Simple Summary
The tachinid fly Drino inconspicuoides parasitizes various lepidopteran larvae, including Noctuidae and Nymphalidae. The adult female oviposits the eggs on the surface of the host, and the hatched larvae immediately penetrate into the host hemocoel and develop there during the larval stage. The caterpillar possesses immune defense mechanisms to eliminate such invaders, but the tachinid larva can avoid these defenses. Within 24 h, the parasitizing larva is surrounded by a black pigmented structure called the “funnel”, and the larva and the funnel are wrapped with a cottony structure called the “cloak”. The funnel is a physically rigid structure that penetrates the epidermis of the host, allowing the larva to breathe through contact with the outside air. This study aimed to clarify the function of the cloak, which has not previously been elucidated. Our findings suggest that formation of the funnel generates ROS, but that these are detoxified in the cloak to enhance the survival of the tachinid larva. The funnel and cloak are composed of host cells. Our study provides insight into the parasitic strategy of a tachinid fly by through which it ingeniously diverts host cells.
Abstract
Drino inconspicuoides (Diptera: Tachinidae) is an endoparasitoid that develops inside the lepidopteran host. When the larva of D. inconspicuoides penetrates into the host, Mythimna separata (Lepidoptera: Noctuidae), the larva creates a cap-like structure, called the funnel, by using host hemocytes, forming a respiratory attachment to permit efficient respiration. A newly described cloudy and cottony structure, called the “cloak”, is formed outside the funnel within 24 h of parasitism. The cloak contains the host fat body and hemocytes. In this study, we aimed to clarify the function of the cloak, which has to date remained unknown. We hypothesized that the funnel generates reactive oxygen species (ROS) through melanization, and that the cloak detoxifies them. We confirmed that the black pigments of the funnel were caused by melanization, which inevitably generates ROS that are potentially harmful to the D. inconspicuoides larva inside the funnel. The cloak showed high activities of antioxidant enzymes, including superoxide dismutase, glutathione peroxidase, and catalase. These results suggest that the cloak scavenged the ROS from the melanized funnel through the diversion of antioxidant enzymes in the fat body, thereby protecting the D. inconspicuoides larva from oxidative damage.
1. Introduction
The Tachinidae are one of the largest groups of dipteran insects, with over 8500 species worldwide [1]. The tachinid fly species are a group of koinobiont parasitoids that allow their host to continue eating food and grow until finally killing it [2,3,4]. Since tachinid flies can utilize a variety of economically important insect pests of crops and forest as hosts, they are considered important biological control agents.
The tachinid fly Drino inconspicuoides (Baranov) parasitizes various lepidopteran insects such as Polygonia caureum (Linnaeus) (Lepidoptera: Nymphalidae), Crocidolomia pavonana (Fabricius) (Lepidoptera: Crambidae), Hyphantria cunea (Drury) (Lepidoptera: Arctiidae), Helicoverpa armigera (Hübner), Mythimna separata (Walker) (Lepidoptera: Noctuidae), and Operophtera brumata (Linnaeus) (Lepidoptera: Geometridae) [5,6,7,8]. We previously established an effective rearing method for D. inconspicuoides [9]. As many tachinid flies tend to be difficult to rear in the laboratory, D. inconspicuoides is a valuable model for studying parasitoid–host interactions.
D. inconspicuoides is a gregarious parasitoid; from two to three up to a dozen or more larvae can develop in a single host [8]. It is an ovolarviparous parasitoid whose embryo develops into the first instar within the egg while still in the mother’s oviduct [10]. After the female lays the incubated egg on the surface of the body of the host, the larva hatches within 1 min and rapidly penetrates the hemocoel of the host, passes through the host epidermis, anchors its bodies beneath the epidermis, and acquires nutrition from hemocytes and the fat body around it. Similar to other tachinid flies, D. inconspicuoides has three larval instars, and the period spent inside the host is approximately eight days [9]. When the D. inconspicuoides larva reaches the third instar, it moves freely in the host hemocoel and feeds on the tissues. It eventually kills the host, exits from the remains, and pupates. The pupal period averages 10 days [9].
Endoparasitoids that grow in the host’s hemocoel must evade the host’s immune system to survive. All insects have immune defense mechanisms that consist of cellular and humoral immune responses [11]. Cellular immune responses, including encapsulation, phagocytosis, and nodulation, are mainly mediated by hemocytes [12,13]. In lepidopteran insects, hemocytes are mainly classified into five types—prohemocytes, plasmatocytes, granulocytes, oenocytoids, and spherule cells [12]. Encapsulation is activated against larger invaders, such as parasitoids, and hemocytes surround the invaders to form hemocytic capsules that kill by suffocation [14,15]. Upon recognition of invaders, granulocytes (an adherent hemocyte type) attach first and form a single layer around the invader. Then, another adherent hemocyte, the plasmatocyte, covers the single layer to form a multi-layered capsule. Finally, granulocytes attach again to the outermost layer and undergo apoptosis, which may be a signal of the completion of capsule formation. Once the invader is entirely enclosed, the inner layer of the capsule is strongly melanized and shows deposits of black pigments [16,17].
In the melanization process, phenoloxidase (PO) is a critical enzyme; it exists in the hemolymph and hemocytes in the form of the precursor prophenoloxidase (proPO) [18]. In response to the intrusion of a foreign substance into the hemocoel, proPO is cleaved into active PO via a serine protease cascade [16]. PO first catalyzes the conversion of tyrosine to 3,4-dihydroxyphenylalanine (DOPA). Melanin with black pigmentation is finally synthesized via several reactions, including oxidation of quinoids mediated by PO [17,19]. During this process, various reactive oxygen species (ROS), such as superoxide anions (O2•−) and hydrogen peroxide (H2O2), are generated [20,21]. Since ROS are toxic, cause an uncontrolled increase in lipid peroxidation, and damage DNA and protein molecules [22,23], they play a vital role in insect immunity. In addition to suffocation, the invader in the hemocytic capsule is eliminated by ROS damage [24,25].
