Detection of La Crosse Virus In Situ and in Individual Progeny to Assess the Vertical Transmission Potential in Aedes albopictus and Aedes aegypti
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Mosquito Maintenance
2.2. Preparation of LACV-Containing Artificial Bloodmeals
2.3. Plaque Assays for the Detection of LACV
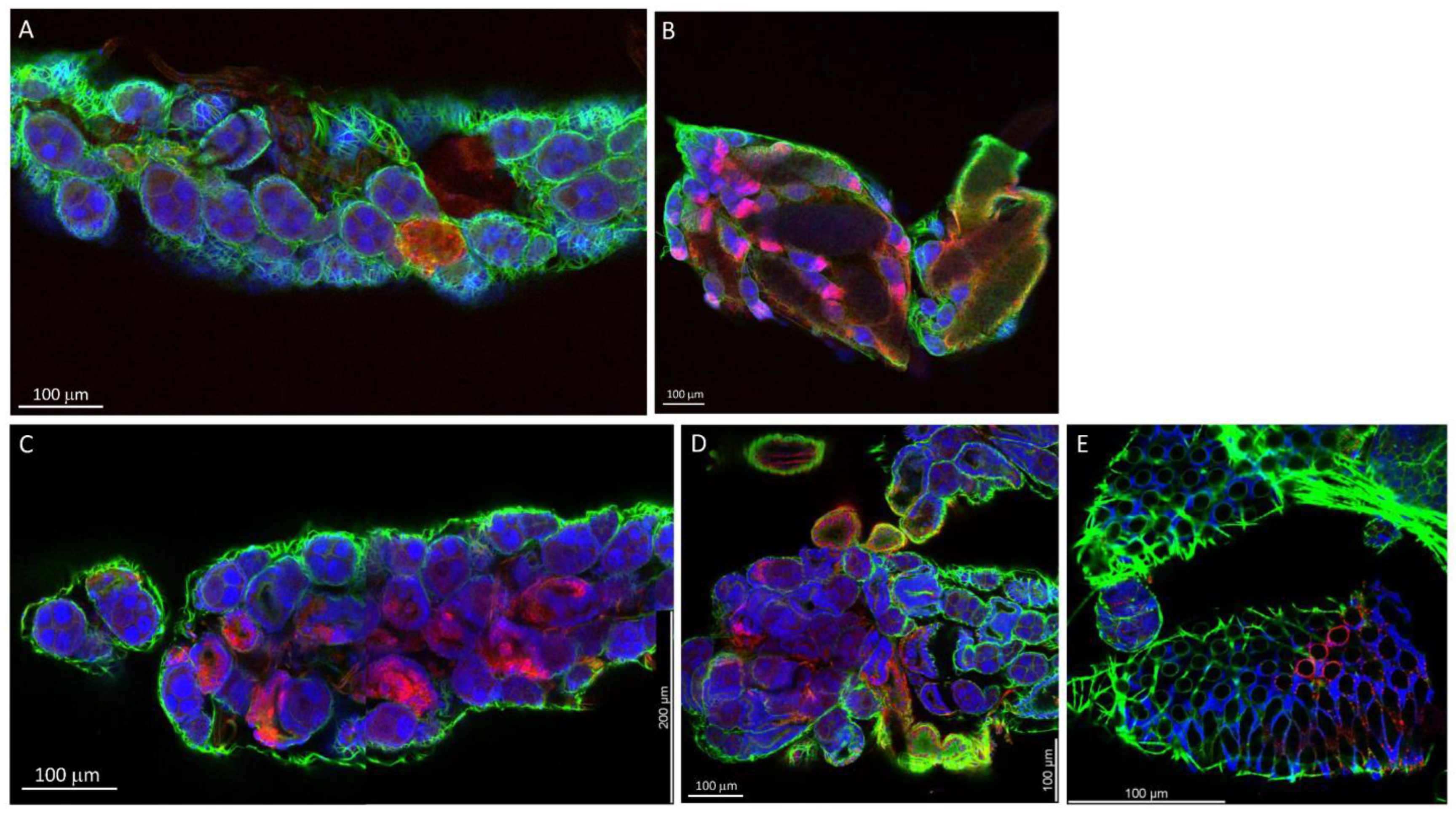
2.4. Immunofluorescence Confocal Microscopy (IFCM) to Detect LACV Antigen in Mosquito Tissues
2.5. Immunohistochemistry (IHC) Assay to Detect LACV Antigen In Situ
2.6. Oviposition of Individual LACV-Infected Females and Rearing of F1 Larvae for LACV Detection
2.7. RNA Extraction and cDNA Synthesis from Larvae
2.8. LACV TaqMan qRT-PCR
2.9. Statistical Analysis
3. Results
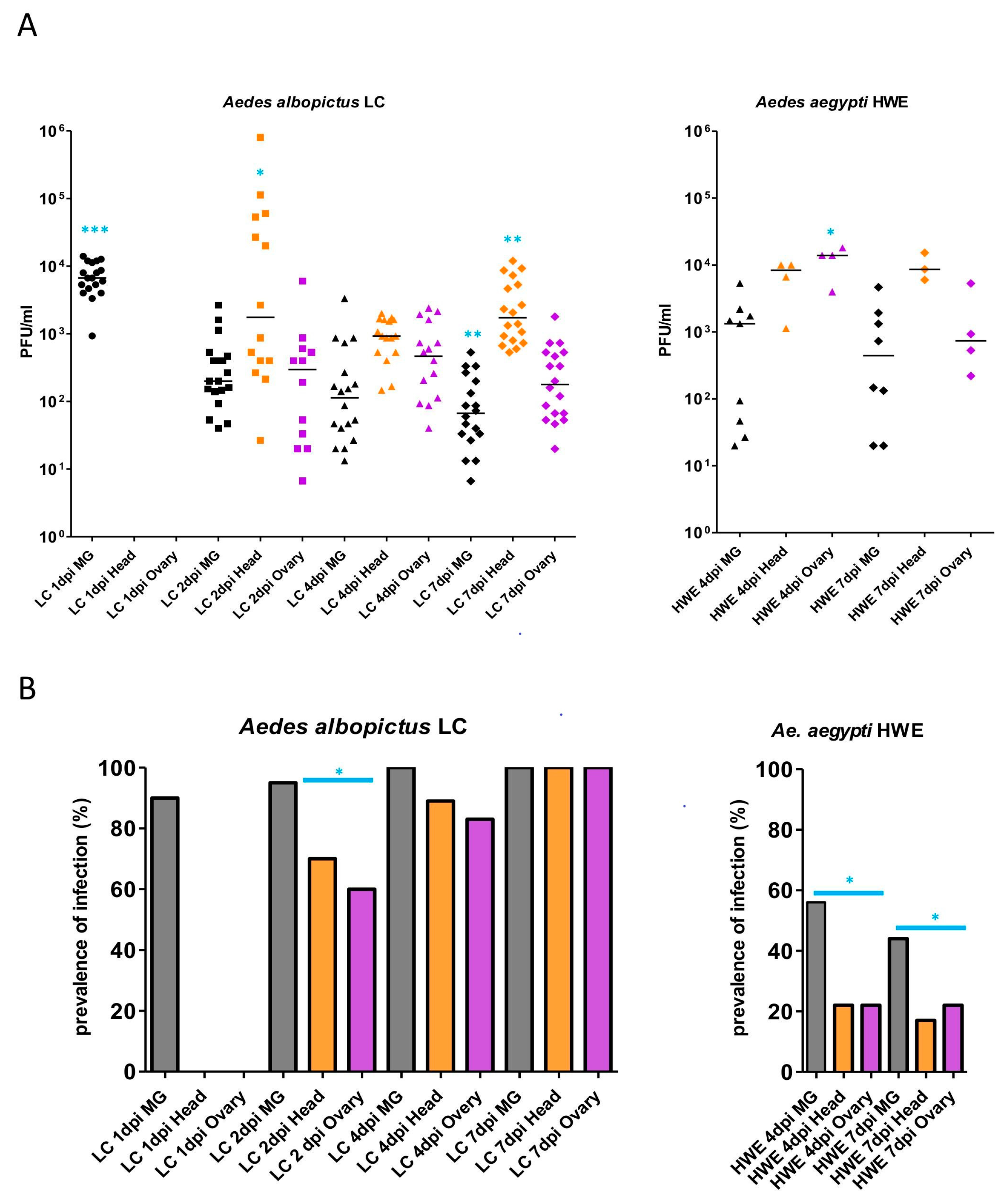
3.1. Detection of LACV in Midguts, Head Tissue, and Ovaries of Ae. aegypti and Ae. albopictus, Which Had Acquired a Single Virus-Containing Bloodmeal
3.2. LACV Vertical Transmission by Ae. albopictus and Ae. aegypti after One or Two Gonotrophic Cycles
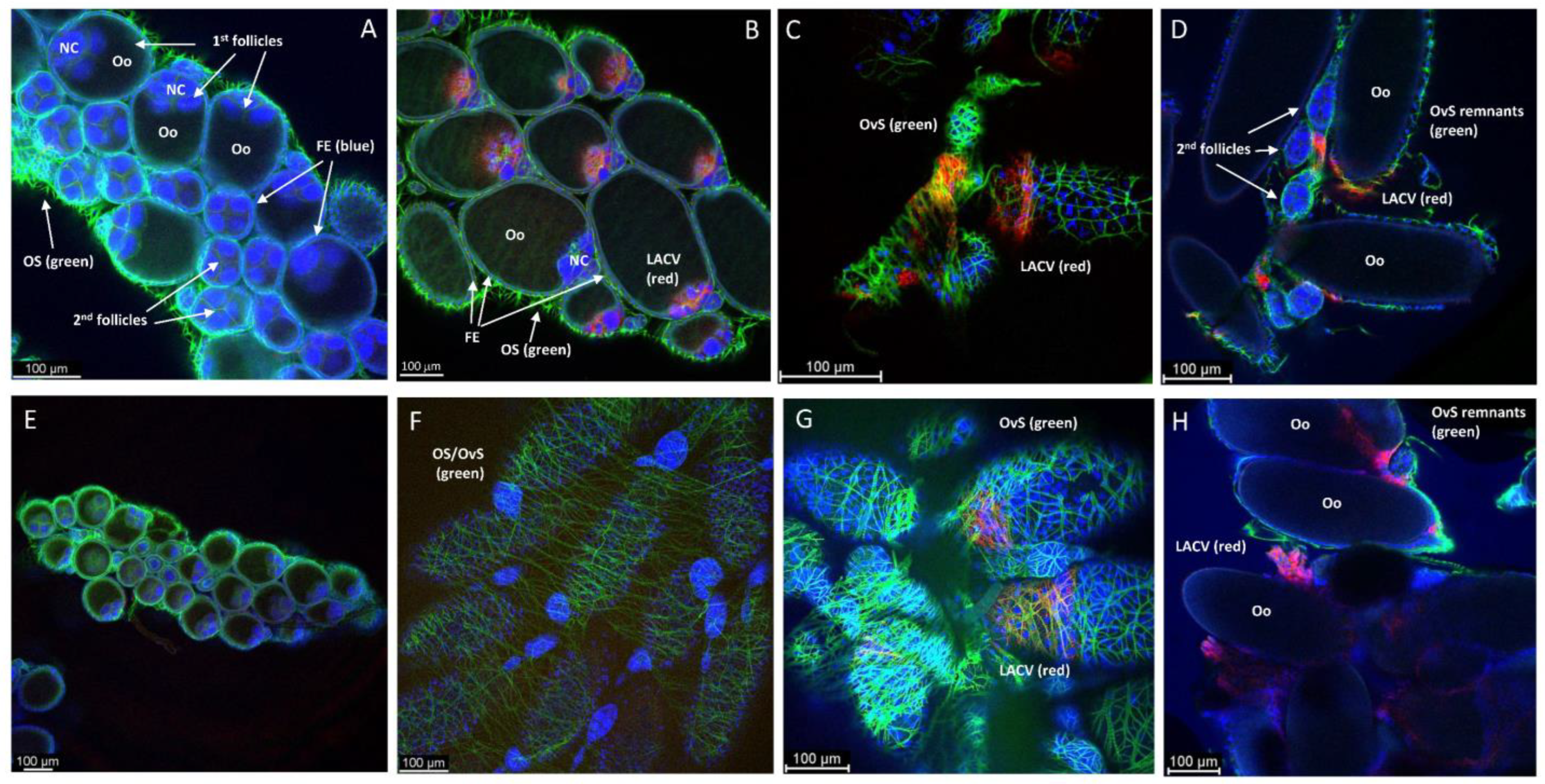
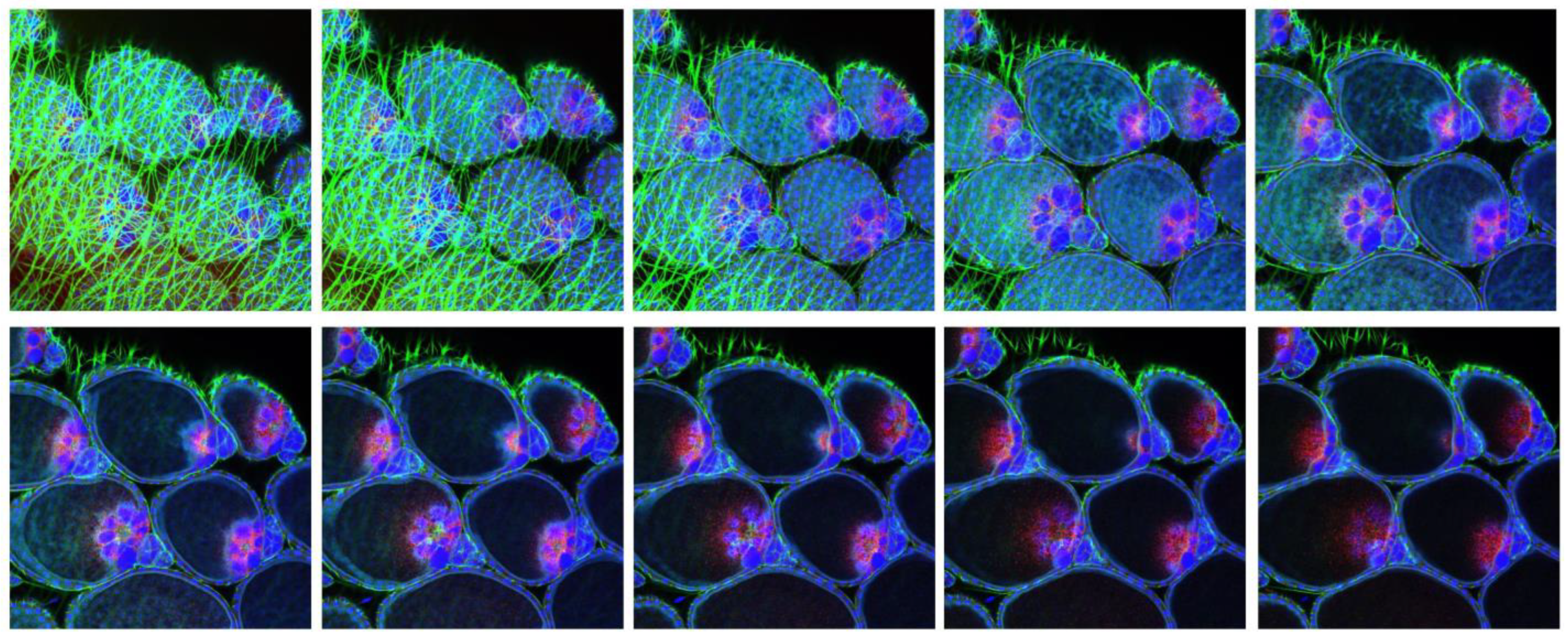
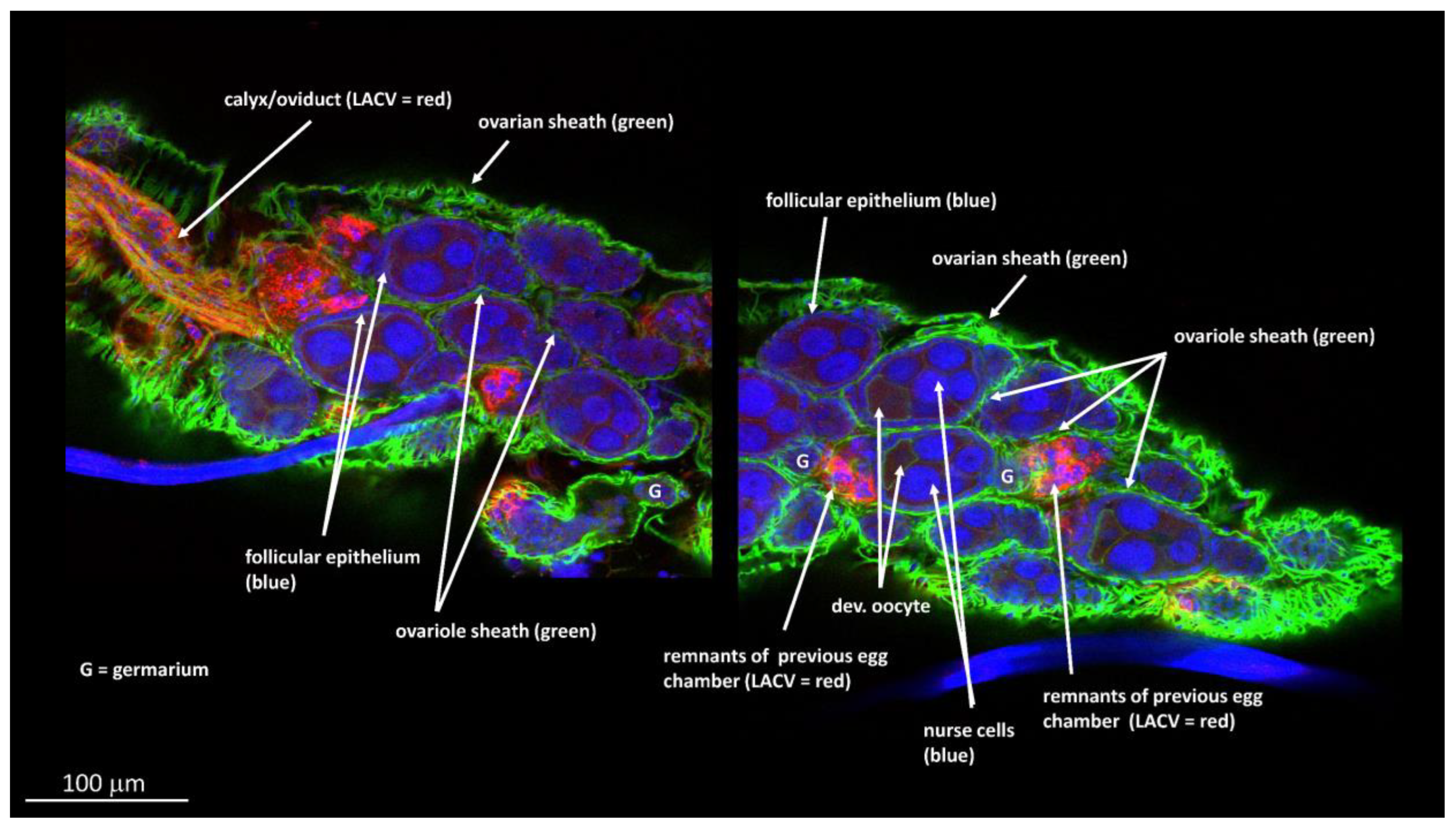
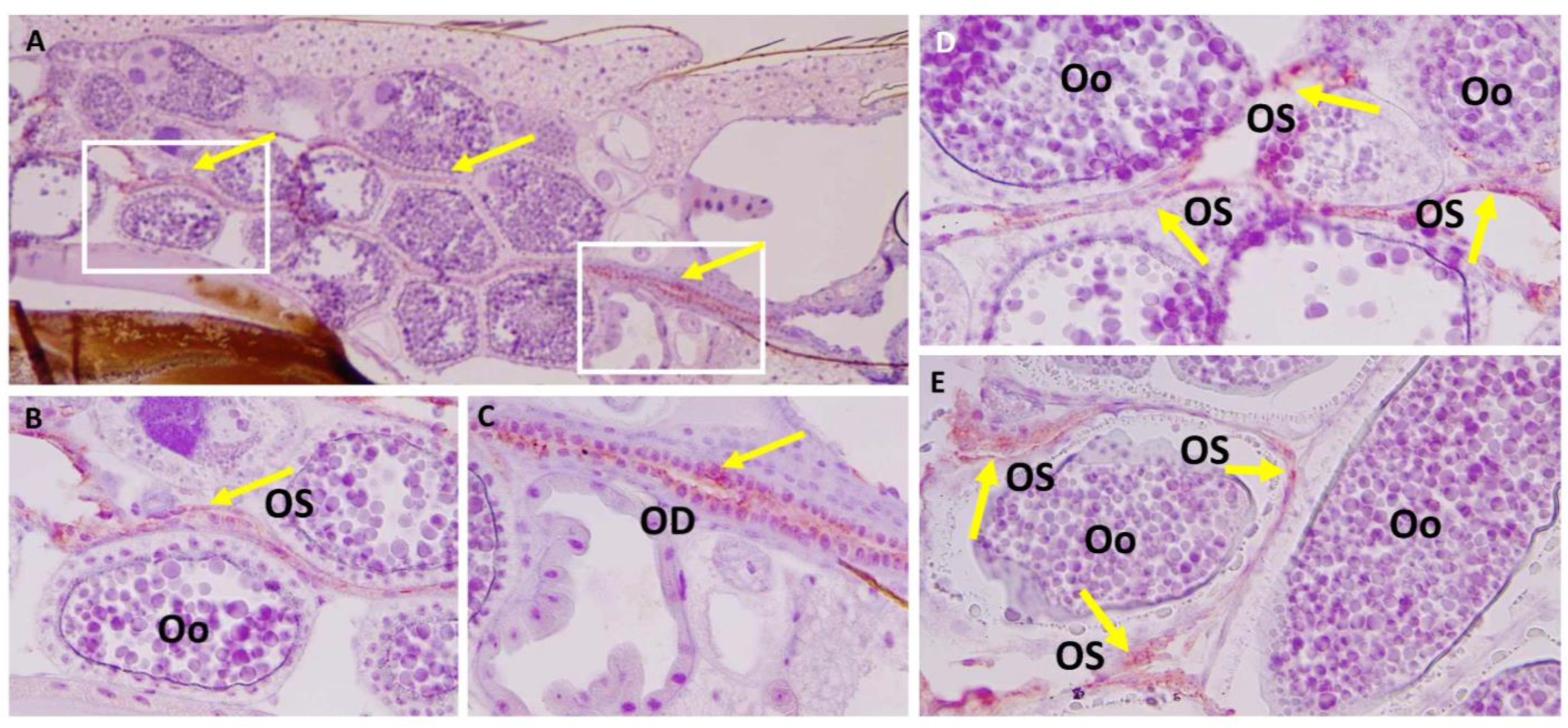
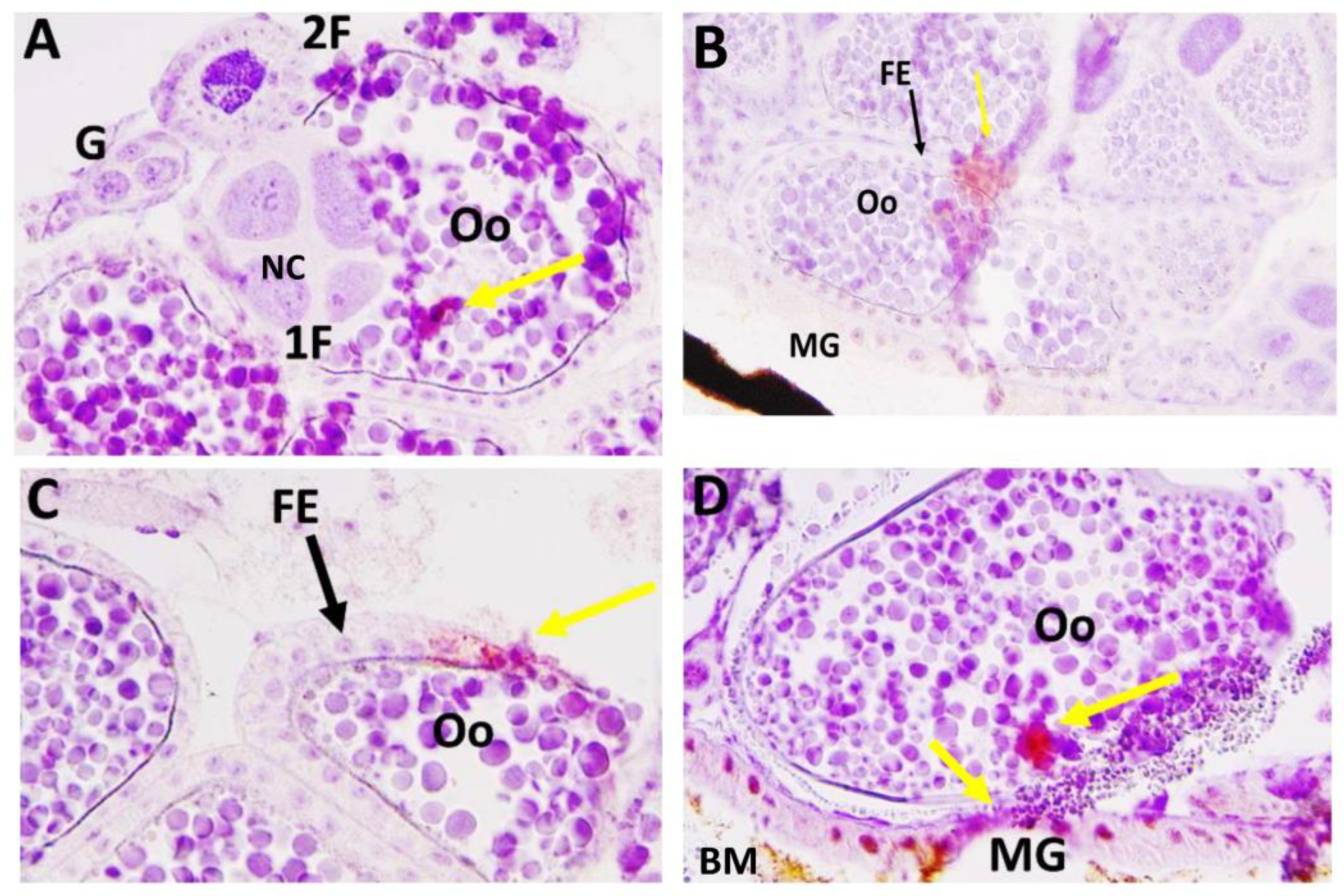
3.3. Ovary Infection Patterns of LACV in HWE and LC Females following Acquisition of a Single Virus-Containing Bloodmeal (BM-1)
3.4. Observing Vertical LACV Transmission by Individual LC and HWE Females
3.5. Ovary Infection Patterns of LACV in Individual HWE and LC Females following Acquisition of BM-2 at 7- or 10-Days Post-BM-1
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tesh, R.B.; Bolling, B.G.; Beaty, B.J. Role of vertical transmission in mosquito-borne arbovirus maintenance and evolution. In Arboviruses–Molecular Biology, Evolution, and Control; Vasilakis, N., Gubler, D.J., Eds.; Caister Academic Press: Norfolk, UK, 2016; pp. 191–217. [Google Scholar]
- Thompson, W.H.; Kalfayan, B.; Anslow, R.O. Isolation of California encephalitis group virus from a fatal human illness. Am. J. Epidemiol. 1965, 81, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Sikes, R.K.; Calisher, C.H.; Smith, J.D. Human infections with La Crosse virus in Georgia, 1982. South. Med. J. 1984, 77, 972–974. [Google Scholar] [CrossRef] [PubMed]
