q. Research status and progress of codling moth in China-CNKI based bibliometrics analysis. China Fruits 2022, 221, 59–65. [Google Scholar] [CrossRef]
- Yang, X.Q.; Zhang, Y.L.; Wang, X.Q.; Dong, H.; Gao, P.; Jia, L.G. Characterization of multiple heat-shock protein transcripts from Cydia pomonella: Their response to extreme temperature and insecticide exposure. J. Agric. Food Chem. 2016, 64, 4288–4298. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Ruan, Y.H. Occurrence characteristics and integrated control of codling moth. Guang Xi Agric. Mech. 2019, 218, 71–74. [Google Scholar]
- Zhang, X.Z. New discovery of Carpocapsa pomonella L. in China. Acta Entomol. Sin. 1957, 4, 467–472. [Google Scholar] [CrossRef]
- Jiang, C.H. Research on the prevention and control of the codling moth. China Fruit Veg. 2016, 36, 28–30. [Google Scholar]
- Ju, D.; Dewer, Y.; Zhang, S.P.; Hu, C.; Li, P.R.; Yang, X.Q. Genome-wide identification, characterization, and expression profiling of ATP-binding cassette (ABC) transporter genes potentially associated with abamectin detoxification in Cydia pomonella. Ecotoxicol. Environ. Saf. 2022, 230, 113152. [Google Scholar] [CrossRef]
- Xu, L. Discussin on the construction of green prevention and control technology system of Cydia pomonella. Prot. For. Sci. Technol. 2022, 219, 81–84. [Google Scholar] [CrossRef]
- Li, C.; Ma, Y.; Wang, S.Q.; Yang, X.F.; Li, X.X. Exploring the occurrence characteristics and integrated control of codling moth. Agric. Technol. Equip. 2021, 383, 170–171. [Google Scholar]
- Peter, W.; Lukasz, S.; Larry, G.; Don, T. Codling moth management and chemical ecology. Annu. Rev. Entomol. 2008, 53, 503–522. [Google Scholar] [CrossRef]
- Ju, D.; Mota-Sanchez, D.; Fuentes-Contreras, E.; Zhang, Y.L.; Wang, X.Q.; Yang, X.Q. Insecticide resistance in the Cydia pomonella (L.): Global status, mechanisms, and research direction. Pestic. Biochem. Physiol. 2021, 178, 104925. [Google Scholar] [CrossRef] [PubMed]
- Reuveny, H.; Cohen, E. Evaluation of mechanisms of azinphos-methyl resistance in the codling moth Cydia pomonella (L.). Arch. Insect Biochem. Physiol. 2004, 57, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Reyes, M.; Franck, P.; Charmillot, P.J.; Ioriatti, C.; Olivares, J.; Pasqualini, E.; Sauphanor, B. Diversity of insecticide resistance mechanisms and spectrum in European populations of the codling moth, Cydia pomonella. Pest Manag. Sci. 2007, 63, 890–902. [Google Scholar] [CrossRef] [PubMed]
- Rachael, M.H.; Peter, L.L.; David, J.R.; Walker, J.T.S.; David, M.S. Combined effects of mating disruption, insecticides, and the sterile insect technique on Cydia pomonella in New Zealand. Insects 2020, 11, 837. [Google Scholar] [CrossRef]
- López-Martínez, G.; Carpenter, J.E.; Hight, S.D.; Hahn, D.A. Low-oxygen atmospheric treatment improves the performance of irradiation-sterilized male cactus moths used in SIT. J. Econ. Entomol. 2014, 107, 185–197. [Google Scholar] [CrossRef]
- Kostas, B.; Marc, J.B.V. Sterile insect technique (SIT) and its applications. Insects 2021, 638. [Google Scholar] [CrossRef]
- Knipling, E.F. The Basic Principles of Insect Population Suppression and Management; United States Department of Agriculture: Washington, DC, USA, 1979. [Google Scholar]
- Tabashnik, B.E.; Liesner, L.R.; Ellsworth, P.C.; Unnithan, G.C.; Fabrick, J.A.; Naranjo, S.E.; Li, X.; Dennehy, T.J.; Antilla, L.; Staten, R.T.; et al. Transgenic cotton and sterile insect releases synergize eradication of pink bollworm a century after it invaded the United States. Proc. Natl. Acad. Sci. USA 2021, 118, e2019115118. [Google Scholar] [CrossRef]
