Fertility Life Table, Thermal Requirements, and Ecological Zoning of Anthonomus grandis grandis Boheman (Coleoptera: Curculionidae) in Brazil
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing
2.2. Biology of A. grandis grandis at Different Temperatures on Artificial Diet
2.3. Statistical Analysis
2.3.1. Biology
2.3.2. Thermal Requirements and Fertility Life Table
2.3.3. Occurrence of A. grandis in Brazil Based on Fertility Life Table
3. Results
3.1. Biology of A. grandis grandis at Different Temperatures
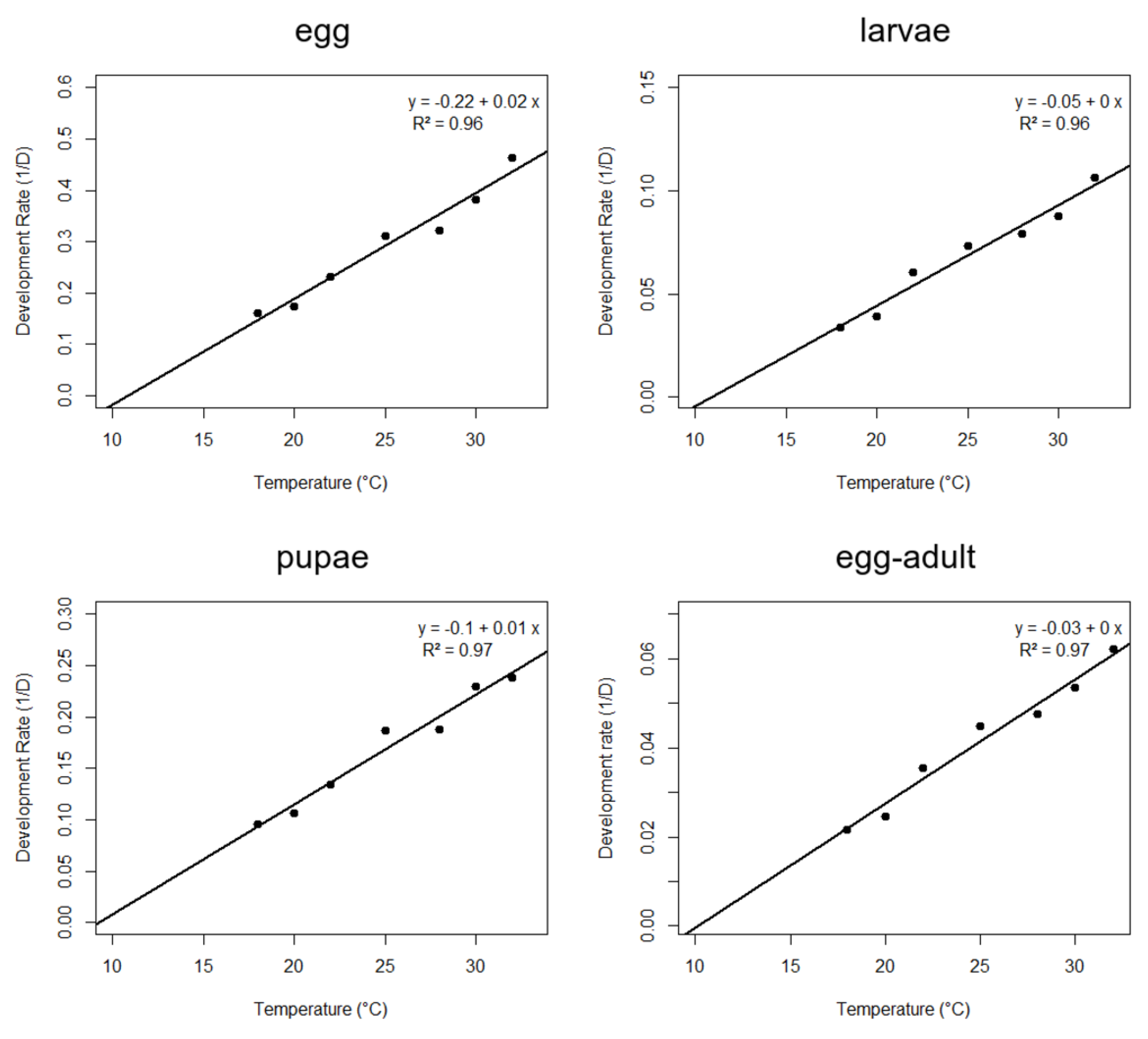
3.2. Thermal Requirements and Fertility Life Table of A. grandis grandis on Artificial Diet at Seven Constant Temperatures
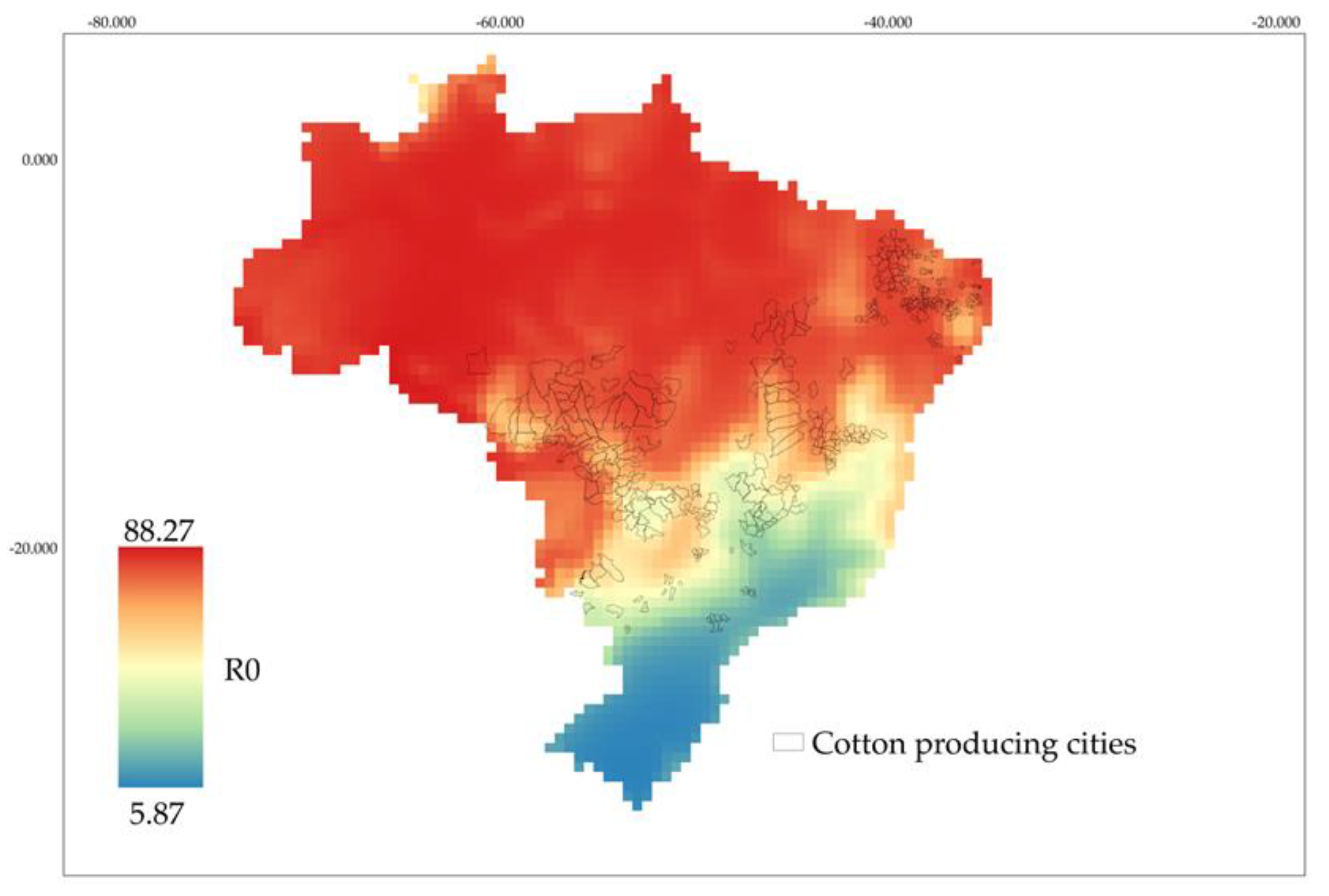
3.3. Occurrence of A. grandis grandis Based on Fertility Life Table in Cotton Fields in Brazil
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boheman, C. Original description of Anthonomus grandis. In Genera et Species Curculionidum; Schoenherr, C.J., Ed.; La Librairie Encyclopedique de Roret: Paris, France, 1843; pp. 232–233. [Google Scholar]
- Howard, L.O. A new cotton insect in Texas. Insect Life 1894, 7, 1–273. [Google Scholar]
- Burke, H.R.; Clark, W.E.; Cate, J.R.; Fryxell, P.A. Origin and Dispersal of the Boll Weevil. Bull. Entomol. Soc. Am. 1986, 32, 228–238. [Google Scholar] [CrossRef]
- Sobrinho, R.B.; Lukefahr, M.J. Bicudo (Anthonomus Grandis Boheman): Nova Ameaça à Cotonicultura Brasileira: Biologia e Controle; EMBRAPA, Centro Nacional de Pesquisa Algodão: Campina Grande, Brazil, 1983; pp. 1–32. [Google Scholar]
- Degrande, P. Bicudo do Algodoeiro: Manejo Integrado; Embrapa: Dourados, Mato Grosso do Sul, Brazil, 1991; pp. 1–142. [Google Scholar]
- Oliveira-Marra, S.O.D.; Guedes, R.N.C.; Bastos, C.S.; Marra, P.H.A.; Vivan, L.M.; Zanine, A.D.M. Insecticide resistance and control failure likelihood among populations of the boll weevil (Anthonomus grandis) from Mato Grosso (Brazil). Acta Sci. Agron. 2019, 41, e42714. [Google Scholar] [CrossRef]