- Vahey, G.M.; Lindsey, N.P.; Staples, J.E.; Hills, S.L. La Crosse virus disease in the United States, 2003–2019. Am. J. Trop. Med. Hyg. 2021, 105, 807–812. [Google Scholar] [CrossRef] [PubMed]
- Pantuwatana, S.; Thompson, W.H.; Watts, D.M.; Hanson, R.P. Experimental infection of chipmunks and squirrels with La Crosse and Trivittatus viruses and biological transmission of La Crosse virus by Aedes triseriatus. Am. J. Trop. Med. Hyg. 1972, 21, 476–481. [Google Scholar] [CrossRef]
- Gauld, L.W.; Hanson, R.P.; Thompson, W.H.; Sinha, S.K. Observations on a natural cycle of La Crosse virus (California group) in Southwestern Wisconsin. Am. J. Trop. Med. Hyg. 1974, 23, 983–992. [Google Scholar] [CrossRef]
- Rowe, R.D.; Odoi, A.; Paulsen, D.; Moncayo, A.C.; Trout Fryxell, R.T. Spatial-temporal clusters of host-seeking Aedes albopictus, Aedes japonicus, and Aedes triseriatus collections in a La Crosse virus endemic county (Knox County, Tennessee, USA). PLoS ONE 2020, 15, e0237322. [Google Scholar] [CrossRef]
- Hopkins, M.C.; Zink, S.D.; Paulson, S.L.; Hawley, D.M. Influence of forest disturbance on La Crosse virus risk in Southwestern Virginia. Insects 2019, 11, 28. [Google Scholar] [CrossRef]
- Bewick, S.; Agusto, F.; Calabrese, J.M.; Muturi, E.J.; Fagan, W.F. Epidemiology of La Crosse virus emergence, Appalachia Region, United States. Emerg. Infect. Dis. 2016, 22, 1921–1929. [Google Scholar] [CrossRef]
- Eastwood, G.; Shepard, J.J.; Misencik, M.J.; Andreadis, T.G.; Armstrong, P.M. Local persistence of novel regional variants of La Crosse virus in the Northeast USA. Parasites Vectors 2020, 13, 569. [Google Scholar] [CrossRef]
- Grimstad, P.R.; Kobayashi, J.F.; Zhang, M.B.; Craig, G.B., Jr. Recently introduced Aedes albopictus in the United States: Potential vector of La Crosse virus (Bunyaviridae: California serogroup). J. Am. Mosq. Control Assoc. 1989, 5, 422–427. [Google Scholar]
- Gerhardt, R.R.; Gottfried, K.L.; Apperson, C.S.; Davis, B.S.; Erwin, P.C.; Smith, A.B.; Panella, N.A.; Powell, E.E.; Nasci, R.S. First isolation of La Crosse virus from naturally infected Aedes albopictus. Emerg. Infect. Dis. 2001, 7, 807–811. [Google Scholar] [CrossRef] [PubMed]