However, the larva of a tachinid fly that successfully parasitizes a host is not enclosed by the hemocytic capsule formed by encapsulation. Instead, a cap-like structure called the “funnel”, which wraps the posterior part of the larva, is formed [26,27,28,29]. The funnel allows the tachinid larva to have direct contact with the outside air via the body wall of the host, thereby preventing death by suffocation. When a freeze-killed D. inconspicuoides larva was experimentally transplanted into a host, the oriental armyworm M. separata, it became surrounded by the hemocytic capsule and melanized by encapsulation [30] (Figure 1a), suggesting that the parasitizing live tachinid larval parasitoids actively form the funnel. The funnel consists of host hemocytes [28,29], which suggests that the funnel is formed in response to active manipulation of the host immune system by parasitoids. The tachinid larva utilizes the host hemocytes, whose original function is to kill the parasitoid via encapsulation to generate a respiratory apparatus. Salt [27] interpreted this strategy of parasitoids as resistance by deflection of the host’s effort.
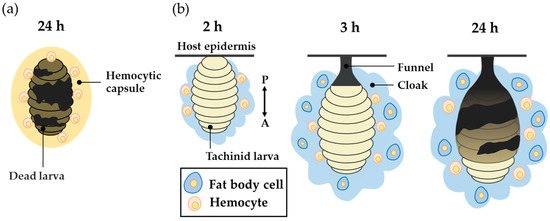
Figure 1.
A schematic view of the encapsulation of transplanted freeze-killed Drino inconspicuoides larva and the early stage of its parasitization in the host, Mythimna separata. (a) The hemocytic capsule formed by encapsulation around freeze-killed D. inconspicuoides larva after 24 h of parasitism. (b) The process of cloak formation around the larva; diagrams from left to right show cloak status at 2, 3, and after 24 h of parasitism. The first-instar D. inconspicuoides is first enveloped by the host hemocytes, after which the fat body of the host covers the hemocyte layer; finally, the two tissues are mixed. The double-headed arrow indicates the anterior–posterior (A/P) axis of the larva.
We previously observed a unique cloudy and cottony structure in connection with this process, which we named the “cloak” [30]. The cloak is not formed around dead D. inconspicuoides larvae or microbeads, but envelops the parasitizing live larva of D. inconspicuoides and its surrounding funnel in a parasitized host, M. separata (Figure 1b). We have clarified the following by employing a combination of techniques using genetic markers. At after 2 h of parasitism, the D. inconspicuoides larva is surrounded by a cloudy material that contains only the host hemocytes. After 3 h, the cottony mass containing the host fat body and hemocytes gradually accumulates around the tachinid larva. The formation of this cloak derived from host materials is completed within 24 h [30]. The purpose of the present study was to clarify the function of the cloak, which has to date remained uninvestigated. We hypothesized that the funnel generates ROS through melanization, and that the cloak detoxifies them. We investigated ROS levels and the activities of antioxidant enzymes, including superoxide dismutase (SOD), glutathione peroxidase (GPx), and catalase in the funnel and cloak.
2. Materials and Methods
2.1. Insect Rearing
We used a colony of D. inconspicuoides originating from parasitized larvae of the fall webworm Hyphantria cunea (Drury) (Lepidoptera: Erebidae), which were collected in Tsukuba, Japan (36°03′ N, 140°05′ E), in September and October 2010. Mythimna separata was supplied from stock cultures stored at Takeda Chemical Industries, Ltd. (Osaka, Japan) [31], and maintained in the Laboratory of Applied Entomology and Zoology, University of Tsukuba, Japan. The parasitoid flies were reared according to the method described by Kalyebi and Nakamura [9], using larvae of M. separata as host insects at 25 °C under a 16 h light: 8 h dark photoperiod. The adult flies were maintained with water and sugar cubes at 20 °C, 60–70% relative humidity, and a 16 h light:8 h dark photoperiod. The larvae of M. separata were fed an artificial diet (Silkmate; Nosan Corporation, Yokohama, Japan).
2.2. Sample Collection
Drino inconspicuoides typically lays multiple eggs on a single caterpillar host. In this experiment, we placed a mated female of D. inconspicuoides in a 9 cm diameter petri dish along with a sixth-instar larva of M. separata, and she was removed after she laid one egg on the caterpillar larvae. After 24 h of parasitism, the hosts were dissected under an MZ12 stereoscopic microscope (Leica Microsystems, Wetzlar, Germany). The tachinid larva, funnel, and cloak were taken together out of the hosts, the tachinid larva was removed out of the funnel, and the cloak was peeled off from the funnel. For further purification of the funnel, it was washed multiple times with phosphate-buffered saline (PBS), and centrifuged to remove any residual cloak components. Hemocytes and the fat body samples were also prepared from the five parasitized hosts from which tachinid larvae were sampled. To collect host cells that form hemocytic capsule formed by encapsulation, a hemocytic capsule was artificially prepared by transplanting micro-polystyrene beads (Φ600 μm; Polysciences, PA, USA) into ice-anesthetized sixth-instar larvae of M. separata. The beads were transplanted into an abdominal leg and the leg was immediately tied with a thread to prevent hemolymph leakage. The microbeads were left in the host hemocoel for 24 h to ensure the full melanization of the artificial hemocytic capsules formed around the beads, and were surgically extracted from the larvae. All the samples were stored at −80 °C until being subjected to PO activity, ROS measurement, and antioxidant enzyme assays.