- Vreysen, M.J.B.; Abd-Alla, A.M.M.; Bourtzis, K.; Bouyer, J.; Caceres, C.; Beer, C.; Oliveira, C.; Maiga, D.H.; Mamai, W.; Nikolouli, K.; et al. The insect pest control laboratory of the joint FAO/IAEA programme: Ten years (2010–2020) of research and development, achievements and challenges in support of the sterile insect technique. Insects 2021, 12, 346. [Google Scholar] [CrossRef]
- Wyss, J.H. Screwworm eradication in the Americas. Ann. N. Y. Acad. Sci. 2000, 916, 186–193. [Google Scholar] [CrossRef]
- Knipple, D.C. Prospects for the use of transgenic approaches to improve the efficacy of the sterile insect technique (SIT) for control of the codling moth Cydia pomonella Linnaeus (Lepidoptera: Tortricidae). Crop Prot. 2013, 44, 142–146. [Google Scholar] [CrossRef]
- Thistlewood, H.M.A.; Judd, G.J.R. Twenty-five years of research experience with the sterile insect technique and area-wide management of codling moth, Cydia pomonella (L.), in Canada. Insects 2019, 10, 292. [Google Scholar] [CrossRef] [PubMed]
- Brower, J.H. Effects of irradiation on reproductive biology of adult tobacco moth, Ephestia elutella. II. J. Ga. Entomol. Soc. 1979, 14, 201–209. [Google Scholar] [CrossRef]
- Bond, J.G.; Aguirre-Ibáñez, S.; Osorio, A.R.; Marina, C.F.; Gómez-Simuta, Y.; Tamayo-Escobar, R.; Dor, A.; Liedo, P.; Carvalho, D.O.; Williams, T. Sexual competitiveness and induced egg sterility by Aedes aegypti and Aedes albopictus gamma-irradiated males: A laboratory and field study in Mexico. Insects 2021, 12, 145. [Google Scholar] [CrossRef]
- Idris, I.; Hussian, K. Effects of gamma radiation and Bacillus thuringiensis on F1 progeny of Cydia pomonella. Hell. Plant Prot. J. 2021, 14, 99–107. [Google Scholar] [CrossRef]
- Yamada, H.; Parker, A.G.; Oliva, C.F.; Balestrino, F.; Gilles, J.R.L. X-ray-induced sterility in Aedes albopictus (Diptera: Culicidae) and male longevity following irradiation. J. Med. Entomol. 2014, 51, 811–816. [Google Scholar] [CrossRef]
- Zhang, J.H.; Li, N.; Zhao, H.Y.; Wang, Y.Q.; Yang, X.Q.; Wu, K.M. Sterility of Cydia pomonella by X ray irradiation as an alternative to gamma radiation for the sterile insect technique. Bull. Entomol. Res. 2023, 113, 72–78. [Google Scholar] [CrossRef]
- Jiang, S.; Fu, X.W.; Jiang, S.S.; Yang, X.M.; Zhao, H.Y.; Wu, K.M. Effect of X-ray irradiation on development, flight, and reproduction of Spodoptera litura. Front. Physiol. 2022, 1441, 947848. [Google Scholar] [CrossRef]
- Hu, C.; Wang, W.; Ju, D.; Chen, G.M.; Tan, X.L.; Mota-Sanchez, D.; Yang, X.Q. Functional characterization of a novel λ-cyhalothrin metabolizing glutathione S-transferase, CpGSTe3, from the codling moth Cydia pomonella. Pest Manag. Sci. 2020, 76, 1039–1047. [Google Scholar] [CrossRef]
- Fried, M. Determination of sterile-insect competitiveness. J. Econ. Entomol. 1971, 64, 869–872. [Google Scholar] [CrossRef]
- Katsoyannos, B.I.; Papadopoulos, N.T.; Hendrichs, J.; Wornoayporn, V. Comparative response to citrus foliage and citrus fruit odour by wild and mass reared sterile Mediterranean fruit fly males of a genetic sexing strain. J. Appl. Entomol. 1999, 123, 139–143. [Google Scholar] [CrossRef]
- White, L.D.; Mantey, K.D. Codling moth: Mating of irradiated and unirradiated laboratory-reared and native moths in the field. J. Econ. Entomol. 1977, 70, 811–812. [Google Scholar] [CrossRef]
- Bloem, S.; Bloem, K.A.; Carpenter, J.E.; Calkins, C.O. Inherited sterility in codling moth (Lepidoptera: Tortricidae): Effect of substerilizing doses of radiation on insect fecundity, fertility, and control. Ann. Entomol. Soc. Am. 1999, 92, 222–229. [Google Scholar] [CrossRef]