- Thompson, W.H.; Beaty, B.J. Venereal transmission of La Crosse (California encephalitis) arbovirus in Aedes triseriatus mosquitoes. Science 1977, 196, 530–531. [Google Scholar] [CrossRef] [PubMed]
- Watts, D.M.; Pantuwatana, S.; DeFoliart, G.R.; Yuill, T.M.; Thompson, W.H. Transovarial transmission of LaCrosse virus (California encephalitis group) in the mosquito, Aedes triseriatus. Science 1973, 182, 1140–1141. [Google Scholar] [CrossRef]
- Beaty, B.J.; Calisher, C.H. Bunyaviridae--natural history. Curr. Top. Microbiol. Immunol. 1991, 169, 27–78. [Google Scholar] [PubMed]
- Miller, B.R.; DeFoliart, G.R.; Yuill, T.M. Vertical transmission of La Crosse virus (California encephalitis group): Transovarial and filial infection rates in Aedes triseriatus (Diptera: Culicidae). J. Med. Entomol. 1977, 14, 437–440. [Google Scholar] [CrossRef]
- Parks, J.J.; Larsen, J.R. A morphological study of the female reproductive system and follicular development in the mosquito Aedes aegypti (L.). Trans. Am. Microsc. Soc. 1965, 84, 88–98. [Google Scholar] [CrossRef]
- King, R.C.; Buening, J. The origin and functioning of insect oocytes and nurse cells. In Comprehensive Insect Physiology, Biochemistry and Pharmacology; Kerkut, G.A., Gilbert, L.I., Eds.; Pergamon Press: Oxford, NY, USA, 1985; Volume 1, pp. 37–82. [Google Scholar]
- dos Santos, D.C.; Gregório, E.A. Ultrastructure of the ovariole sheath in Diatraea saccharalis (Lepidoptera: Pyralidae). Biocell 2002, 26, 229–235. [Google Scholar] [CrossRef]
- Cruickshank, W.J. The ultrastructure and functions of the ovariole sheath and tunica propria in the flour moth. J. Insect Physiol. 1973, 19, 577–592. [Google Scholar] [CrossRef]
- Isoe, J.; Koch, L.E.; Isoe, Y.E.; Rascón, A.A., Jr.; Brown, H.E.; Massani, B.B.; Miesfeld, R.L. Identification and characterization of a mosquito-specific eggshell organizing factor in Aedes aegypti mosquitoes. PLoS Biol. 2019, 17, e3000068. [Google Scholar] [CrossRef]
- Lehane, M.J.; Laurence, B.R. Development of the calyx and lateral oviduct during oogenesis in Aedes aegypti. Cell Tissue Res. 1978, 193, 125–137. [Google Scholar] [CrossRef]
