Life Table Parameters of the Tomato Leaf Miner Tuta absoluta (Lepidoptera: Gelechiidae) on Five Tomato Cultivars in China
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Tomato Cultivars
2.3. Life Table Study
2.4. Life Table Analysis
2.5. Population Projection
2.6. Statistical Analysis
3. Results
3.1. Developmental Duration, Fecundity, and Offspring Sex Ratio of T. absoluta on the Five Tomato Cultivars
3.2. Life Table Analysis
3.3. Prediction of the Population Growth of T. absoluta on the Five Tomato Cultivars
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Desneux, N.; Wajnberg, E.; Wyckhuys, K.A.G.; Burgio, G.; Arpaia, S.; Narváez-Vasquez, C.A.; González-Cabrera, J.; Catalán Ruescas, D.; Tabone, E.; Frandon, J.; et al. Biological invasion of European tomato crops by Tuta absoluta: Ecology, geographic expansion and prospects for biological control. J. Pest Sci. 2010, 83, 197–215. [Google Scholar] [CrossRef]
- Desneux, N.; Luna, M.G.; Guillemaud, T.; Urbaneja, A. The invasive south American tomato pinworm, Tuta absoluta, continues to spread in Afro-Eurasia and beyond: The new threat to tomato world production. J. Pest Sci. 2011, 84, 403–408. [Google Scholar] [CrossRef]
- Campos, M.R.; Biondi, A.; Adiga, A.; Guedes, R.N.C.; Desneux, N. From the western palaearctic region to beyond: Tuta absoluta 10 years after invading Europe. J. Pest. Sci. 2017, 90, 787–796. [Google Scholar] [CrossRef]
- Biondi, A.; Guedes, R.N.C.; Wan, F.H.; Desneux, N. Ecology, worldwide spread, and management of the invasive south American tomato pinworm, Tuta absoluta: Past, present, and future. Annu. Rev. Entomol. 2018, 63, 239–258. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Bayram, Y.; Shaltiel-Harpaz, L.; Sohrabi, F.; Saji, A.; Esenali, U.T.; Jalilov, A.; Ali, A.; Shashank, P.R.; Ismoilov, K.; et al. Tuta absoluta continues to disperse in Asia: Damage, ongoing management and future challenges. J. Pest Sci. 2019, 92, 1317–1327. [Google Scholar] [CrossRef]
- Han, P.; Lavoir, A.V.; Rodriguez-Saona, C.; Desneux, N. Bottom-up forces in agroecosystems and their potential impact on arthropod pest management. Annu. Rev. Entomol. 2022, 67, 239–259. [Google Scholar] [CrossRef] [PubMed]
- Suckling, D.M.; Charles, J.G.; Kay, M.K.; Kean, J.M.; Burnip, G.M.; Chhagan, A.; Noble, A.; Barrington, A.M. Host range testing for risk assessment of a sexually dimorphic polyphagous invader, painted apple moth: Risk assessment of painted apple moth. Agric. For. Entomol. 2014, 16, 1–13. [Google Scholar] [CrossRef]
- Pereyra, P.C.; Sánchez, N.E. Effect of two solanaceous plants on developmental and population parameters of the tomato leaf miner, Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). Neotrop. Entomol. 2006, 35, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Taleh, M.; Rafiee-Dastjerdi, H.; Naseri, B.; Sheikhi-Garjan, A.; Talebi-Jahromi, K.; Ebadollahi, A. A laboratory assessment of the lethal and sub-lethal effects of four insecticides considered for commercial control of the tomato leaf miner, Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). Afr. Entomol. 2021, 29, 370–380. [Google Scholar] [CrossRef]
- Krechemer, F.S.; Foerster, L.A. Development, reproduction, survival, and demographic patterns of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) on different commercial tomato cultivars. Neotrop. Entomol. 2017, 46, 694–700. [Google Scholar] [CrossRef]
- Silva, G.A.; Queiroz, E.A.; Arcanjo, L.P.; Lopes, M.C.; Araújo, T.A.; Galdino, T.S.V.; Samuels, R.I.; Rodrigues-Silva, N.; Picanço, M.C. Biological performance and oviposition preference of tomato pinworm Tuta absoluta when offered a range of solanaceous host plants. Sci. Rep. 2021, 11, 1153. [Google Scholar] [CrossRef] [PubMed]
- Caparros Megido, R.; Brostaux, Y.; Haubruge, E.; Verheggen, F.J. Propensity of the tomato leafminer, Tuta absoluta (Lepidoptera: Gelechiidae), to develop on four potato plant varieties. Am. J. Potato Res. 2013, 90, 255–260. [Google Scholar] [CrossRef]