- Rolim, G.G.; Barros, E.M.; Barbosa, P.R.; Arruda, L.S.; Torres, J.B. Sublethal effects of insect growth regulators on boll weevil (Coleoptera: Curculionidae). J. Econ. Entomol. 2019, 112, 2222–2228. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.B.; Rolim, G.G.; Arruda, L.S.; Santos, M.P.; Leite, S.A.; Neves, R.C.S. Insecticides in Use and Risk of Control Failure of Boll Weevil (Coleoptera: Curculionidae) in the Brazilian Cerrado. Neotrop. Entomol. 2022, 51, 613–627. [Google Scholar] [CrossRef]
- CONAB (Companhia Nacional de Abastecimento). Acompanhamento da Safra Brasileira de Grãos, 11° Levantamento, Safra 2021/2022; Ministério da Agricultura, Pecuária e Abastecimento: Brasília, Brazil, 2022. [Google Scholar]
- Oliveira, C.M.; Auad, A.M.; Mendes, S.M.; Frizzas, M.R. Economic impact of exotic insect pests in Brazilian agriculture. J. Appl. Entomol. 2012, 137, 1–15. [Google Scholar] [CrossRef]
- Degrande, P.; Santos, W.; Silva, A. Programa nacional contra o bicudo. Cultiv. Gd. Cult. 2004, 68, 8–10. [Google Scholar]
- Azambuja, R.; Degrande, E.D. Trinta anos do bicudo-do-algodoeiro no Brasil. Arq. Inst. Biol. 2014, 4, 1–81. [Google Scholar] [CrossRef]
- Paula, D.P.; Claudino, D.; Timbó, R.V.; Miranda, J.E.; Bemquerer, M.P.; Ribeiro, A.C.J.; Sujii, E.R.; Fontes, E.M.G.; Pires, C.S.S. Reproductive dormancy in boll-weevil from populations of the Midwest of Brazil. J. Econ. Entomol. 2013, 106, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Pires, C.S.S.; Pimenta, M.; Mata, R.A.; Souza, L.M.; Paula, D.P.; Sujii, E.R.; Fontes, E.M.G. Survival pattern of the boll weevil during cotton fallow in Midwestern Brazil. Pesqui. Agropecuária Bras. 2017, 52, 149–160. [Google Scholar] [CrossRef]
- Stinner, R.; Butler, G., Jr.; Bacheler, J.; Tuttle, C. Simulation of temperature-dependent development in population dynamics models. Can. Entomol. 1975, 107, 1167–1174. [Google Scholar] [CrossRef]