- Blomefield, T.L.; Bloem, S.; Carpenter, J.E. Effect of radiation on fecundity and fertility of codling moth Cydia pomonella (Linnaeus) (Lepidoptera: Tortricidae) from South Africa. J. Appl. Entomol. 2010, 134, 216–220. [Google Scholar] [CrossRef]
- Proverbs, M.D.; Newton, J.R. Influence of gamma radiation on the development and fertility of the codling moth, Carpocapsa pomonella (L.) (Lepidoptera: Olethreutidae). Can. J. Zool. 1962, 40, 401–420. [Google Scholar] [CrossRef]
- Bakri, A.; Mehta, K.; Lance, D.R. Sterilizing Insects with Ionizing Radiation. In Sterile Insect Technique; Dyck, V.A., Hendrichs, J., Robinson, A., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 233–268. [Google Scholar]
- Brower, J.H. Recovery of fertility by irradiated males of the Indian meal moth. J. Econ. Entomol. 1976, 69, 273–276. [Google Scholar] [CrossRef]
- Curtis, C.F.; Langley, P. Sterility Induction in Tsetse. In Proceedings of the Symposium: Sterile Insect Technique and Radiation in Insect Control, Neuherberg, Germany, 29 June–3 July 1981; Food and Agriculture Organization of the United Nations: Rome, Italy; International Atomic Energy Agency: Vienna, Austria, 1982; pp. 169–183. [Google Scholar]
- Dyck, V.A.; Hendrichs, J.; Robinson, A.S. Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management; Springer: Cham, The Netherlands, 2005; pp. 303–539. [Google Scholar]
- Carpenter, J.E.; Gross, H.R. Suppression of feral Helicoverpa zea (Lepidoptera: Noctuidae) populations following the infusion of inherited sterility from released substerilemales. Environ. Entomol. 1993, 22, 1084–1091. [Google Scholar] [CrossRef]
- Papadopoulos, N.T.; Katsoyannos, B.I.; Kouloussis, N.A.; Hendrichs, J. Effect of orange peel substances on mating competitiveness of male Ceratitis capitata. Entomol. Exp. Appl. 2001, 99, 253–261. [Google Scholar] [CrossRef]
- Horner, R.M.; Walker, J.T.S.; Rogers, D.J.; Lo, P.L.; Suckling, D.M. Use of the sterile insect technique in New Zealand: Benefits and constraints. N. Z. Plant Prot. 2016, 69, 296–304. [Google Scholar] [CrossRef]
- Anisimov, A.I.; Lazurkina, N.V.; Shvedov, A.N. Influence of radiation-induced genetic damage on the suppressive effect of inherited sterility in the codling moth (Lepidoptera: Tortricidae). Ann. Entomol. Soc. Am. 1989, 82, 769–777. [Google Scholar] [CrossRef]
- Shah, M.A.; Mir, S.A.; Pala, S.A. Enhancing food safety and stability through irradiation: A review. J. Microbiol. Biotechnol. Food Sci. 2014, 3, 371–378. [Google Scholar]
- Marec, F.A.; Vreysen, M.J.B. Advances and challenges of using the Sterile Insect Technique for the management of pest Lepidoptera. Insects 2019, 10, 371. [Google Scholar] [CrossRef] [PubMed]
- North, D.T. Inherited sterility in Lepidoptera. Annu. Rev. Entomol. 1975, 20, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Marec, F.A.; Tothová, K.S.; Traut, W. Meiotic pairing of sex chromosome fragments and its relation to atypical transmission of a sex-linked marker in Ephestia kuehniella (Insecta, Lepidoptera). Heredity 2001, 87, 659–671. [Google Scholar] [CrossRef] [PubMed]
- Marec, F.; Traut, W. Analysis of Structural Rearrangements of Lepidoptera Chromosomes using the Centrifugation Spreading Technique. In Management of Insect Pests: Nuclear and Related Molecular and Genetic Techniques; Proceedings Series—International Atomic Energy Agency; IAEA: Vienna, Austria, 1993; pp. 243–250. [Google Scholar]
- Jiang, S.; Sun, X.T.; Ge, S.S.; Yang, X.M.; Wu, K.M. Mating competitiveness of male Spodoptera frugiperda (Smith) irradiated by X-rays. Insects 2023, 14, 137. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; He, L.M.; He, W.; Zhao, H.Y.; Yang, X.M.; Yang, X.Q.; Wu, K.M. Effects of X-ray irradiation on the fitness of the established invasive pest fall armyworm Spodoptera frugiperda. Pest Manag. Sci. 2022, 78, 2806–2815. [Google Scholar] [CrossRef] [PubMed]