- Bawin, T.; Dujeu, D.; De Backer, L.; Fauconnier, M.L.; Lognay, G.; Delaplace, P.; Francis, F.; Verheggen, F.J. Could alternative solanaceous hosts act as refuges for the tomato leafminer, Tuta absoluta? Arthropod-Plant Interact. 2015, 9, 425–435. [Google Scholar] [CrossRef]
- Campos, M.R.; Amiens-Desneux, E.; Béarez, P.; Soares, M.A.; Ponti, L.; Biondi, A.; Harwood, J.D.; Desneux, N. Impact of low temperature and host plant on Tuta absoluta. Entomol. Exp. Appl. 2021, 169, 984–996. [Google Scholar] [CrossRef]
- Sawadogo, M.W.; Dabire, R.A.; Ahissou, B.R.; Bonzi, S.; Somda, I.; Nacro, S.; Martin, C.; Legrève, A.; Verheggen, F.J. Comparison of life history traits and oviposition preferences of Tuta absoluta for twelve common tomato varieties in Burkina Faso. Physiol. Entomol. 2022, 47, 55–61. [Google Scholar] [CrossRef]
- Gharekhani, G.H.; Salek-Ebrahimi, H. Life table parameters of Tuta absoluta (Lepidoptera: Gelechiidae) on different varieties of tomato. J. Econ. Entomol. 2014, 107, 1765–1770. [Google Scholar] [CrossRef] [PubMed]
- Rostami, E.; Madadi, H.; Abbasipour, H.; Allahyari, H.; Cuthbertson, A.G.S. Life table parameters of the tomato leaf miner Tuta absoluta (Lepidoptera: Gelechiidae) on different tomato cultivars. J. Appl. Entomol. 2017, 141, 88–96. [Google Scholar] [CrossRef]
- Ghaderi, S.; Fathipour, Y.; Asgari, S. Susceptibility of seven selected tomato cultivars to Tuta absoluta (Lepidoptera: Gelechiidae): Implications for its management. J. Econ. Entomol. 2017, 110, 421–429. [Google Scholar] [CrossRef]
- Negi, S.; Sharma, P.L.; Sharma, K.C.; Verma, S.C. Effect of host plants on developmental and population parameters of invasive leafminer, Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). Phytoparasitica 2018, 46, 213–221. [Google Scholar] [CrossRef]
- Kanle Satishchandra, N.; Chakravarthy, A.K.; Özgökçe, M.S.; Atlihan, R. Population growth potential of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) on tomato, potato, and eggplant. J. Appl. Entomol. 2019, 143, 518–526. [Google Scholar] [CrossRef]
- Heidari, N.; Sedaratian-Jahromi, A.; Ghane-Jahromi, M.; Zalucki, M.P. How bottom-up effects of different tomato cultivars affect population responses of Tuta absoluta (Lep.: Gelechiidae): A case study on host plant resistance. Arthropod-Plant Interact. 2020, 14, 181–192. [Google Scholar] [CrossRef]
- Aslan, B.; Birgücü, A.K. Population parameters of the tomato leaf miner Tuta absoluta (Lepidoptera: Gelechiidae) on wild tomato species. Plant Prot. Sci. 2022, 58, 315–325. [Google Scholar] [CrossRef]
- Abbes, K.; Harbi, A.; Elimem, M.; Hafsi, A.; Chermiti, B. Bioassay of three solanaceous weeds as alternative hosts for the invasive tomato leafminer Tuta absoluta (Lepidoptera: Gelechiidae) and insights on their carryover potential. Afr. Entomol. 2016, 24, 334–342. [Google Scholar] [CrossRef]
- Younes, A.A.; Zohdy, N.Z.M.; Abul Fadl, H.A.; Fathy, R. Microbial biopesticides affected age-stage life table of the tomato leaf miner, Tuta absoluta (Lepidoptera: Gelechiidae). Egypt. J. Biol. Pest Control 2018, 28, 10. [Google Scholar] [CrossRef]
- Younes, A.A.; Zohdy, N.Z.M.; Abulfadl, H.A.; Fathy, R. Life table parameters of the tomato leafminer, Tuta absoluta (Lepidoptera: Gelechiidae), on three solanaceous host plants. Afr. Entomol. 2019, 27, 461. [Google Scholar] [CrossRef]
- Erb, M.; Meldau, S.; Howe, G.A. Role of phytohormones in insect-specific plant reactions. Trends Plant Sci. 2012, 17, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Mouttet, R.; Kaplan, I.; Bearez, P.; Amiens-Desneux, E.; Desneux, N. Spatiotemporal patterns of induced resistance and susceptibility linking diverse plant parasites. Oecologia 2013, 173, 1379–1386. [Google Scholar] [CrossRef] [PubMed]
- De Azevedo, S.M.; Ventura Faria, M.; Maluf, W.R.; Barneche De Oliveira, A.C.; De Freitas, J.A. Zingiberene-mediated resistance to the South American tomato pinworm derived from Lycopersicon hirsutum var. hirsutum. Euphytica 2003, 134, 347–351. [Google Scholar] [CrossRef]
- FAOSTAT (The Food and Agriculture Organization of the United Nations). 2021. FAOSTAT Statistics Database. Rome, Italy, Updated 27 December 2021. Available online: http://www.fao.org/faostat (accessed on 20 October 2023).