- Haddad, M.L.; Parra, J.R.P. Métodos Para Estimar os Limites Térmicos e a Faixa Òtima de Desenvolvimento das Diferentes Fases do Ciclo Evolutivo dos Insetos; FEALQ: Piracicaba, Brazil, 1984; pp. 1–10. [Google Scholar]
- Wagner, T.; Wu, H.; Sarper, P.; Schoolfiel, R.; Coulson, R. Modeling insect development rates: A literature review and application of a biophysical. Ann. Entomol. Soc. Am. 1984, 77, 208–225. [Google Scholar] [CrossRef]
- Sharpe, P.; DeMichele, D. Reaction kinetics of poikilotherm development. J. Theor. Biol. 1977, 64, 649–670. [Google Scholar] [CrossRef] [PubMed]
- Haddad, M.L.; Parra, J.R.P.; Moraes, R.C.B. Métodos Para Estimar os Limites Térmicos Inferior e Superior de Desenvolvimento dos Insetos; FEALQ: Piracicaba, Brazil, 1999; pp. 1–29. [Google Scholar]
- Campbell, A.B.; Frazer, D.; Gilbert, N.; Gutierrez, A.P.; Mackauer, M. Temperature requirements of some aphids and their parasites. J. Appl. Ecol. 1974, 11, 431–438. [Google Scholar] [CrossRef]
- Garcia, A.G.; Parra, J.R.P. Métodos de Determinação de Limites Térmicos e Constante Térmica Para Insetos; Esalq: Piracicaba, Brazil, 2022; pp. 1–25. [Google Scholar]
- Sandhu, H.S.; Nuessly, G.S.; Webb, S.E.; Cherry, R.H.; Gilbert, R.A. Temperature-dependent reproductive and life table parameters of Elasmopalpus lignosellus (Lepidoptera: Pyralidae) on sugarcane. Fla. Entomol. 2013, 96, 380–390. [Google Scholar] [CrossRef]
- Martins, J.C.; Picanço, M.C.; Bacci, L.; Guedes, R.N.C.; Santana, P.A.; Ferreira, D.O.; Chediak, M. Life table determination of thermal requirements of the tomato borer Tuta absoluta. J. Pest Sci. 2016, 89, 897–908. [Google Scholar] [CrossRef]
- Lacerda, L.F.A.; Coelho, A., Jr.; Garcia, A.G.; Sentelhas, P.C.; Parra, J.R.P. Biology at Different Temperatures, Thermal Requirements, and Ecological Zoning of Opogona sacchari (Lepidoptera: Tineidae). J. Econ. Entomol. 2019, 112, 1676–1682. [Google Scholar] [CrossRef]