- Buening, J. The Insect Ovary: Ultrastructure, Previtellogenic Growth and Evolution; Chapman and Hall: London, UK, 1994; p. 400. [Google Scholar]
- Hagedorn, H.H.; Kunkel, J.G. Vitellogenin and vitellin in insects. Ann. Rev. Entomol. 1979, 24, 475–505. [Google Scholar] [CrossRef]
- Rosen, L. Mechanism of vertical transmission of the dengue virus in mosquitoes. Comptes Rendus Acad. Sci. III 1987, 304, 347–350. [Google Scholar]
- Rosen, L. Further observations on the mechanism of vertical transmission of flaviviruses by Aedes mosquitoes. Am. J. Trop. Med. Hyg. 1988, 39, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Rosen, L.; Lien, J.C.; Shroyer, D.A.; Baker, R.H.; Lu, L.C. Experimental vertical transmission of Japanese encephalitis virus by Culex tritaeniorhynchus and other mosquitoes. Am. J. Trop. Med. Hyg. 1989, 40, 548–556. [Google Scholar] [CrossRef]
- Labuda, M.; Ciampor, F.; Kozuch, O. Experimental model of transovarial transmission of Tahyna virus in Aedes aegypti mosquitoes. Acta Virol. 1983, 27, 245–250. [Google Scholar]
- Bergren, N.A.; Kading, R.C. The Ecological significance and implications of transovarial transmission among the vector-borne bunyaviruses: A review. Insects 2018, 9, 173. [Google Scholar] [CrossRef]
- Piedra, L.A.; Martinez, L.C.; Ruiz, A.; Vazquez, J.R.; Guzman, M.G.; Rey, J.; Bisset, J.A. First record of natural transovarial transmission of dengue virus in Aedes albopictus from Cuba. Am. J. Trop. Med. Hyg. 2021, 106, 582–584. [Google Scholar] [CrossRef]
- Sánchez-Vargas, I.; Harrington, L.C.; Doty, J.B.; Black, W.C., 4th; Olson, K.E. Demonstration of efficient vertical and venereal transmission of dengue virus type-2 in a genetically diverse laboratory strain of Aedes aegypti. PLoS Negl. Trop. Dis. 2018, 12, e0006754. [Google Scholar] [CrossRef]
- Heath, C.J.; Grossi-Soyster, E.N.; Ndenga, B.A.; Mutuku, F.M.; Sahoo, M.K.; Ngugi, H.N.; Mbakaya, J.O.; Siema, P.; Kitron, U.; Zahiri, N.; et al. Evidence of transovarial transmission of chikungunya and dengue viruses in field-caught mosquitoes in Kenya. PLoS Negl. Trop. Dis. 2020, 14, e0008362. [Google Scholar] [CrossRef]