- Bloem, B.; Carpenter, J.E.; Hendrik, H. Radiation biology and inherited sterility in false codling moth (Lepidoptera: Tortricidae). J. Econ. Entomol. 2003, 96, 1724–1731. [Google Scholar] [CrossRef]
- Proverbs, M.D.; Newton, J.R.; Logan, D.M. Suppression of codling moth, Laspeyresia pomonella (Lepidoptera: Olethreutidae), by release of sterile and partially sterile moths. Can. Entomol. 1978, 110, 1095–1102. [Google Scholar] [CrossRef]
- Mastrangelo, T.; Kovaleski, A.; Botteon, V.; Scopel, W.; Costa, M.D.L.Z. Optimization of the sterilizing doses and overflooding ratios for the South American fruit fly. PloS ONE 2018, 13, e0201026. [Google Scholar] [CrossRef]
- Rendon, P.; McInnis, D.; Lance, D.; Stewart, J. Medfly (Diptera: Tephritidae) genetic sexing: Large-scale field comparison of males-only and bisexual sterile fly releases in Guatemala. J. Econ. Entomol. 2004, 97, 1547–1553. [Google Scholar] [CrossRef]
- Judd, G.J.; Gardiner, M.G. Temperature, irradiation and delivery as factors affecting spring-time flight activity and recapture of mass-reared male codling moths released by the Okanagan-Kootenay sterile insect programme. J. Entomol. Soc. Br. Columbia 2006, 103, 19–32. [Google Scholar]
- Bloem, K.; Fielding, L. Okanagan-Kootenay Sterile Insect Release Program Strategic Plan. Unpublished Report Prepared for the Okanagan-Kootenay SIR Board, October 1996. Available online: https://www.oksir.org/wp-content/uploads/2016/08/Strategic-Plan-12_June_2.pdf (accessed on 5 July 2023).
- Bloem, K.A.; Bloem, S. SIT for Codling Moth Eradication in British Columbia, Canada, Sterile Insect Technique; IAEA: Pulau Pinang, Malaysia, 2000; pp. 207–214. [Google Scholar]
- Chersoni, L.; Checcucci, A.; Malfacini, M.; Puggiol, A.; Balestrino, F.; Carrieri, M.; Piunti, I.; Dindo, M.L.; Mattarelli, P.; Bellini, R. The possible role of microorganisms in mosquito mass rearing. Insects 2021, 12, 645. [Google Scholar] [CrossRef] [PubMed]
| Matching Ratio (IM:NM:NF) 1 | Total Egg Production | Number of Eggs Laid/Female | Hatching Rate % | Competitive Capacity (C) | |
|---|---|---|---|---|---|
| Observed Value | Expected Value (E) | ||||
| 0:1:1 | 300.33 ± 147.70 ab | 45.25 ± 9.92 ab | 64.15 ± 10.45 a | 33.03 | 0.0088 |
| 1:0:1 | 102.33 ± 12.01 b | 23.68 ± 1.80 b | 1.91 ± 2.59 b | ||
| 1:1:1 | 534 ± 165.76 a | 66.75 ± 20.72 a | 63.49 ± 3.57 a | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Huang, S.; Zhao, S.; Wang, X.; Yang, X.; Zhao, H.; Gao, P.; Li, Y.; Yang, X. The Effect of X-ray Irradiation on the Fitness and Field Adaptability of the Codling Moth: An Orchard Study in Northeast China. Insects 2023, 14, 615. https://doi.org/10.3390/insects14070615
Zhang J, Huang S, Zhao S, Wang X, Yang X, Zhao H, Gao P, Li Y, Yang X. The Effect of X-ray Irradiation on the Fitness and Field Adaptability of the Codling Moth: An Orchard Study in Northeast China. Insects. 2023; 14(7):615. https://doi.org/10.3390/insects14070615
Chicago/Turabian StyleZhang, Jinghan, Shengwang Huang, Shici Zhao, Xingya Wang, Xianming Yang, Huiyuan Zhao, Ping Gao, Yuting Li, and Xueqing Yang. 2023. "The Effect of X-ray Irradiation on the Fitness and Field Adaptability of the Codling Moth: An Orchard Study in Northeast China" Insects 14, no. 7: 615. https://doi.org/10.3390/insects14070615
APA StyleZhang, J., Huang, S., Zhao, S., Wang, X., Yang, X., Zhao, H., Gao, P., Li, Y., & Yang, X. (2023). The Effect of X-ray Irradiation on the Fitness and Field Adaptability of the Codling Moth: An Orchard Study in Northeast China. Insects, 14(7), 615. https://doi.org/10.3390/insects14070615