- Zhang, G.F.; Ma, D.Y.; Wang, Y.S.; Gao, Y.H.; Liu, W.X.; Zhang, R.; Fu, W.J.; Xian, X.Q.; Wang, J.; Kuang, M.; et al. First report of the South American tomato leafminer, Tuta absoluta (Meyrick), in China. J. Integr. Agric. 2020, 19, 1912–1917. [Google Scholar] [CrossRef]
- Zhang, G.F.; Xian, X.Q.; Zhang, Y.B.; Liu, W.X.; Liu, H.; Feng, X.D.; Ma, D.Y.; Wang, Y.S.; Gao, Y.H.; Zhang, R.; et al. Outbreak of the South American tomato leafminer, Tuta absoluta, in the Chinese mainland: Geographic and potential host range expansion. Pest Manag. Sci. 2021, 77, 5475–5488. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Tian, X.C.; Wang, H.; Casteňé, C.; Arnó, J.; Collatz, J.; Romeis, J.; Wu, S.R.; Xian, X.Q.; Liu, W.X.; et al. Host selection behavior of the host-feeding parasitoid Necremnus tutae on Tuta absoluta. Entomol. Gen. 2022, 42, 445–456. [Google Scholar] [CrossRef]
- Chi, H. TWOSEX-MSChart: A Computer Program for the Age Stage, Two-Sex Life Table Analysis. Taichung (Taiwan): National Chung Hsing University. 2018. Available online: http://140.120.197.173/Ecology/Download/TWOSEX-MSChart.rar (accessed on 23 October 2023).
- Chi, H.; Liu, H. Two new methods for the study of insect population ecology. Bull. Inst. Zool. Acad. Sin. 1985, 24, 225–240. [Google Scholar]