- Moraes, A.; Cortelli, F.C.; Miranda, T.B.; Aquino, D.R.; Cortelli, J.R.; Guimarães, M.I.A.; Costa, F.O.; Cortelli, S.C. Transovarial transmission of dengue 1 virus in Aedes aegypti larvae: Real-time PCR analysis in a Brazilian city with high mosquito population density. Can. J. Microbiol. 2018, 64, 393–400. [Google Scholar] [CrossRef]
- da Costa, C.F.; Dos Passos, R.A.; Lima, J.B.P.; Roque, R.A.; de Souza Sampaio, V.; Campolina, T.B.; Secundino, N.F.C.; Pimenta, P.F.P. Transovarial transmission of DENV in Aedes aegypti in the Amazon basin: A local model of xenomonitoring. Parasites Vectors 2017, 10, 249. [Google Scholar] [CrossRef] [PubMed]
- Cruz, L.C.; Serra, O.P.; Leal-Santos, F.A.; Ribeiro, A.L.; Slhessarenko, R.D.; Santos, M.A. Natural transovarial transmission of dengue virus 4 in Aedes aegypti from Cuiabá, State of Mato Grosso, Brazil. Rev. Soc. Bras. Med. Trop. 2015, 48, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Li, C.X.; Guo, X.X.; Deng, Y.Q.; Xing, D.; Sun, A.J.; Liu, Q.M.; Wu, Q.; Dong, Y.D.; Zhang, Y.M.; Zhang, H.D.; et al. Vector competence and transovarial transmission of two Aedes aegypti strains to Zika virus. Emerg. Microbes Infect. 2017, 6, e23. [Google Scholar] [CrossRef] [PubMed]
- Tingström, O.; Wesula Lwande, O.; Näslund, J.; Spyckerelle, I.; Engdahl, C.; Von Schoenberg, P.; Ahlm, C.; Evander, M.; Bucht, G. Detection of Sindbis and Inkoo virus RNA in genetically typed mosquito larvae sampled in northern Sweden. Vector Borne Zoonotic Dis. 2016, 16, 461–467. [Google Scholar] [CrossRef]
- Chandler, L.J.; Blair, C.D.; Beaty, B.J. La Crosse virus infection of Aedes triseriatus (Diptera: Culicidae) ovaries before dissemination of virus from the midgut. J. Med. Entomol. 1998, 35, 567–572. [Google Scholar] [CrossRef]
- Willis, F.S.; Nasci, R.S. Aedes albopictus (Diptera: Culicidae) population density and structure in southwest Louisiana. J. Med. Entomol. 1994, 31, 594–599. [Google Scholar] [CrossRef]
- Wendell, M.D.; Wilson, T.G.; Higgs, S.; Black, W.C. Chemical and gamma-ray mutagenesis of the white gene in Aedes aegypti. Insect Mol. Biol. 2000, 9, 119–125. [Google Scholar] [CrossRef]
- Miller, B.R.; DeFoliart, G.R.; Yuill, T.M. Aedes triseriatus and La Crosse virus: Lack of infection in eggs of the first ovarian cycle following oral infection of females. Am. J. Trop. Med. Hyg. 1979, 28, 897–901. [Google Scholar] [CrossRef]
