Current and Future Habitat Suitability Models for Four Ticks of Medical Concern in Illinois, USA
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Tick Occurrence Data
2.2. Environmental Covariates
2.3. Model Fitting and Evaluation
3. Results
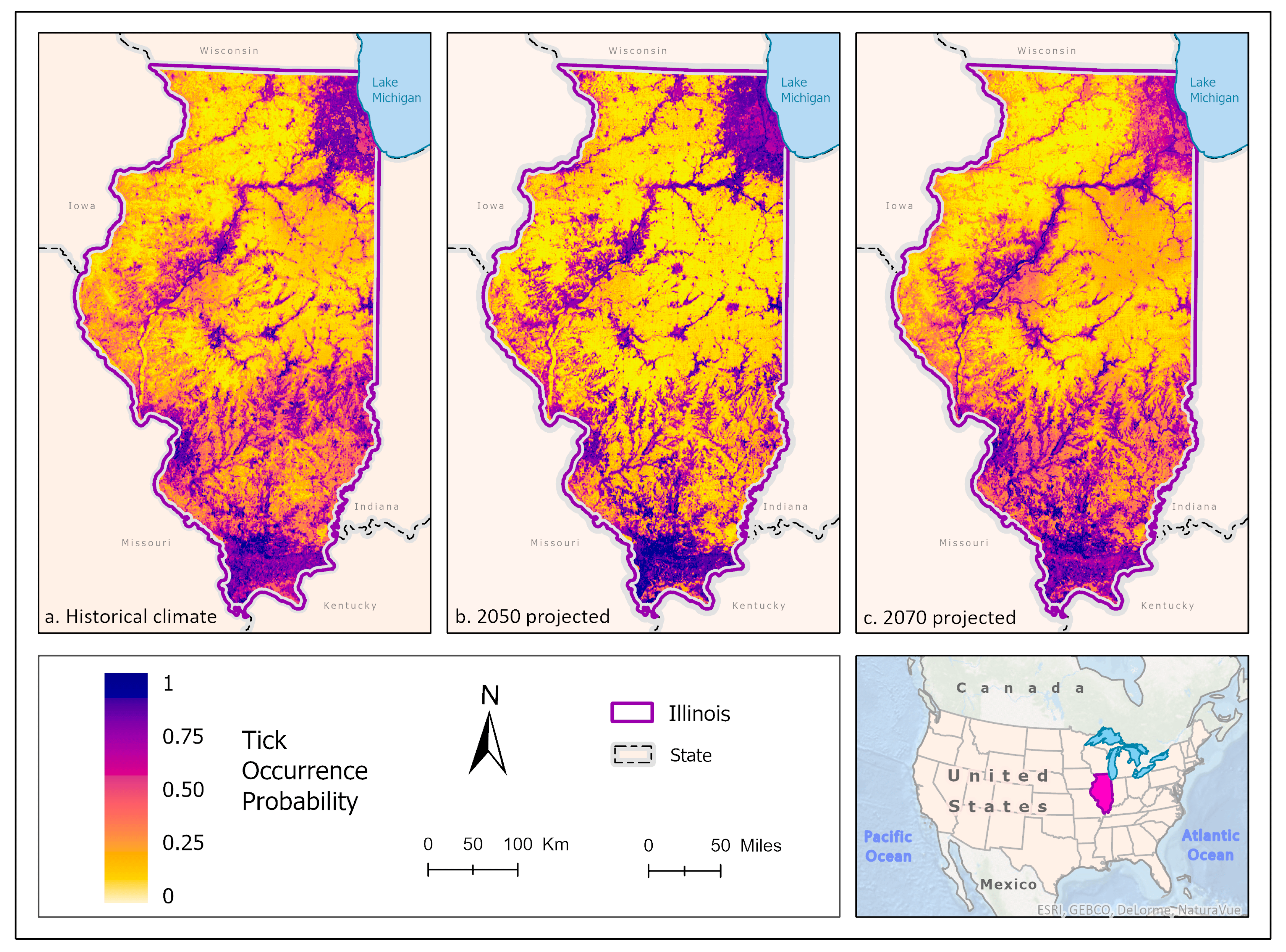
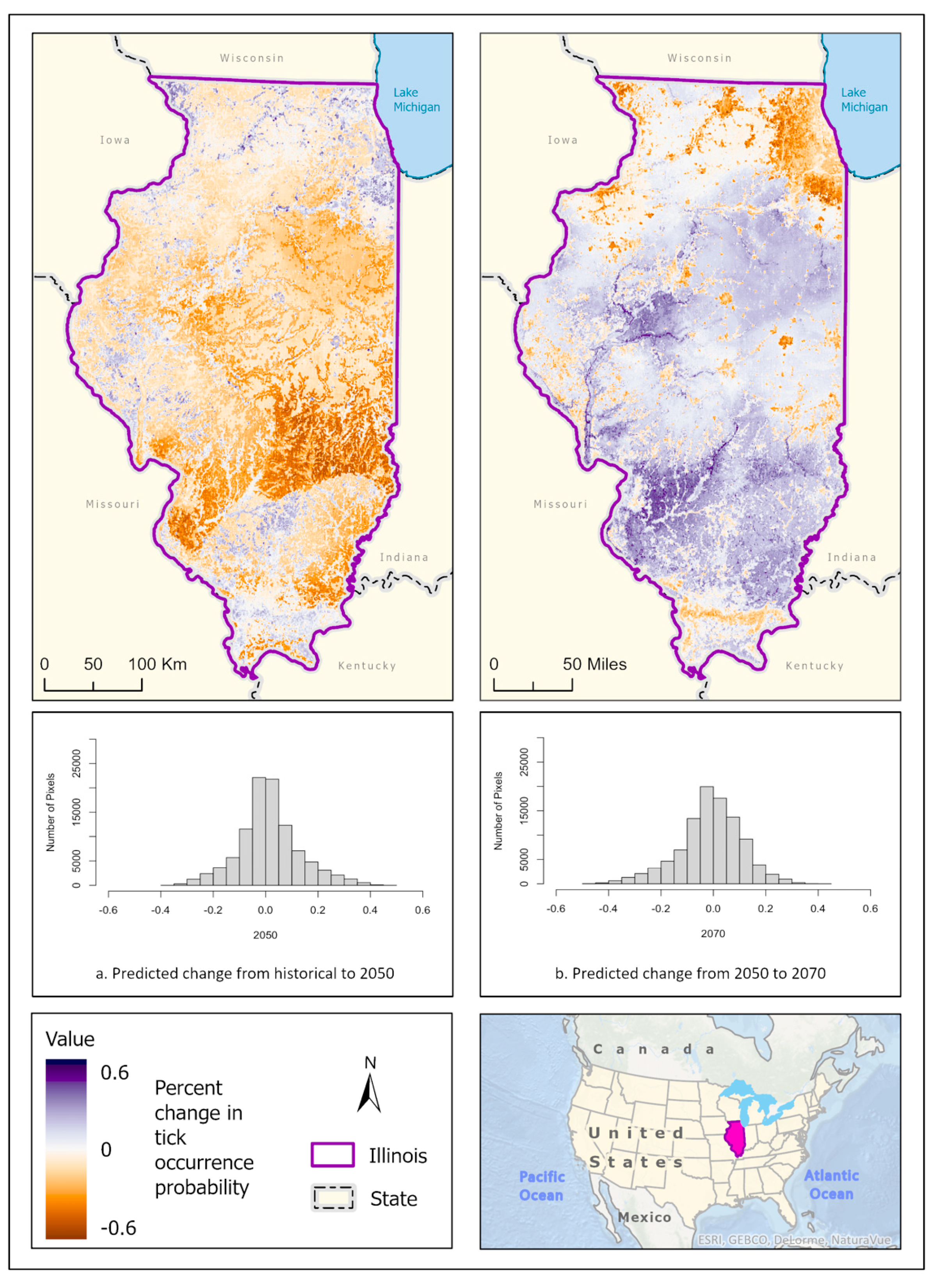
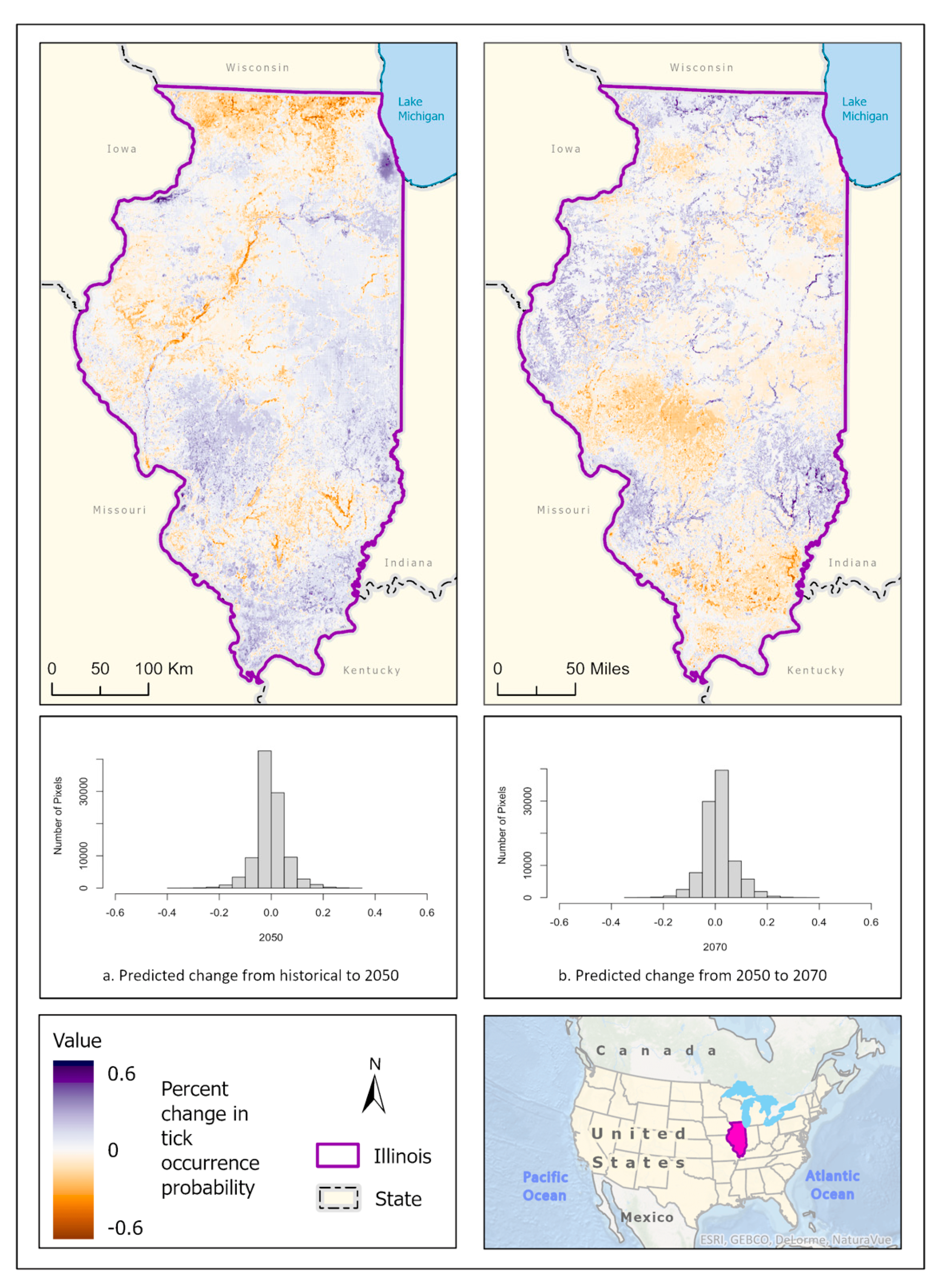
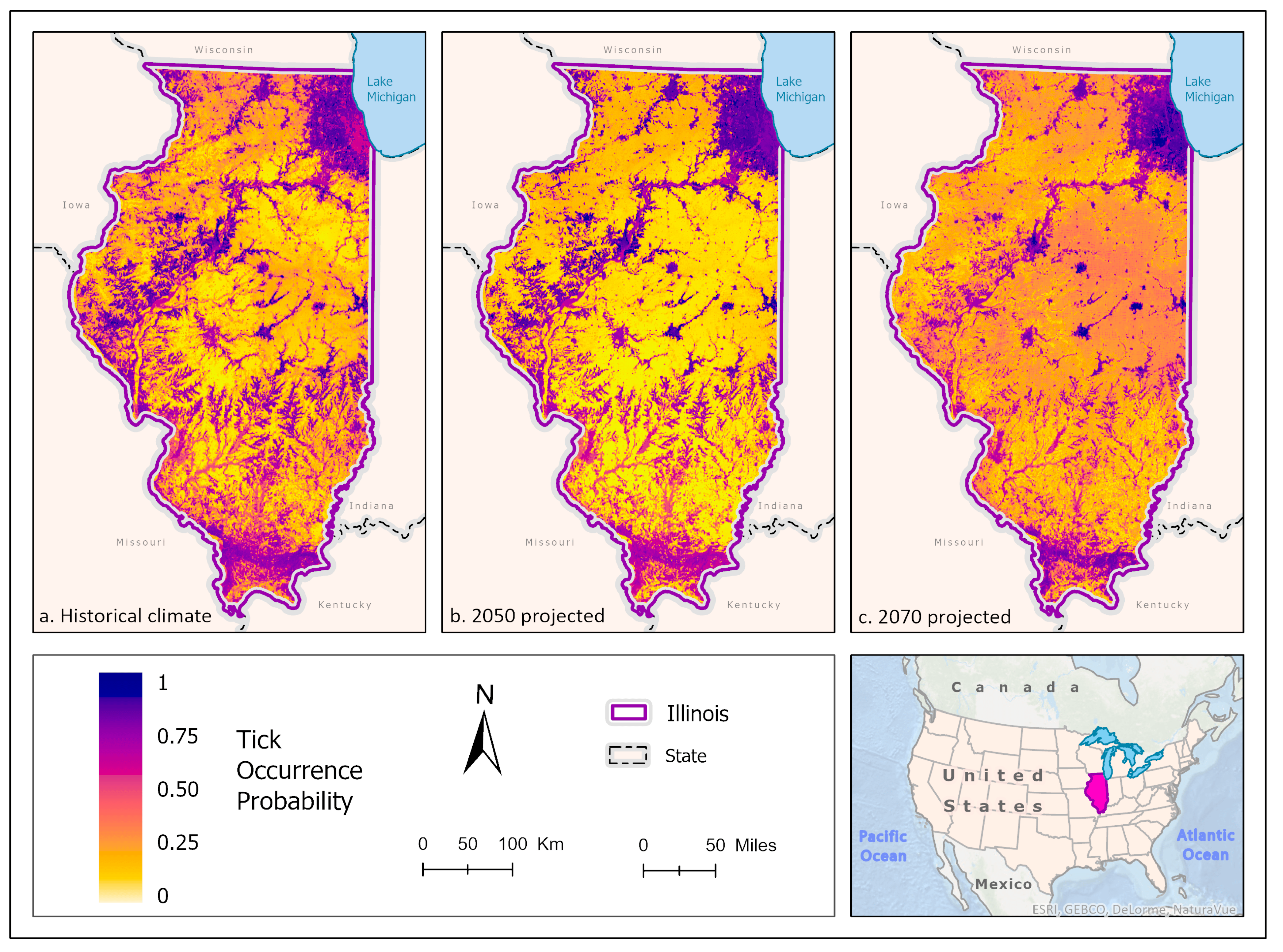
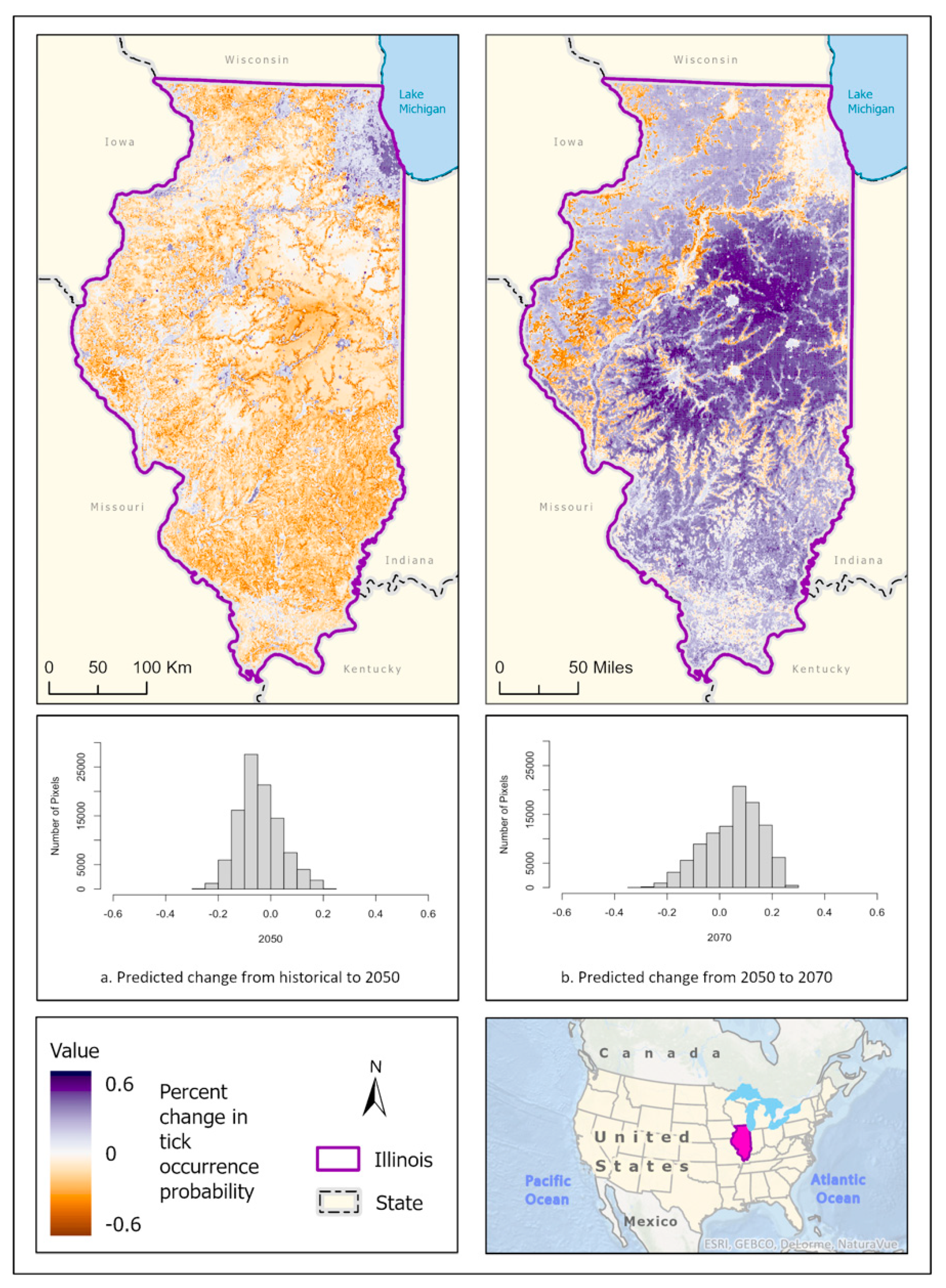
3.1. Ixodes scapularis Models
3.2. Amblyomma americanum Models
3.3. Dermacentor variabilis Models
3.4. Amblyomma maculatum Models
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Diuk-Wasser, M.A.; VanAcker, M.C.; Fernandez, M.P. Impact of Land Use Changes and Habitat Fragmentation on the Eco-Epidemiology of Tick-Borne Diseases. J. Med. Entomol. 2021, 58, 1546–1564. [Google Scholar] [CrossRef] [PubMed]