- Chi, H. TIMING-MSChart: A Computer Program for the Population Projection Based on Age-Stage, Two-Sex Life Table. Taichung (Taiwan): National Chung Hsing University. 2021. Available online: http://140.120.197.173/Ecology/Download/TWOSEX-MSChart.rar (accessed on 23 October 2023).
- Chi, H. Timing of control based on the stage structure of pest populations: A simulation approach. J. Econ. Entomol. 1990, 83, 1143–1150. [Google Scholar] [CrossRef]
- Akca, I.; Ayvaz, T.; Yazici, E.; Smith, C.L.; Chi, H. Demography and population projection of Aphis fabae (Hemiptera: Aphididae): With additional comments on life table research criteria. J. Econ. Entomol. 2015, 108, 1466–1478. [Google Scholar] [CrossRef]
- Erb, S.L.; Bourchier, R.S.; Van Frankenhuyzen, K.; Smith, S.M. Sublethal effects of Bacillus thuringiensis Berliner subsp. Kurstaki on Lymantria dispar (Lepidoptera: Lymantriidae) and the tachinid parasitoid Compsilura concinnata (Diptera: Tachinidae). Environ. Entomol. 2001, 30, 1174–1181. [Google Scholar] [CrossRef]
- Sedaratian, A.; Fathipour, Y.; Talaei-Hassanloui, R. Deleterious effects of Bacillus thuringiensis on biological parameters of Habrobracon hebetor parasitizing Helicoverpa armigera. BioControl 2014, 59, 89–98. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Zhang, H.L.; Yang, N.W.; Wang, J.J.; Wan, F.H. Income resources and reproductive opportunities change life history traits and the egg/time limitation trade-off in a synovigenic parasitoid. Ecol. Entomol. 2014, 39, 723–731. [Google Scholar] [CrossRef]
- Benelli, G.; Giunti, G.; Tena, A.; Desneux, N.; Caselli, A.; Canale, A. The impact of adult diet on parasitoid reproductive performance. J. Pest Sci. 2017, 90, 807–823. [Google Scholar] [CrossRef]
- Hoedjes, K.M.; Rodrigues, M.A.; Flatt, T. Amino acid modulation of lifespan and reproduction in Drosophila. Curr. Opin. Insect Sci. 2017, 23, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Amarendra, K.P.; Tripathi, C.P.M. Effect of temperature on the development, fecundity, progeny sex ratio and life-table of Campoletis chlorideae, an endolarval parasitoid of the pod borer, Helicoverpa armigera. BioControl 2008, 53, 461–471. [Google Scholar] [CrossRef]
- Amarasekare, P.; Coutinho, R.M. Effects of temperature on intraspecific competition in ectotherms. Am. Nat. 2014, 184, E50–E65. [Google Scholar] [CrossRef] [PubMed]
- Casas, J.; Pincebourde, S.; Mandon, N.; Vannier, F.; Poujol, R.; Giron, D. Lifetime nutrient dynamics reveal simultaneous capital and income breeding in a parasitoid. Ecology 2005, 86, 545–554. [Google Scholar] [CrossRef][Green Version]