- Rosen, L.; Shroyer, D.A.; Lien, J.C. Transovarial transmission of Japanese encephalitis virus by Culex tritaeniorhynchus mosquitoes. Am. J. Trop. Med. Hyg. 1980, 29, 711–712. [Google Scholar] [CrossRef]
- Sim, S.; Jupatanakul, N.; Ramirez, J.L.; Kang, S.; Romero-Vivas, C.M.; Mohammed, H.; Dimopoulos, G. Transcriptomic profiling of diverse Aedes aegypti strains reveals increased basal-level immune activation in dengue virus-refractory populations and identifies novel virus-vector molecular interactions. PLoS Negl. Trop. Dis. 2013, 7, e2295. [Google Scholar] [CrossRef]
- Hughes, M.T.; Gonzalez, J.A.; Reagan, K.L.; Blair, C.D.; Beaty, B.J. Comparative potential of Aedes triseriatus, Aedes albopictus, and Aedes aegypti (Diptera: Culicidae) to transovarially transmit La Crosse virus. J. Med. Entomol. 2006, 43, 757–761. [Google Scholar] [CrossRef] [PubMed]
- Bara, J.J.; Parker, A.T.; Muturi, E.J. Comparative susceptibility of Ochlerotatus japonicus, Ochlerotatus triseriatus, Aedes albopictus, and Aedes aegypti (Diptera: Culicidae) to La Crosse virus. J. Med. Entomol. 2016, 53, 1415–1421. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Kantor, A.M.; Lin, J.; Passarelli, A.L.; Clem, R.J.; Franz, A.W. Infection pattern and transmission potential of chikungunya virus in two New World laboratory-adapted Aedes aegypti strains. Sci. Rep. 2016, 6, 24729. [Google Scholar] [CrossRef] [PubMed]
- Kantor, A.M.; Lin, J.; Wang, A.; Thompson, D.C.; Franz, A.W.E. Infection pattern of Mayaro virus in Aedes aegypti (Diptera: Culicidae) and transmission potential of the virus in mixed infections with chikungunya virus. J. Med. Entomol. 2019, 56, 832–843. [Google Scholar] [CrossRef] [PubMed]
- Dubrulle, M.; Mousson, L.; Moutailler, S.; Vazeille, M.; Failloux, A.B. Chikungunya virus and Aedes mosquitoes: Saliva is infectious as soon as two days after oral infection. PLoS ONE 2009, 4, e5895. [Google Scholar] [CrossRef]
- Thangamani, S.; Huang, J.; Hart, C.E.; Guzman, H.; Tesh, R.B. Vertical transmission of Zika virus in Aedes aegypti mosquitoes. Am. J. Trop. Med. Hyg. 2016, 95, 1169–1173. [Google Scholar] [CrossRef]