- Parra, J.R.P.; Coelho, A., Jr.; Cuervo-Rugno, J.B.; Garcia, A.G.; Moral, R.A.; Specht, A.; Neto, D.D. Important pest species of the Spodoptera complex: Biology, thermal requirements and ecological zoning. J. Pest Sci. 2022, 95, 169–186. [Google Scholar] [CrossRef]
- Eligio, V.V.; Lozano, L.B.; Castaneda, R.P.; Garciá, G.G.; Villalon, M.L. Regional-Scale Spatio-Temporal Analysis of Anastrepha ludens (Diptera: Tephritidae) Populations in the Citrus Region of Santa Engracia, Tamaulipas, Mexico. J. Econ. Entomol. 2015, 108, 1655–1664. [Google Scholar] [CrossRef]
- Byrne, D.; Pickard, A.J. Neogeography and the democratization of GIS: A metasynthesis of qualitative research. Inf. Commun. Soc. 2016, 11, 1505–1522. [Google Scholar] [CrossRef]
- Norris, R.F.; Chen, C.; Kogan, M. Concepts in Integrated Pest Management; Prentice Hall: Upper Saddle River, NJ, USA, 2003. [Google Scholar]
- Parra, J.R.P. A biologia de insetos e o manejo de pragas: Da criação em laboratório à aplicação em campo. In Bases e Técnicas do Manejo de Insetos; Guedes, J.C., Costa, I.D., Castiglioni, E., Eds.; UFSM: Santa Maria, Brazil, 2000; pp. 1–29. [Google Scholar]
- Parra, J.R.P. Técnicas de Criação de Insetos Para Programas de Controle Biológico; FEALQ: Piracicaba, Brazil, 2001; p. 137. [Google Scholar]
- Vanderzant, E.S.; Davich, T.B. Laboratory rearing of the boll weevil: A satisfactory larval diet and oviposition studies. J. Econ. Entomol. 1958, 51, 288–291. [Google Scholar] [CrossRef]
- Sterling, W.L.; Adkisson, P.L. An artificial diet for laboratory cultures of boll weevil larvae and adults. J. Econ. Entomol. 1966, 59, 1074–1077. [Google Scholar] [CrossRef]
- Lindig, O.H. A replacement for cottonseed meal and meats in boll weevil diets. J. Econ. Entomol. 1979, 72, 291–292. [Google Scholar] [CrossRef]
- Monnerat, R.G.; Dias, S.C.; de Oliveira Neto, O.; Nobre, S.; Silva-Werneck, J.; Sá, M. Criação massal do bicudo do algodoeiro Anthonomus grandis em laboratório. Embrapa Recur. Genéticos Biotecnol. 2000, 29, 1–22. [Google Scholar]