- Jervis, M.A.; Ellers, J.; Harvey, J.A. Resource acquisition, allocation, and utilization in parasitoid reproductive strategies. Annu. Rev. Entomol. 2008, 53, 361–385. [Google Scholar] [CrossRef]
- Richard, R.; Casas, J. Stochasticity and controllability of nutrient sources in foraging: Host-feeding and egg resorption in parasitoids. Ecol. Monogr. 2009, 79, 465–483. [Google Scholar] [CrossRef]
- Rosenheim, J.A. Stochasticity in reproductive opportunity and the evolution of egg limitation in insects. Evolution 2011, 65, 2300–2312. [Google Scholar] [CrossRef] [PubMed]
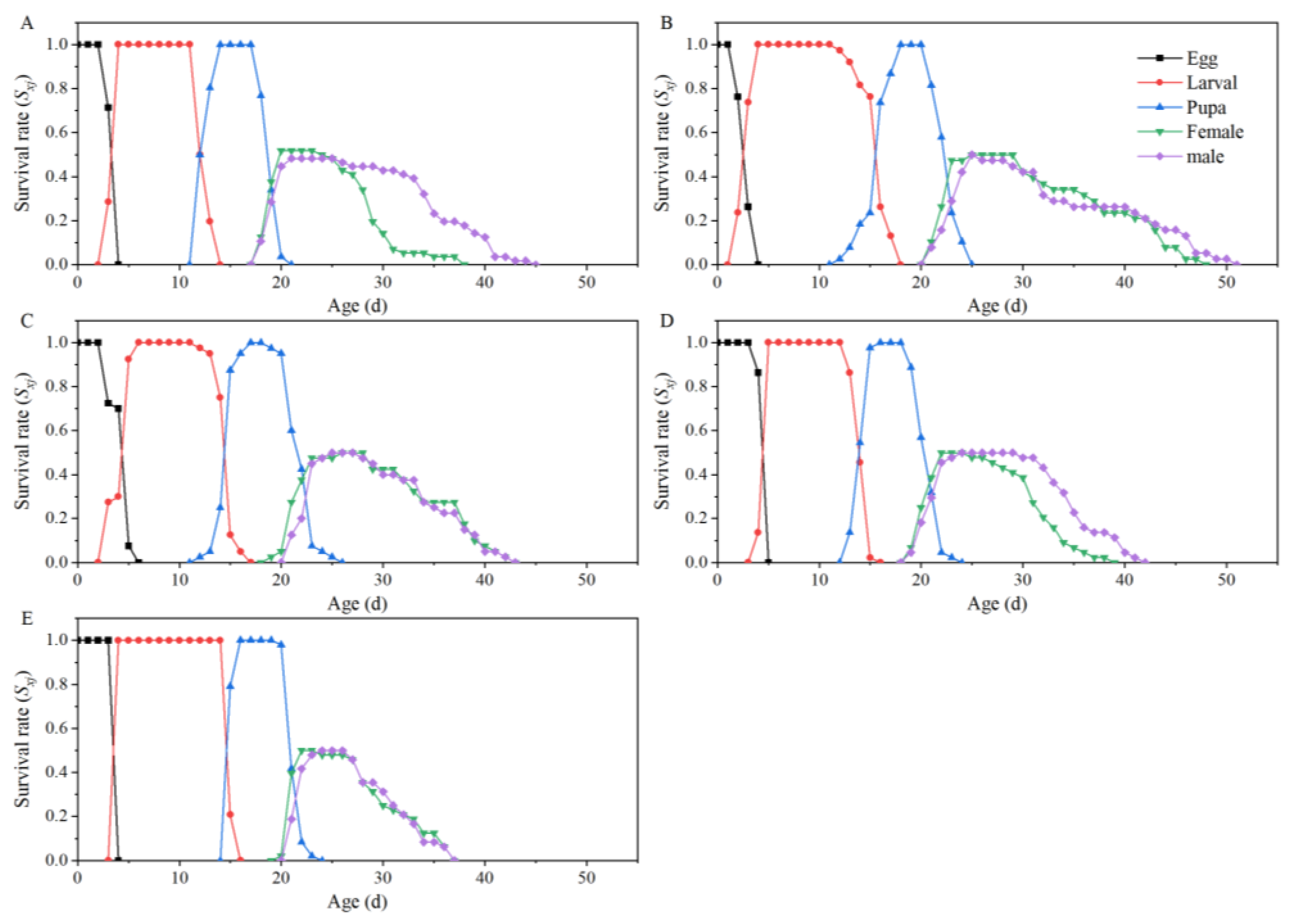
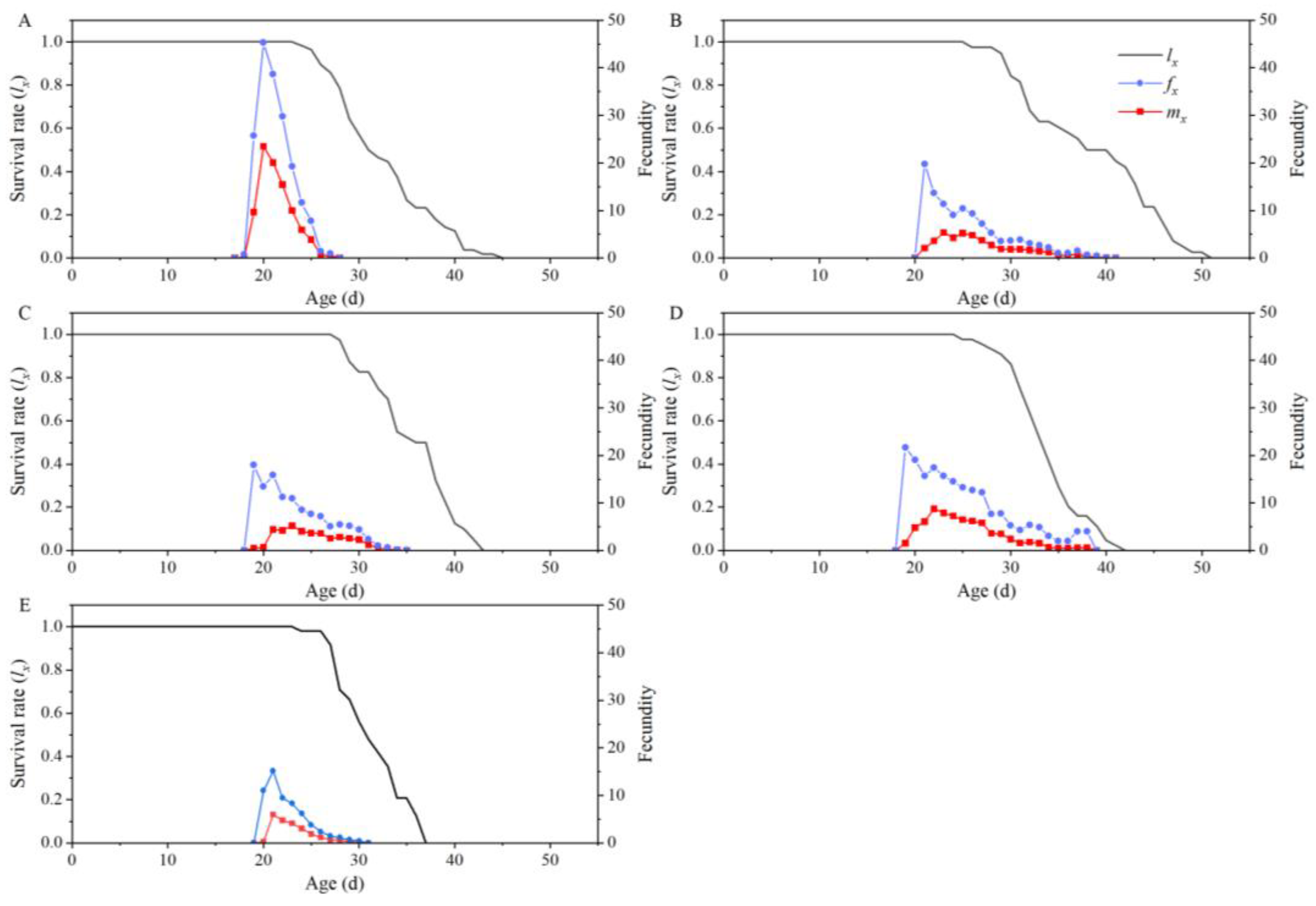
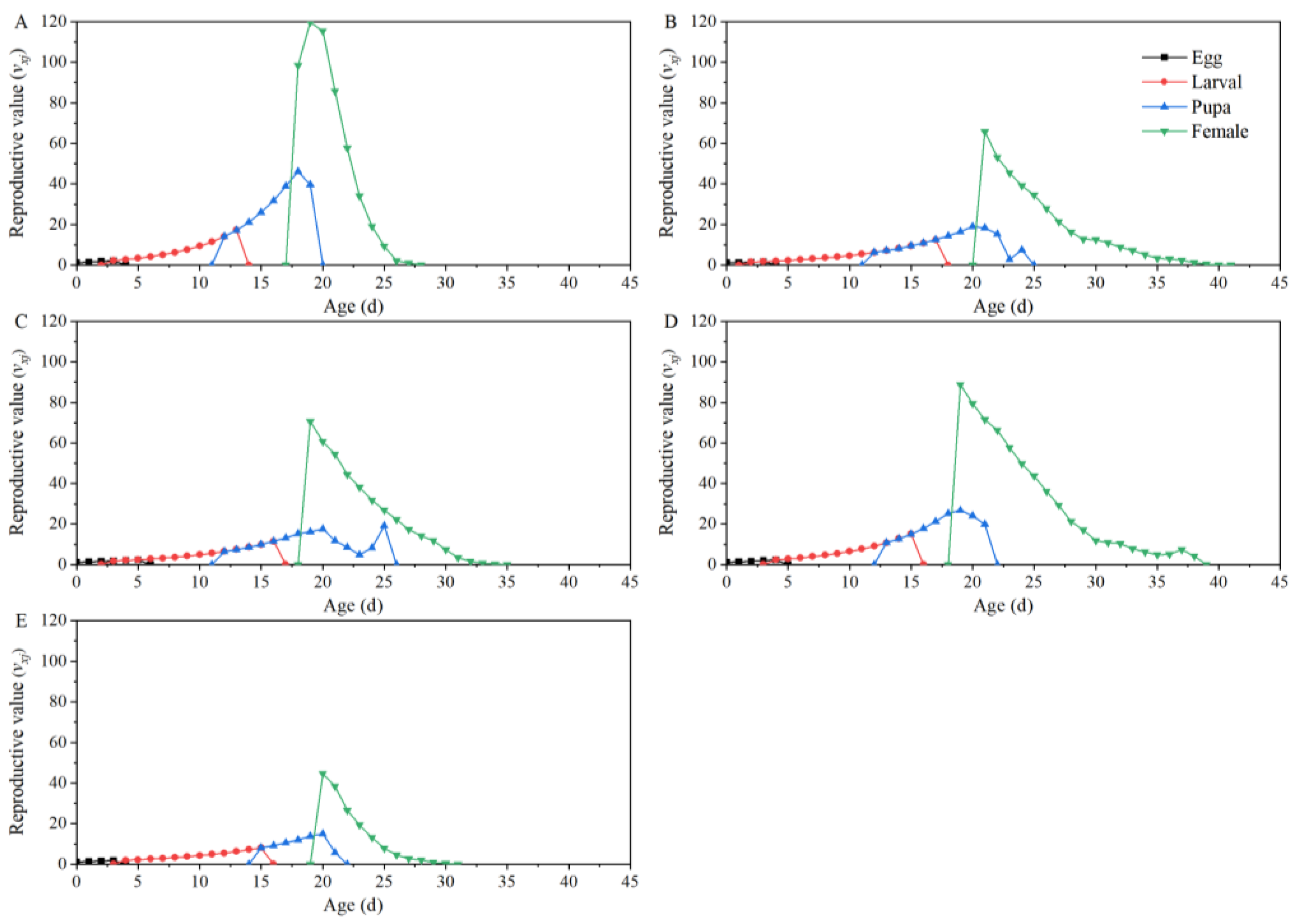
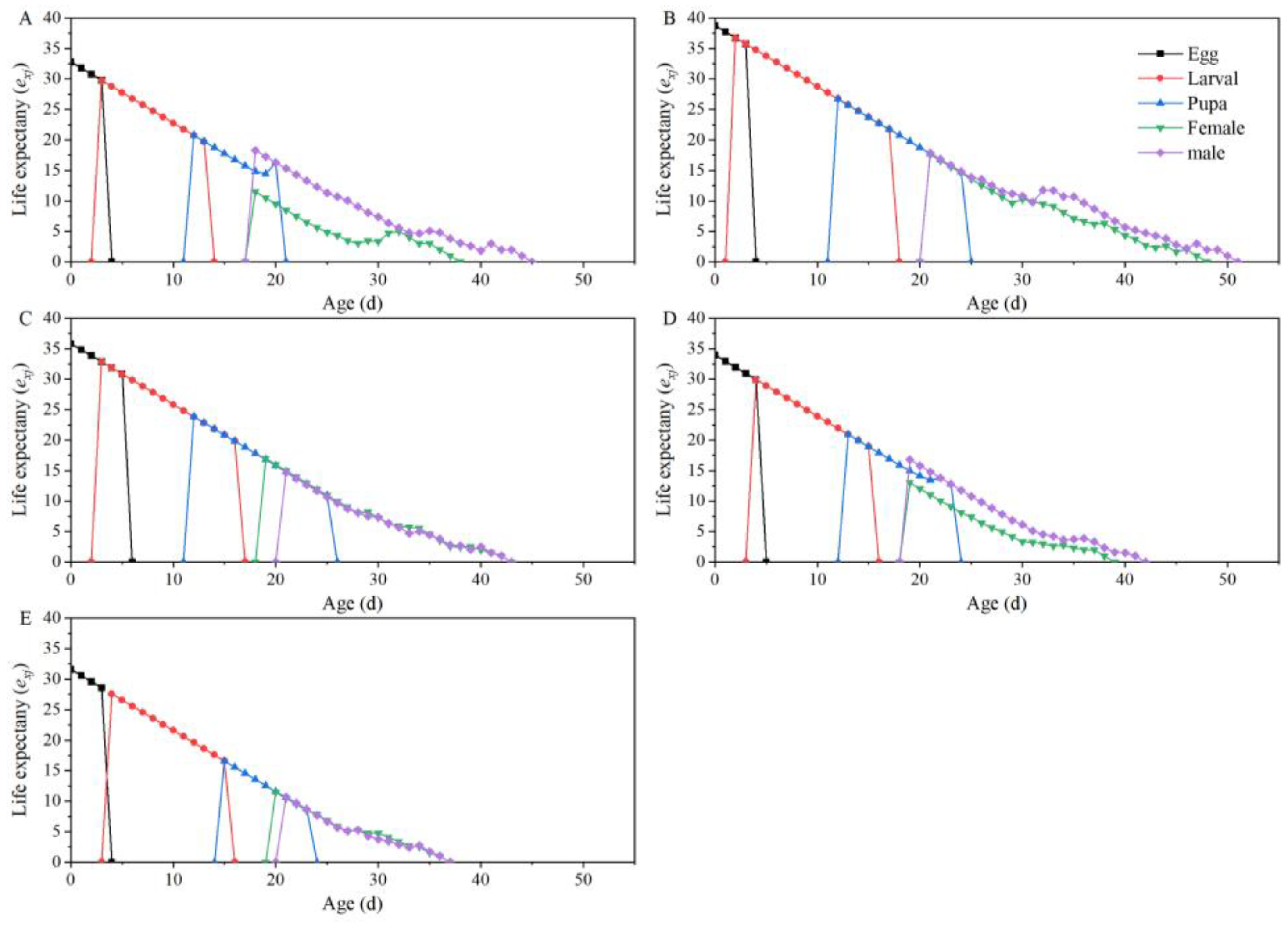

| Parameters | Dafen | Th1902 | Heinz1015 | Th9 | Dimen2272 |
|---|---|---|---|---|---|
| Duration of Egg stage | 3.71 ± 0.06 c | 3.03 ± 0.12 d | 4.5 ± 0.16 a | 4.86 ± 0.05 a | 4 ± 0 c |
| Duration of Larva stage | 8.98 ± 0.1 e | 12.84 ± 0.13 a | 10.35 ± 0.12 c | 9.48 ± 0.08 d | 11.21 ± 0.06 b |
| Duration of Pupa stage | 6.45 ± 0.1 bc | 6.87 ± 0.15 ab | 7.25 ± 0.142 a | 6.5 ± 0.11 bc | 6.29 ± 0.08 c |
| Duration of preadult stage | 19.14 ± 0.11 d | 22.74 ± 0.20 a | 22.10 ± 0.21 b | 20.84 ± 0.17 c | 21.50 ± 0.11 b |