2.3. Measurement of PO Activity
PO activity was measured following the method described by Laughton and Siva-Jothy [32]. The samples were homogenized in 40 µL PBS and centrifuged at 6000× g for 1 min. The supernatant (20 μL) was mixed with 20 µL of 5.75 mM L-DOPA (Sigma-Aldrich, St. Louis, MO, USA) and then diluted with 960 µL distilled water. After 20 min, the optical absorbance at 490 nm was read in a plate reader (Biochrom, Cambridge, UK). One unit of PO activity was defined as ΔA490 = 0.001 after 10 min of incubation. PO activity was normalized by the amount of total proteins measured using the Qubit Protein Assay Kit (Thermo Fisher Scientific, Waltham, MA, USA) and a Quantus Fluorometer (Promega, Madison, WI, USA).
2.4. Measurement of ROS Content
ROS levels were determined using the OxiSelect InVitro ROS/RNS Assay Kit Green Fluorescence (Cell Biolabs, San Diego, CA, USA) according to the manufacturer’s instructions. ROS in the samples react with dichlorodihydrofluorescin (DCFH), rapidly oxidizing to the highly fluorescent 2′, 7′-dichlorodihydrofluorescein (DCF). Each sample was homogenized in 40 µL PBS on ice, centrifuged at 10,000× g for 5 min, and the supernatant was allowed to react with the provided DCFH probe for 45 min at 25 °C. Oxidation levels were measured from the fluorescence emission at 530 nm when excited at 480 nm using a microplate reader (Multiskan Ascent, Thermo Fisher Scientific). The amount of ROS was normalized by the amount of total proteins and expressed in terms of nmol of DCF per mg of protein.
2.5. Antioxidant Enzyme Assay
The activities of three antioxidant enzymes, SOD, GPx, and catalase, were analyzed. SOD activity was measured using the Colorimetric EpiQuik Superoxide Dismutase Activity/Inhibition Assay Kit (EpiGentek, Farmingdale, NY, USA). The principle of this assay is based on the formation of a colored water-soluble formazan upon reduction of a dye with superoxide anions. The samples were incubated with a water-soluble tetrazolium salt, and the optical absorbance at 470 nm was measured. The amount of SOD required to inhibit the rate of reduction of cytochrome c by 50% was defined as 1 unit of activity. GPx activity was detected using the Amplite Fluorimetric Glutathione Peroxidase Assay Kit (AAT Bioquest, Sunnyvale, CA, USA). This assay is based on the oxidation of glutathione (GSH) to oxidized glutathione (GSSG) catalyzed by glutathione peroxidase. Each sample was incubated with an NADP probe; then, the fluorescence emission at 480 nm when excited at 420 nm was measured. One unit of GPx was defined as the amount of enzyme capable of oxidizing 1.0 µmol GSH to GSSG per min. Catalase activity was measured using the DetectX Catalase Fluorescent Assay Kit (Arbor Assays, Ann Arbor, MI, USA). The principle of this assay is based on the reaction of horseradish peroxidase (HRP) with the substrate in the presence of hydrogen peroxide to convert the colorless substrate into a fluorescent product. Each sample was mixed with HRP, and the fluorescence emission at 590 nm when excited at 520 nm was measured. One unit of catalase activity was defined as the amount of enzyme that decomposed 1.0 µmol H2O2 per min. All activities of the studied antioxidative enzymes were normalized by the amount of total proteins.
2.6. Statistical Analysis
Statistically significant differences between groups were assessed using a one-way analysis of variance (ANOVA) followed by the Tukey–Kramer multiple comparison test. All analyses were conducted using the R software (version 4.0.3) [33]. p-values < 0.01 were considered statistically significant.
3. Results
3.1. Melanization of the Funnel around D. inconspicuoides Larva
The D. inconspicuoides larva in the host is surrounded by host material. The funnel adhering to the larva features black pigmentations, whereas the cloak does not. The pigmentation of the funnel is thought to be due to melanization, similar to that in the hemocytic capsule formed by encapsulation, but this has not been experimentally demonstrated. To confirm that the black pigmentation on the funnel is derived from melanin, we measured the PO activity that mediates melanization. After removing the tachinid larvae and structures surrounding them from the host after 24 h of parasitism, we isolated the blackened funnel and the cottony cloak from the D. inconspicuoides larvae (Figure 2a,b). The funnel showed a significantly higher PO activity than the cloak (ANOVA, F2,12 = 43.5, p < 0.01; Tukey–Kramer’s test, Figure 2c). To obtain a hemocytic capsule consisting of only host materials, we prepared artificial capsules by transplanting microbeads into M. separata. We used a microbead instead of a freeze-killed D. inconspicuoides larva, as separating the dead larva and host-derived parts was not feasible. The activity of the funnel was comparable to that of the artificial capsule that imitated the hemocytic capsule intended to kill the parasitoid. These observations suggest that the funnel is melanized and potentially harmful to the D. inconspicuoides larva.
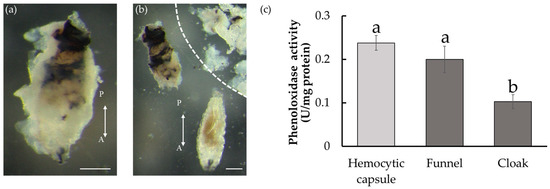
Figure 2.