- Tesh, R.B.; Shroyer, D.A. The mechanism of arbovirus transovarial transmission in mosquitoes: San Angelo virus in Aedes albopictùs. Am. J. Trop. Med. Hyg. 1980, 29, 1394–1404. [Google Scholar] [CrossRef]
- Shroyer, D.A. Transovarial maintenance of San Angelo virus in sequential generations of Aedes albopictus. Am. J. Trop. Med. Hyg. 1986, 35, 408–417. [Google Scholar] [CrossRef]
- Manuel, M.; Missé, D.; Pompon, J. Highly efficient vertical transmission for Zika virus in Aedes aegypti after long extrinsic incubation time. Pathogens 2020, 9, 366. [Google Scholar] [CrossRef]
- Lequime, S.; Paul, R.E.; Lambrechts, L. Determinants of arbovirus vertical transmission in mosquitoes. PLoS Pathog. 2016, 12, e1005548. [Google Scholar] [CrossRef]
- Nag, D.K.; Payne, A.F.; Dieme, C.; Ciota, A.T.; Kramer, L.D. Zika virus infects Aedes aegypti ovaries. Virology 2021, 561, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Romoser, W.S.; Oviedo, M.N.; Lerdthusnee, K.; Patrican, L.A.; Turell, M.J.; Dohm, D.J.; Linthicum, K.J.; Bailey, C.L. Rift Valley fever virus-infected mosquito ova and associated pathology: Possible implications for endemic maintenance. Res. Rep. Trop. Med. 2011, 2, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Tesh, R.B.; Cornet, M. The location of San Angelo virus in developing ovaries of transovarially infected Aedes albopictus mosquitoes as revealed by fluorescent antibody technique. Am. J. Trop. Med. Hyg. 1981, 30, 212–218. [Google Scholar] [CrossRef]
- Bergren, N.A.; Borland, E.M.; Hartman, D.A.; Kading, R.C. Laboratory demonstration of the vertical transmission of Rift Valley fever virus by Culex tarsalis mosquitoes. PLoS Negl. Trop. Dis. 2021, 15, e0009273. [Google Scholar] [CrossRef] [PubMed]
- Freier, J.E.; Rosen, L. Vertical transmission of dengue viruses by Aedes mediovittatus. Am. J. Trop. Med. Hyg. 1988, 39, 218–222. [Google Scholar] [CrossRef]
- Turell, M.J.; Hardy, J.L.; Reeves, W.C. Stabilized infection of California encephalitis virus in Aedes dorsalis, and its implications for viral maintenance in nature. Am. J. Trop. Med. Hyg. 1982, 31, 1252–1259. [Google Scholar] [CrossRef]