| Adult male longevity | 17.04 ± 0.86 a | 15.74 ± 1.75 a | 13.20 ± 1.02 ab | 14.73 ± 0.72 a | 9.83 ± 0.72 b |
| Adult female longevity | 10.45 ± 0.56 c | 16.26 ± 1.38 a | 14.30 ± 0.98 ab | 11.45 ± 0.70 bc | 10.33 ± 0.80 c |
| Average oviposition period | 5.00 ± 0.25 c | 9.74 ± 0.87 a | 8.10 ± 0.81 ab | 10.32 ± 0.74 a | 5.75 ± 0.51 bc |
| Lifetime Fecundity(eggs/female) | 172.17 ± 7.50 a | 80.89 ± 6.41 c | 74.05 ± 9.24 cd | 132.5 ± 11.22 b | 44.88 ± 4.89 d |
| Offspring sex ratio (female/male) | 1.07 ± 0.06 a | 0.92 ± 0.05 a | 1.01 ± 0.07 a | 0.98 ± 0.04 a | 1.10 ± 0.10 a |
| Parameters | Dafen | Th1902 | Heinz1015 | Th9 | Dimen2272 |
|---|---|---|---|---|---|
| Net reproductive rate (R0) | 89.16 ± 12.10 a | 40.45 ± 7.24 bc | 37.03 ± 7.40 cd | 66.25 ± 11.43 ab | 22.44 ± 4.02 d |
| Gross reproduction rate (GRR) | 89.54 ± 12.17 a | 44.10 ± 8.16 bc | 38.25 ± 7.65 cd | 71.43 ± 12.99 ab | 22.98 ± 4.16 d |
| Intrinsic rate of increase (r, per day) | 0.2034 ± 0.0063 a | 0.1404 ± 0.0073 c | 0.1432 ± 0.0082 c | 0.1696 ± 0.0073 b | 0.1310 ± 0.0076 c |
| Finite rate of increase (λ, per day) | 1.2256 ± 0.0077 a | 1.1507 ± 0.0084 c | 1.1539 ± 0.0095 c | 1.1848 ± 0.0086 b | 1.1400 ± 0.0086 c |
| Mean generation time (days) | 22.07 ± 0.17 d | 26.36 ± 0.44 a | 25.22 ± 0.34 b | 24.73 ± 0.26 b | 23.74 ± 0.17 c |