Host-derived structures formed around Drino inconspicuoides larva developing in Mythimina separata caterpillars and phenoloxidase activity. (a) Larva after 24 h of parasitism. The parasitized larva is encased in the white cottony cloak. (b) Larva (bottom), the funnel (upper left), and the cloak peeled off from the funnel (upper right; zoned by a dotted line). The funnel under the cloak was rigid with black pigmentation, particularly in the posterior region. Scale bars = 0.25 mm. Double-headed arrows indicate the anterior–posterior (A/P) axes of the larvae. (c) Phenoloxidase activity of the hemocytic capsule, the funnel, and the cloak. Each value is expressed as the mean ± standard deviation of samples (n = 5). Different letters above each bar denote significant differences (p < 0.01).
3.2. Production of ROS in the Funnel around D. inconspicuoides Larvae
Since melanization inevitably causes the production of ROS, which can be a host defensive response to cause damage to the parasitoid, we measured the levels of ROS in the funnel and cloak. ROS include O2•−, ·OH, and H2O2, which were quantified collectively. ROS content in the melanized funnel was as high as in the artificial hemocytic capsule (ANOVA, F2,22 = 66.89, p < 0.01; Tukey–Kramer’s test, Figure 3). In contrast, ROS levels in the cloak were significantly lower than those in the funnel. The low ROS levels of the cloak may be explained by low PO activity, but it is also possible that the cloak exhibits antioxidant effects of actively removing ROS.
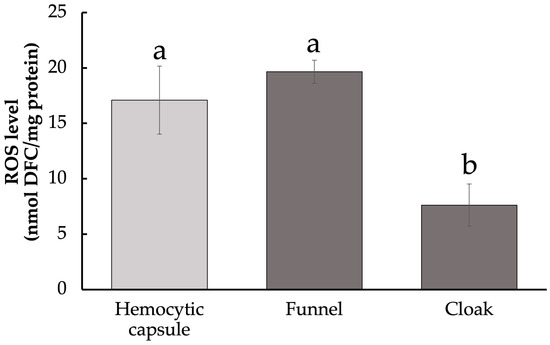
Figure 3.
Levels of reactive oxygen species in host-derived structures surrounding developing Drino inconspicuoides larva. Values are expressed as mean ± standard deviation (Hemocytic capsule, n = 5; Funnel, Cloak, n = 10). Different letters above each bar denote significant differences (p < 0.01).
3.3. Activities of Three Antioxidant Enzymes in the Cloak
In animals, ROS are scavenged by many enzymatic antioxidants, such as SOD, GPx, catalase, and glutathione-S-transferase, and some non-enzymatic antioxidants, including ascorbic acid, thiols, and 𝛼-tocopherols [34,35]. We examined the activities of three antioxidant enzymes in the funnel and cloak to examine whether the cloak actively removes ROS.
O2•− is the first ROS molecule generated during the melanization process and converted into H2O2 by SOD in an early step of the ROS detoxification pathway. SOD activity of the cloak was higher than that of the funnel and comparable to that of the hemocytic capsule (ANOVA, F4,20 = 215.4, p < 0.01; Tukey–Kramer’s test, Figure 4a), suggesting that conversion of O2•− to H2O2 occurred mainly in the cloak rather than in the funnel. The hemocytic capsule formed by encapsulation and the respiratory funnel are derived from hemocytes, whereas the cloak contains fat body tissue in addition to hemocyte-derived structures. We, therefore, also measured the SOD activities of hemocytes and the fat body isolated from M. separata larvae. SOD activity was significantly higher in hemocytes than in the fat body, suggesting that the high activity in the cloak is associated with the contained hemocytes.
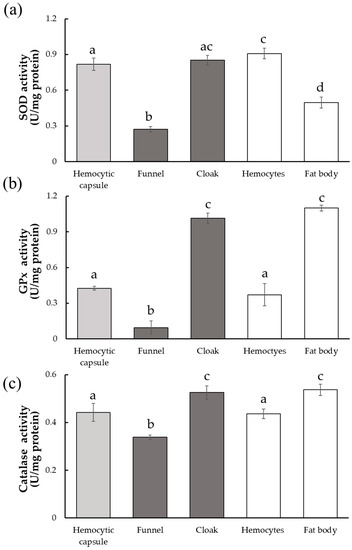
Figure 4.
Activities of three antioxidant enzymes in host-derived structures surrounding developing Drino inconspicuoides larva. (a) Superoxidase dismutase (SOD) activity; (b) Glutathione peroxidase (GPx) activity; (c) Catalase activity. Each value is expressed as the mean ± standard deviation of samples (n = 5). Different letters above each bar denote significant differences (p < 0.01).
GPx and catalase are enzymes that act independently on H2O2, but both of them convert H2O2 into non-toxic H2O. GPx activity was much higher in the cloak than in the funnel (ANOVA, F4,20 = 293.2, p < 0.01; F4,20 = 58.47, p < 0.01; Tukey–Kramer’s test, Figure 4b,c). Upon comparing the host tissues, the activity in the fat body was higher than that in the hemocytes, suggesting that the high GPx activity in the cloak is mainly due to the presence of the host fat body. Catalase activity also showed a trend similar to that of GPx (ANOVA, F4,20 = 293.2, p < 0.01; Tukey–Kramer’s test, Figure 4c), although the differences in activity between the tissues were less than those observed for GPx activity.