- Monnerat, R.G.; Nobre, S.D.N.; Neto, O.B.O.; Schimidt, F.G.V.; Dias, S.; Lauman, R.; Sá, M.F.G.; Sujii, E.R. Parâmetros Biônomicosdo Bicudo-do-Algodoeiro (Anthonomus grandis) Criado em Dieta Artificial Para a Realização de Bioensaios. Boletim de Pesquisa e Desenvolvimento; Embrapa: Brasília, Brazil, 2002; pp. 1–29. [Google Scholar]
- Sappington, T.; Spurgeon, D. Preferred technique for adult sex determination of the boll weevil (Coleoptera: Curculionidae). Ann. Entomol. Soc. Am. 2000, 93, 610–615. [Google Scholar] [CrossRef]
- Burr, I.W.; Foster, L.A. A Test for Equality of Variances; Purdue University: West Lafayette, IN, USA, 1972; pp. 1–26. [Google Scholar]
- Shapiro, S.S.; Wilk, M.B. An Analysis of Variance Test for Normality (Complete Samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2008. [Google Scholar]
- Chi, H.; You, M.S.; Athhann, R.; Smith, C.L.; Kavousi, A.; Özgökçe, M.S.; Güncan, A.; Tuan, S.J.; Fu, J.W.; Xu, Y.Y.; et al. Age-Stage, Two-Sex Life Table: An Introduction to Theory, Data Analysis, and Application. Entomol. Gen. 2023, 40, 103–124. [Google Scholar] [CrossRef]
- Chi, H. Life-table analysis incorporating both sexes and variable development rates among individuals. Environ. Entomol. 1988, 17, 26–34. [Google Scholar] [CrossRef]
- Chi, H.; Liu, H. Two new methods for the study of insect population ecology. Bull. Inst. Zool. Acad. Sin. 1985, 24, 225–240. [Google Scholar]
- INMET. Normais Climatológicas do Brasil, 1961–2020; Instituto Nacional de Meteorologia—INMET, Ministério da Agricultura, Pecuária e Abastecimento—MAPA: Brasília, Brazil, 2020. [Google Scholar]
- QGIS Development Team. QGIS Geographic Information System. Open Source Geospatial Foundation. Available online: http://qgis.org (accessed on 12 October 2022).
- SIDRA-IBGE. 2023. SIDRA-IBGE. Available online: https://sidra.ibge.gov.br/tabela/1612#resultado (accessed on 12 October 2022).