| Doubling time (days) | 3.41 ± 0.11 c | 4.94 ± 0.27 a | 4.84 ± 0.29 a | 4.09 ± 0.18 b | 5.29 ± 0.32 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Zhang, C.; Shen, Y.; Gao, H.; Zhang, G.; Liu, W.; Jiang, H.; Zhang, Y. Life Table Parameters of the Tomato Leaf Miner Tuta absoluta (Lepidoptera: Gelechiidae) on Five Tomato Cultivars in China. Insects 2024, 15, 208. https://doi.org/10.3390/insects15030208
Yang H, Zhang C, Shen Y, Gao H, Zhang G, Liu W, Jiang H, Zhang Y. Life Table Parameters of the Tomato Leaf Miner Tuta absoluta (Lepidoptera: Gelechiidae) on Five Tomato Cultivars in China. Insects. 2024; 15(3):208. https://doi.org/10.3390/insects15030208
Chicago/Turabian StyleYang, Hesen, Chi Zhang, Yuyang Shen, Haifeng Gao, Guifen Zhang, Wanxue Liu, Hongbo Jiang, and Yibo Zhang. 2024. "Life Table Parameters of the Tomato Leaf Miner Tuta absoluta (Lepidoptera: Gelechiidae) on Five Tomato Cultivars in China" Insects 15, no. 3: 208. https://doi.org/10.3390/insects15030208
APA StyleYang, H., Zhang, C., Shen, Y., Gao, H., Zhang, G., Liu, W., Jiang, H., & Zhang, Y. (2024). Life Table Parameters of the Tomato Leaf Miner Tuta absoluta (Lepidoptera: Gelechiidae) on Five Tomato Cultivars in China. Insects, 15(3), 208. https://doi.org/10.3390/insects15030208