4. Discussion
Parasitoid larvae developing inside hosts that lack countermeasures against host immune defenses are typically surrounded by hemocytic capsules formed by encapsulation and are suffocated. In addition, the ROS generated during melanization inside the hemocytic capsule are thought to contribute to the elimination of parasitoids. The artificial hemocytic capsule we prepared in this study had high PO activity. They contained high levels of ROS (Figure 2c and Figure 3), indicating that the ROS generated during melanization accumulate within the capsules and cause damage to the invaders. In this study, we first measured the PO activity in the funnel and the findings indicate that its black pigmentation was derived from melanization, as was that of the hemocytic capsule (Figure 2c). Melanization inevitably leads to the production of ROS, which are harmful to the tachinid larva in the funnel. We postulated that the cloak formed around the funnel detoxified these ROS.
The first ROS produced during melanization is O2•−, which converted to H2O2 by SOD [36]. Figure 5a summarizes the putative mechanism of the detoxification of the ROS by the cloak. ROS levels were higher in the funnel than in the cloak (Figure 3), whereas SOD activity was lower in the funnel than in the cloak (Figure 4a), suggesting that the high ROS levels in the funnel are attributable mainly to O2•−. The high SOD activity in the cloak suggests that H2O2 is produced using the O2•− in the funnel as the substrate. In the cloak, the activities of GPx and catalase, both of which convert H2O2 to non-toxic H2O [37,38], were higher than those in the funnel (Figure 4b,c), suggesting that the detoxification of O2•− to H2O through H2O2 takes place in the cloak. ROS levels in the cloak were low (Figure 3), possibly because H2O2 is rapidly converted to H2O in the cloak. It is conceivable that melanization of the funnel produces O2•−, which is detoxified into H2O in the cloak. This prevents the O2•− from the funnel from seriously damaging D. inconspicuoides larvae inside the funnel. Measurements of GPx and catalase activity (Figure 4b,c) suggest that GPx plays a more dominant role than catalase in detoxifying H2O2 in the cloak. On the other hand, the hemocytic capsule formed by encapsulation had high SOD activity but low GPx and catalase activities (Figure 4a–c); this indicates that the O2•− generated during melanization around the invaders is converted to H2O2 but not detoxified to H2O as it is in the cloak. H2O2, which is more cytotoxic than O2•− [39], is known to be a major cause of oxidative damage [40,41], and its elimination is critical to the survival of parasitoid wasps [23]. This highly toxic H2O2 in the hemocytic capsule acts to efficiently kill the invader in the capsule (Figure 5b).
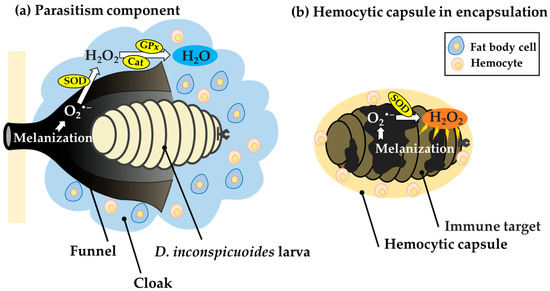
Figure 5.
Comparison of putative metabolic pathways of reactive oxygen species (ROS) in host-derived structures surrounding developing Drino inconspicuoides larvae and hemocytic capsules during encapsulation. (a) In the structure formed around the D. inconspicuoides larva, O2•−, a type of ROS, is generated from the melanization of the funnel. The O2•− is detoxified to H2O via H2O2 owing to the high SOD, GPx, and catalase activities of the cloak containing the host fat body, allowing the tachinid larva to avoid damage from the ROS. (b) In the hemolytic capsule in encapsulation, melanization activated around invaders leads to the production of O2•−, which is converted into highly toxic H2O2 by SOD. However, H2O2 is not detoxified into H2O due to low activities of GPx and catalase in the capsules and thus causes damage to invaders as a toxic molecule. SOD: superoxide dismutase; GPx: Glutathione peroxidase; Cat: Catalase.
We previously examined the origin of the cells contained in the funnel and cloak by performing PCR using specific primer sets for the M. separata and D. inconspicuoides [30]. The result revealed that both structures exclusively contained host cells and no cells derived from the tachinid fly. In general, SOD, GPx, and catalase lack a signal peptide necessary for extracellular secretion [42,43,44,45,46]. This implies that the origin of the antioxidant enzymes detected in the funnel and cloak are derived from the host cells. They are synthesized by the host cells, and not secreted from the tachinid larva.
The hemocytic capsule and funnel are made of the host’s hemocytes, but the SOD activity of the funnel was significantly lower than that of hemocytic capsule and hemocytes. This may suggest that SOD is downregulated in the hemocytes constituting the funnel. Reducing conversion of less toxic O2•− into highly toxic H2O2 by suppressing the SOD activity in the funnel is beneficial for the tachinid larvae. It has been reported that parasitism of E. bombycis affects the activities of antioxidant enzymes, including SOD and catalase, in the silkworm Bombyx mori (Linnaeus) (Lepidoptera: Bombycidae) [47]. Tachinid larvae may also be capable of influencing enzyme expression in hemocytes of their hosts.
The formation of the cloak outside the funnel raises the question of who is inducing the cloak formation. One possibility is that the tachinid larva induces the cloak to efficiently release the O2•− from the funnel. Another possibility is that the host forms the cloak as a defensive reaction to detoxify O2•− that leaks out of the funnel as a consequence of the suppression of SOD activity by the tachinid larva. We recently discovered that the salivary gland of D. inconspicuoides larvae contains substances that attract cells of the host fat body (data not shown); we therefore suggest that it is more likely that the tachinid larva, rather than the host, induces cloak formation to efficiently detoxify ROS produced in the funnel.