- Fye, R.E.; Patana, R.; McAda, W.C. Developmental periods for boll weevils reared at several constant and fluctuating temperatures. J. Econ. Entomol. 1969, 62, 1402–1405. [Google Scholar] [CrossRef]
- Cole, C.L.; Adkisson, P.L. Effects of constant and variable temperature regimes of the survival and rate of increase of the boll weevil. Southwest. Entomol. 1982, 7, 50–55. [Google Scholar]
- Greenberg, S.M.; Setamou, M.; Sappington, T.W.; Liu, T.X.; Coleman, R.; Armstrong, J.S. Temperature-dependent development and reproduction of the boll weevil (Coleoptera: Curculionidae). Insect Sci. 2005, 12, 449–459. [Google Scholar] [CrossRef]
- Cole, C.L.; Adkisson, P.L. Life history and fecundity of the boll weevil reared in constant and variable temperature regimens. Southwest. Entomol. 1981, 64, 298–302. [Google Scholar]
- Parra, J.R.P.; Pazzini, A.R.; Haddad, M. 2012. Nutritional indices for measuring insect food intake and utilization. In Insect Bioecology and Nutrition for Integrated Pest Management; Panizzi, A.R., Parra, J.R.P., Eds.; CCR Press: Boca Raton, FL, USA, 2012; pp. 13–51. [Google Scholar]
- Fye, R.E.; Bonham, C.D. Summer temperatures of the soil surface and their effect on survival of boll weevils in fallen cotton squares. J. Econ. Entomol. 1970, 63, 1599–1602. [Google Scholar] [CrossRef]
- Ribeiro, P.D.A.; Suji, E.R.; Diniz, I.R.; de Medeiros, M.A.; Salgado-Labouriau, M.L.; Branco, M.C.; Pires, C.S.S.; Fontes, E.M.G. Alternative food sources and overwintering feeding behavior of the boll weevil, Anthonomus grandis Boheman (Coleoptera: Curculionidea) under the tropical conditions of Central Brazil. Neotrop. Entomol. 2010, 39, 28–34. [Google Scholar] [CrossRef]
- Brazzel, J.R.; Newsom, L.D. Diapause in Anthonomus grandis Boh. J. Econ. Entomol. 1959, 52, 603–611. [Google Scholar] [CrossRef]
- Spurgeon, D.W.; Raulston, J.R. Boll weevil (Coleoptera: Curculionidae) reproductive development as a function of temperature. Environ. Entomol. 1998, 27, 675–681. [Google Scholar] [CrossRef]
- Spurgeon, D.W.; Suh, C.P.C.; Esquivel, J.F. Diapause response of the boll weevil (Coleoptera: Curculionae) to feeding period duration and cotton square size. J. Insect Sci. 2018, 18, 1–9. [Google Scholar] [CrossRef]

| Temperature (°C) | Survival (%) | |||
|---|---|---|---|---|
| Egg | Larva | Pupa | Total (Egg-Adult) | |
| 18 | 45.0 ± 0.8 d | 68.4 ± 7.5 c | 75.7 ± 8.1 a | 23.3 ± 2.1 c |
| 20 | 54.2 ± 1.9 cd | 72.8 ± 7.2 bc | 75.1 ± 5.6 a | 29.2 ± 4.5 c |
| 22 | 65.0 ± 3.4 b | 89.3 ± 2.0 b | 85.5 ± 3.4 a | 49.6 ± 4.5 ab |
| 25 | 75.8 ± 3.1 a | 95.4 ± 2.2 a | 84.1 ± 5.2 a | 60.8 ± 6.8 a |
| 28 | 68.3 ± 2.1 ab | 90.4 ± 4.1 ab | 83.6 ± 1.9 a | 51.6 ± 3.1 ab |