- Savage, H.M.; Burkhalter, K.L.; Godsey, M.S., Jr.; Panella, N.A.; Ashley, D.C.; Nicholson, W.L.; Lambert, A.J. Bourbon Virus in Field-Collected Ticks, Missouri, USA. Emerg. Infect. Dis. 2017, 23, 2017–2022. [Google Scholar] [CrossRef]
- Paddock, C.D.; Goddard, J. The Evolving Medical and Veterinary Importance of the Gulf Coast Tick (Acari: Ixodidae). J. Med. Entomol. 2015, 52, 230–252. [Google Scholar] [CrossRef]
- Raghavan, R.K.; Koestel, Z.L.; Boorgula, G.; Hroobi, A.; Ganta, R.; Harrington, J., Jr.; Goodin, D.; Stich, R.W.; Anderson, G. Unexpected Winter Questing Activity of Ticks in the Central Midwestern United States. PLoS ONE 2021, 16, e0259769. [Google Scholar] [CrossRef] [PubMed]
- Molaei, G.; Eisen, L.M.; Price, K.J.; Eisen, R.J. Range Expansion of Native and Invasive Ticks, a Looming Public Health Threat. J. Infect. Dis. 2022, 226, 370–373. [Google Scholar] [CrossRef] [PubMed]
- Rochlin, I. Modeling the Asian Longhorned Tick (Acari: Ixodidae) Suitable Habitat in North America. J. Med. Entomol. 2019, 56, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Hook, S.A.; Jeon, S.; Niesobecki, S.A.; Hansen, A.P.; Meek, J.I.; Bjork, J.K.H.; Dorr, F.M.; Rutz, H.J.; Feldman, K.A.; White, J.L.; et al. Economic Burden of Reported Lyme Disease in High-Incidence Areas, United States, 2014–2016. Emerg. Infect. Dis. 2022, 28, 1170–1179. [Google Scholar] [CrossRef]
- Ogden, N.H.; Radojevic, M.; Wu, X.; Duvvuri, V.R.; Leighton, P.A.; Wu, J. Estimated Effects of Projected Climate Change on the Basic Reproductive Number of the Lyme Disease Vector Ixodes Scapularis. Environ. Health Perspect. 2014, 122, 631–638. [Google Scholar] [CrossRef]
- Bacon, E.A.; Kopsco, H.; Gronemeyer, P.; Mateus-Pinilla, N.; Smith, R.L. Effects of Climate on the Variation in Abundance of Three Tick Species in Illinois. J. Med. Entomol. 2022, 59, 700–709. [Google Scholar] [CrossRef]