The mechanisms by which parasitoids evade host immune defenses have been studied in greater detail in parasitic wasps than in tachinid flies. Most females of endoparasitoid wasps that deposit their eggs into the host inject immune-suppressive factors, such as polydnaviruses and venom, to suppress PO activity, production of ROS, and encapsulation [48,49,50,51,52]. In contrast, many species of tachinid flies cannot inject such immune-suppressive factors, requiring the hatched larvae to evade the host’s immune defenses on their own, for which they have developed various strategies. In D. inconspicuoides and Exorista bombycis (Louis) (Diptera: Tachinidae), the larvae secrete immune-suppressive factors [53,54]. As a strategy that is not found in parasitic wasps, some tachinid species migrate and develop in sites where host hemocytes cannot reach them, such as the salivary glands, ganglia, or midgut [55,56]. Creating a breathing funnel is also unique to tachinid flies. This strategy seems to be sophisticated in that the tachinid larvae utilize the host’s materials to protect themselves, making it difficult for the hosts to develop countermeasures with their own materials.
The high antioxidant enzyme activity in the cloak suggests that it contributes to the scavenging of toxic ROS from the funnel, which provide the D. inconspicuoides larvae a suitable environment to develop. The presence of cloak has only been reported in D. inconspicuoides, and it remains to be investigated whether cloak formation is also present in other parasitoids. Future studies on the molecular mechanisms of transformation of host tissues into funnels and cloaks in D. inconspuoides, and possibly in other parasitoids, will lead to a more comprehensive understanding of parasitic strategies of tachinid flies.
Author Contributions
Conceptualization, K.Z. and S.F.; formal analysis, K.Z. and S.F.; data curation, K.Z.; writing—original draft preparation, K.Z.; writing—review and editing, K.Z. and S.F.; project administration, S.F. and S.N.; funding acquisition, S.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Japan Society for the Promotion of Science KAKENHI, grant number JP17K08157.
Data Availability Statement
The datasets and analysis protocols used during the current study are available from the corresponding author on request.
Acknowledgments
We thank Tetsuhiko Sasaki for constructive criticism of the manuscript.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this paper.
References
- Stireman, J.O., III; Cerretti, P.; O’Hara, J.E.; Blaschke, J.D.; Moulton, J.K. Molecular phylogeny and evolution of world Tachinidae (Diptera). Mol. Phylogenet. Evol. 2019, 139, 106358. [Google Scholar] [CrossRef] [PubMed]
- Dindo, M.L. Tachinid parasitoids: Are they to be considered as koinobionts? BioControl 2011, 56, 249–255. [Google Scholar] [CrossRef]
- Irwin, M.E.; Schlinger, E.I.; Thompson, F.C. Diptera, True Flies. In The Natural History of Madagascar; Goodman, S.M., Benstead, J.P., Eds.; University of Chicago Press: Chicago, IL, USA, 2003; pp. 692–702. [Google Scholar]
- Stireman, J.O., III; Singer, M.S. Determinants of parasitoid–host associations: Insights from a natural tachinid–lepidopteran community. Ecology 2003, 84, 296–310. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, L.; Yang, H.; Sappington, T.W.; Cheng, Y. Biocontrol of the oriental armyworm, Mythimna separata, by the tachinid fly Exorista civilis is synergized by Cry1Ab protoxin. Sci. Rep. 2016, 6, 26873. [Google Scholar] [CrossRef]
- Cherry, A.; Cock, M.; van den Berg, H.; Kfir, R. Biological control of Helicoverpa armigera in Africa. In Biological Ontrol in IPM Systems in Africa; CAB International: Wallingford, UK, 2003; pp. 329–346. [Google Scholar]
- Broadley, H.J.; Kelly, E.A.; Elkinton, J.S.; Kula, R.R.; Boettner, G.H. Identification and impact of hyperparasitoids and predators affecting Cyzenis albicans (Tachinidae), a recently introduced biological control agent of winter moth (Operophtera brumata L.) in the northeastern USA. Biol. Control 2018, 121, 99–108. [Google Scholar] [CrossRef]
- Shima, H. A host-parasite catalog of Tachinidae (Diptera) of Japan. Makunagi/Acta Dipterol. 2006, 31, 1–108. [Google Scholar]
- Kalyebi, A.; Nakamura, S. The biology of the parasitoid fly Drino inconspicuoides (Diptera: Tachinidae) in the host Mythimna separata (Lepidoptera: Noctuidae). Appl. Entomol. Zool. 2006, 41, 365–370. [Google Scholar] [CrossRef]
- Baranov, N. Messages about gezucktete oriental larvaevoriden (Insecta: Diptera). Entomol. Nachr. 1934, 8, 41–49. [Google Scholar]
- Yang, L.; Qiu, L.M.; Fang, Q.; Stanley, D.W.; Ye, G.Y. Cellular and humoral immune interactions between Drosophila and its parasitoids. Insect Sci. 2021, 28, 1208–1227. [Google Scholar] [CrossRef]