| 30 | 59.2 ± 0.8 bc | 77.3 ± 3.6 bc | 81.3 ± 5.7 a | 37.2 ± 3.9 bc |
| 32 | 52.5 ± 2.2 cd | 66.4 ± 3.4 c | 63.9 ± 3.7 a | 22.1 ± 2.5 c |
| Temperature (°C) | Pre-Oviposition (Days) | Fecundity | Sex Ratio | Longevity (Days) | |
|---|---|---|---|---|---|
| Females | Males | ||||
| 18 | 10.2 ± 0.13 a | 33.7 ± 3.1 ab | 0.51 ± 0.04 a | 115.0 ± 2.8 a | 111.0 ± 4.9 a |
| 20 | 6.50 ± 0.15 b | 99.3 ± 10.6 cd | 0.43 ± 0.09 a | 72.9 ± 2.2 b | 66.5 ± 1.9 b |
| 22 | 6.10 ± 0.13 b | 156.0 ± 12.1 d | 0.43 ± 0.07 a | 75.2 ± 1.9 b | 64 ± 3.1 b |
| 25 | 5.56 ± 0.08 c | 251.0 ± 15.8 e | 0.50 ± 0.04 a | 56.1 ± 1.4 c | 55.1 ± 1.3 c |
| 28 | 4.93 ± 0.04 d | 234.0 ± 17.9 e | 0.45 ± 0.05 a | 47.7 ± 1.4 d | 41.0 ± 1.9 d |
| 30 | 4.52 ± 0.11 d | 70.2 ± 9.6 bc | 0.51 ± 0.10 a | 34.4 ± 1.2 e | 31.9 ± 1.4 e |
| 32 | 3.38 ± 0.18 e | 9.0 ± 1.5 a | 0.49 ± 0.06 a | 14.2 ± 1.3 f | 11.8 ± 0.8 f |
| T (°C) | R0 | T | rm | ʎ |
|---|---|---|---|---|
| 18 | 3.367 ± 0.972 a | 72.948 ± 1.613 a | 0.016 ± 0.004 a | 1.016 ± 0.004 a |
| 20 | 9.932 ± 2.896 b | 64.874 ± 1.035 b | 0.034 ± 0.005 b | 1.035 ± 0.005 b |
| 22 | 28.623 ± 5.921 c | 50.782 ± 0.850 c | 0.065 ± 0.004 c | 1.067 ± 0.004 c |
| 25 | 74.766 ± 11.411 d | 40.754 ± 0.521 d | 0.105 ± 0.004 d | 1.111 ± 0.004 d |
| 28 | 74.722 ± 11.446 d | 40.755 ± 0.528 d | 0.105 ± 0.004 d | 1.111 ± 0.004 d |
| 30 | 11.681 ± 2.841 e | 30.179 ± 0.568 e | 0.084 ± 0.008 b | 1.083 ± 0.008 c |
| 32 | 0.675 ± 0.239 f | 22.419 ± 0.989 f | −2.084 ± 0.018 e | 0.979 ± 0.001 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, F.P.; Diniz, A.J.F.; Parra, J.R.P. Fertility Life Table, Thermal Requirements, and Ecological Zoning of Anthonomus grandis grandis Boheman (Coleoptera: Curculionidae) in Brazil. Insects 2023, 14, 582. https://doi.org/10.3390/insects14070582
Pereira FP, Diniz AJF, Parra JRP. Fertility Life Table, Thermal Requirements, and Ecological Zoning of Anthonomus grandis grandis Boheman (Coleoptera: Curculionidae) in Brazil. Insects. 2023; 14(7):582. https://doi.org/10.3390/insects14070582
Chicago/Turabian StylePereira, Fernanda Polastre, Alexandre José Ferreira Diniz, and José Roberto Postali Parra. 2023. "Fertility Life Table, Thermal Requirements, and Ecological Zoning of Anthonomus grandis grandis Boheman (Coleoptera: Curculionidae) in Brazil" Insects 14, no. 7: 582. https://doi.org/10.3390/insects14070582
APA StylePereira, F. P., Diniz, A. J. F., & Parra, J. R. P. (2023). Fertility Life Table, Thermal Requirements, and Ecological Zoning of Anthonomus grandis grandis Boheman (Coleoptera: Curculionidae) in Brazil. Insects, 14(7), 582. https://doi.org/10.3390/insects14070582