- Graham, D.H.; Holmes, J.L.; Higgs, S.; Beaty, B.J.; Black, W.C., 4th. Selection of refractory and permissive strains of Aedes triseriatus (Diptera: Culicidae) for transovarial transmission of La Crosse virus. J. Med. Entomol. 1999, 36, 671–678. [Google Scholar] [CrossRef]
| Mosquito | Number of Bloodmeals 1 | LACV-Infected Larva Pools (%) 2 |
|---|---|---|
| Ae. albopictus LC | BM-1 only | 0/15 (0) |
| BM-2: 10-days post-BM-1 | 4/7 (57%) | |
| Ae. aegypti HWE | BM-1 only | 0/27 (0) |
| BM-2: 10-days post-BM-1 | 1/18 (6%) |
| Mosquito | Days between BM-1 and BM-2 Ingestion 1 | Midgut Infection Rate (%) 2 | Ovary Infection Rate (%) 3 | Proportion of Females that Oviposited (%) | Average No. of Eggs Oviposited per Female 4 | Hatch Rate in % | VTR 5 |
|---|---|---|---|---|---|---|---|
| Aedes albopictus LC | 7 | 12/13 (92%) | 4/12 (33%) | 5/13 (38%) | 11 ± 2.2 | 32 ± 15.4 | 1/4 (25%) |
| 10 | 15/16 (94%) | 6/15 (40%) | 8/16 (50%) | 18 ± 8.9 | 48 ± 12.8 | 1/6 (17%) | |
| Aedes aegypti HWE | 7 | 6/11 (55%) | 5/6 (83%) | 10/11 (91%) | 87 ± 9.9 | 64 ± 9.3 | 0/5 (0) |
| 10 | 3/12 (25%) | 2/3 (67%) | 8/12 (67%) | 95 ± 12.7 | 45 ± 11.4 | 0/2 (0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darby, C.S.; Featherston, K.M.; Lin, J.; Franz, A.W.E. Detection of La Crosse Virus In Situ and in Individual Progeny to Assess the Vertical Transmission Potential in Aedes albopictus and Aedes aegypti. Insects 2023, 14, 601. https://doi.org/10.3390/insects14070601
Darby CS, Featherston KM, Lin J, Franz AWE. Detection of La Crosse Virus In Situ and in Individual Progeny to Assess the Vertical Transmission Potential in Aedes albopictus and Aedes aegypti. Insects. 2023; 14(7):601. https://doi.org/10.3390/insects14070601
Chicago/Turabian StyleDarby, Christie S., Kyah M. Featherston, Jingyi Lin, and Alexander W. E. Franz. 2023. "Detection of La Crosse Virus In Situ and in Individual Progeny to Assess the Vertical Transmission Potential in Aedes albopictus and Aedes aegypti" Insects 14, no. 7: 601. https://doi.org/10.3390/insects14070601
APA StyleDarby, C. S., Featherston, K. M., Lin, J., & Franz, A. W. E. (2023). Detection of La Crosse Virus In Situ and in Individual Progeny to Assess the Vertical Transmission Potential in Aedes albopictus and Aedes aegypti. Insects, 14(7), 601. https://doi.org/10.3390/insects14070601