- Lavine, M.D.; Strand, M.R. Insect hemocytes and their role in cellular immune responses. Insect Biochem. Mol. Biol. 2002, 32, 1237–1242. [Google Scholar] [CrossRef]
- Strand, M.R. The insect cellular immune response. Insect Sci. 2008, 15, 1–14. [Google Scholar] [CrossRef]
- Ratner, S.; Vinson, S.B. Phagocytosis and encapsulation: Cellular immune responses in arthropoda. Am. Zool. 1983, 23, 185–194. [Google Scholar] [CrossRef]
- Ratcliffe, N.A.; Gagen, S.J. Studies on the in vivo cellular reactions of insects: An ultrastructural analysis of nodule formation in Galleria mellonella. Tissue Cell 1977, 9, 73–85. [Google Scholar] [CrossRef]
- Boucias, D.G.; Pendland, J.C. Insect Immune Defense System, Part III: Prophenoloxidase cascade and post-attachment processes of phagocytosis. In Principles of Insect Pathology; Springer: Boston, MA, USA, 1998; pp. 499–537. [Google Scholar]
- Dubovskiy, I.M.; Kryukova, N.A.; Glupov, V.V.; Ratcliffe, N.A. Encapsulation and nodulation in insects. Invertebr. Surviv. J. 2016, 13, 229–246. [Google Scholar] [CrossRef]
- Liu, Y.T.; Chang, C.I.; Hseu, J.R.; Liu, K.F.; Tsai, J.M. Immune responses of prophenoloxidase and cytosolic manganese superoxide dismutase in the freshwater crayfish Cherax quadricarinatus against a virus and bacterium. Mol. Immunol. 2013, 56, 72–80. [Google Scholar] [CrossRef]
- Nappi, A.J.; Christensen, B.M. Melanogenesis and associated cytotoxic reactions: Applications to insect innate immunity. Insect Biochem. Mol. Biol. 2005, 35, 443–459. [Google Scholar] [CrossRef]
- Komarov, D.A.; Slepneva, I.A.; Dubovskii, I.M.; Grizanova, E.V.; Khramtsov, V.V.; Glupov, V.V. Generation of superoxide radical and hydrogen peroxide in insect hemolymph in the course of immune response. Dokl. Biol. Sci. 2006, 411, 482–485. [Google Scholar] [CrossRef]
- Whitten, M.M.; Ratcliffe, N.A. In vitro superoxide activity in the haemolymph of the West Indian leaf cockroach, Blaberus discoidalis. J. Insect Physiol. 1999, 45, 667–675. [Google Scholar] [CrossRef]
- Collin, F. Chemical basis of reactive oxygen species reactivity and involvement in neurodegenerative diseases. Int. J. Mol. Sci. 2019, 20, 2407. [Google Scholar] [CrossRef] [PubMed]
- Nappi, A.J.; Vass, E.; Frey, F.; Carton, Y. Superoxide anion generation in Drosophila during melanotic encapsulation of parasites. Eur. J. Cell Biol. 1995, 68, 450–456. [Google Scholar]
- Dubovskii, I.M.; Grizanova, E.V.; Chertkova, E.A.; Slepneva, I.A.; Komarov, D.A.; Vorontsova, Y.L.; Glupov, V.V. Generation of reactive oxygen species and activity of antioxidants in hemolymph of the moth larvae Galleria mellonella (L.) (Lepidoptera: Piralidae) at development of the process of encapsulation. J. Evol. Biochem. Physiol. 2010, 46, 35–43. [Google Scholar] [CrossRef]
- Nappi, A.J.; Vass, E. Melanogenesis and the generation of cytotoxic molecules during insect cellular immune reactions. Pigm. Cell Res. 1993, 6, 117–126. [Google Scholar] [CrossRef]
- Stireman, J.O., III; O’Hara, J.E.; Wood, D.M. Tachinidae: Evolution, behavior, and ecology. Annu. Rev. Entomol. 2006, 51, 525–555. [Google Scholar] [CrossRef] [PubMed]
- Salt, G. The resistance of insect parasitoids to the defense reactions of their hosts. Biol. Rev. 1968, 43, 200–232. [Google Scholar] [CrossRef] [PubMed]
- Michalková, V.; Valigurová, A.; Dindo, M.L.; Vanhara, J. Larval morphology and anatomy of the parasitoid Exorista larvarum (Diptera: Tachinidae), with an emphasis on cephalopharyngeal skeleton and digestive tract. J. Parasitol. 2009, 95, 544–554. [Google Scholar] [CrossRef]
- Valigurová, A.; Michalková, V.; Koník, P.; Dindo, M.L.; Gelnar, M.; Vaňhara, J. Penetration and encapsulation of the larval endoparasitoid Exorista larvarum (Diptera: Tachinidae) in the factitious host Galleria mellonella (Lepidoptera: Pyralidae). Bull. Entomol. Res. 2014, 104, 203–212. [Google Scholar] [CrossRef]
- Yamashita, K.; Zhang, K.; Ichiki, R.; Nakamura, S.; Furukawa, S. Novel host immune evasion strategy of the endoparasitoid Drino inconspicuoides. Bull. Èntomol. Res. 2019, 109, 643–648. [Google Scholar] [CrossRef]
- Takabayashi, J.; Noda, T.; Takahashi, S. Effect of kairomones in the host searching behavior of Apanteles Kariyai WATANABE (Hymenoptera: Braconidae), a parasitoid of the common armyworm, Pseudaletia Separata WALKER (Lepidoptera: Noctuidae), 1: Presence of arresting stimulants produced by the host larvae. Appl. Entomol. Zool. 1985, 20, 484–489. [Google Scholar] [CrossRef]
- Laughton, A.M.; Siva-Jothy, M.T. A standardised protocol for measuring phenoloxidase and prophenoloxidase in the honey bee, Apis mellifera. Apidologie 2010, 42, 140–149. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; R Core Team: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 5 April 2023).
- Arrigoni, O.; De Tullio, M.C. Ascorbic acid: Much more than just an antioxidant. Biochim. Biophys. Acta. 2002, 1569, 1–9. [Google Scholar] [CrossRef]
- Felton, G.W.; Summers, C.B. Antioxidant systems in insects. Arch. Insect Biochem. Physiol. 1995, 29, 187–197. [Google Scholar] [CrossRef]
- Fridovich, I. Superoxide radical and superoxide dismutases. Annu. Rev. Biochem. 1995, 64, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Deng, D.; Chen, W. Inhibitors and activators of SOD, GSH-Px, and CAT. Enzym. Inhib. Act. 2017, 29, 207–224. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Free radicals, reactive oxygen species and human disease: A critical evaluation with special reference to atherosclerosis. Br. J. Exp. Pathol. 1989, 70, 737–757. [Google Scholar] [PubMed]
- Sutton, H.C.; Winterbourn, C.C. On the participation of higher oxidation states of iron and copper in fenton reactions. Free Radic. Biol. Med. 1989, 6, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Ward, J.F.; Evans, J.W.; Limoli, C.L.; Calabro-Jones, P.M. Radiation and hydrogen peroxide induced free radical damage to DNA. Br. J. Cancer Suppl. 1987, 8, 105–112. [Google Scholar]
- Li, H.B.; Dai, C.G.; Zhang, C.R.; He, Y.F.; Hu, Y. Cloning and expression profiling of catalase gene in the Oriental Armyworm, Mythimna separata (Lepidoptera: Noctuidae). Acta Entomol. Sin. 2018, 61, 178–187. [Google Scholar] [CrossRef]
- Li, H.B.; Dai, C.G.; He, Y.F.; Hu, Y. Characterization and expression of genes encoding superoxide dismutase in the Oriental Armyworm, Mythimna separata (Lepidoptera: Noctuidae). J. Econ. Entomol. 2019, 112, 2381–2388. [Google Scholar] [CrossRef]
- Lu, Y.; Bai, Q.; Zheng, X.; Lu, Z. Expression and enzyme activity of catalase in Chilo suppressalis (Lepidoptera: Crambidae) is responsive to environmental stresses. J. Econ. Entomol. 2017, 110, 1803–1812. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Zhu, Z.; Ma, W.; Lei, C. The molecular characterization of antioxidant enzyme genes in Helicoverpa armigera adults and their involvement in response to ultraviolet—A stress. J. Insect Physiol. 2012, 58, 1250–1258. [Google Scholar] [CrossRef] [PubMed]
- Ou, J.; Deng, H.M.; Zheng, S.C.; Huang, L.H.; Feng, Q.L.; Liu, L. Transcriptomic analysis of developmental features of Bombyx mori wing disc during metamorphosis. BMC Genom. 2014, 15, 820. [Google Scholar] [CrossRef] [PubMed]
- Makwana, P.; Pradeep, A.N.; Hungund, S.P.; Ponnuvel, K.M.; Trivedy, K. The dipteran parasitoid Exorista bombycis induces pro- and anti-oxidative reactions in the silkworm Bombyx mori: Enzymatic and genetic analysis. Arch. Insect Biochem. Physiol. 2017, 94, e21373. [Google Scholar] [CrossRef] [PubMed]
- Lavine, M.D.; Beckage, N.E. Polydnaviruses: Potent mediators of host insect immune dysfunction. Parasitol. Today 1995, 11, 368–378. [Google Scholar] [CrossRef]
- Pennacchio, F.; Strand, M.R. Evolution of developmental strategies in parasitic Hymenoptera. Annu. Rev. Entomol. 2006, 51, 233–258. [Google Scholar] [CrossRef]
- Kryukova, N.A.; Dubovskiy, I.M.; Chertkova, E.A.; Vorontsova, Y.L.; Slepneva, I.A.; Glupov, V.V. The effect of Habrobracon hebetor venom on the activity of the prophenoloxidase system, the generation of reactive oxygen species and encapsulation in the haemolymph of Galleria mellonella larvae. J. Insect Physiol. 2011, 57, 796–800. [Google Scholar] [CrossRef]
- Moreau, S.J.; Asgari, S. Venom proteins from parasitoid wasps and their biological functions. Toxins 2015, 7, 2385–2412. [Google Scholar] [CrossRef]
- Teng, Z.W.; Xu, G.; Gan, S.Y.; Chen, X.; Fang, Q.; Ye, G.Y. Effects of the endoparasitoid Cotesia chilonis (Hymenoptera: Braconidae) parasitism, venom, and calyx fluid on cellular and humoral immunity of its host Chilo suppressalis (Lepidoptera: Crambidae) larvae. J. Insect Physiol. 2016, 85, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Schwier, N.; Zhang, K.; Nakamura, S.; Furukawa, S. Larvae of the tachinid fly, Drino inconspicuoides (Diptera: Tachinidae), suppress melanization in host lepidopteran insects. J. Asia. Pac. Entomol. 2021, 24, 1050–1054. [Google Scholar] [CrossRef]
- Makwana, P.; Dubey, H.; Pradeep, A.N.R.; Sivaprasad, V.; Ponnuvel, K.M.; Mishra, R.K. Dipteran endoparasitoid infestation actively suppressed host defense components in hemocytes of silkworm Bombyx mori for successful parasitism. Anim. Gene 2021, 22, 200118. [Google Scholar] [CrossRef]
- Clausen, C.P. Entomophagous Insects; McGraw-Hill Book Co, Inc.: New York, NY, USA; London, UK, 1940; p. 688. [Google Scholar]
- Ichiki, R.; Shima, H. Immature life of Compsilura concinnata (Meigen) (Diptera: Tachinidae). Ann. Entomol. Soc. Am. 2003, 96, 161–167. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