- Allan, B.F.; Keesing, F.; Ostfeld, R.S. Effect Of Forest Fragmentation on Lyme Disease Risk. Conserv. Biol. 2003, 17, 267–272. [Google Scholar] [CrossRef]
- Brownstein, J.S.; Holford, T.R.; Fish, D. Effect of Climate Change on Lyme Disease Risk in North America. Ecohealth 2005, 2, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Berger, K.A.; Ginsberg, H.S.; Gonzalez, L.; Mather, T.N. Relative Humidity and Activity Patterns of Ixodes Scapularis (Acari: Ixodidae). J. Med. Entomol. 2014, 51, 769–776. [Google Scholar] [CrossRef]
- Berger, K.A.; Ginsberg, H.S.; Dugas, K.D.; Hamel, L.H.; Mather, T.N. Adverse Moisture Events Predict Seasonal Abundance of Lyme Disease Vector Ticks (Ixodes scapularis). Parasites Vectors 2014, 7, 181. [Google Scholar] [CrossRef]
- Ostfeld, R.S.; Brunner, J.L. Climate Change and Ixodes Tick-Borne Diseases of Humans. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20140051. [Google Scholar] [CrossRef] [PubMed]
- Rynkiewicz, E.C.; Clay, K. Tick Community Composition in Midwestern US Habitats in Relation to Sampling Method and Environmental Conditions. Exp. Appl. Acarol. 2014, 64, 109–119. [Google Scholar] [CrossRef]
- Heske, E.J. Mammalian Abundances on Forest-Farm Edges versus Forest Interiors in Southern Illinois: Is There an Edge Effect? J. Mammal. 1995, 76, 562–568. [Google Scholar] [CrossRef]
- Ferrell, A.M.; Brinkerhoff, R.J. Using landscape analysis to test hypotheses about drivers of tick abundance and infection prevalence with Borrelia burgdorferi. Int. J. Environ. Res. Public Health 2018, 15, 737. [Google Scholar] [CrossRef] [PubMed]
- Noden, B.H.; Dubie, T. Involvement of Invasive Eastern Red Cedar (Juniperus virginiana) in the Expansion of Amblyomma Americanum in Oklahoma. J. Vector Ecol. 2017, 42, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Flenniken, J.M.; Tuten, H.C.; Rose Vineer, H.; Phillips, V.C.; Stone, C.M.; Allan, B.F. Environmental Drivers of Gulf Coast Tick (Acari: Ixodidae) Range Expansion in the United States. J. Med. Entomol. 2022, 59, 1625–1635. [Google Scholar] [CrossRef]
- Randolph, S.E.; Storey, K. Impact of Microclimate on Immature Tick-Rodent Host Interactions (Acari: Ixodidae): Implications for Parasite Transmission. J. Med. Entomol. 1999, 36, 741–748. [Google Scholar] [CrossRef]
- Rydzewski, J.; Mateus-Pinilla, N.; Warner, R.E.; Hamer, S.; Weng, H.-Y. Ixodes Scapularis and Borrelia Burgdorferi among Diverse Habitats within a Natural Area in East-Central Illinois. Vector Borne Zoonotic Dis. 2011, 11, 1351–1358. [Google Scholar] [CrossRef]
- Lockwood, B.H.; Stasiak, I.; Pfaff, M.A.; Cleveland, C.A.; Yabsley, M.J. Widespread Distribution of Ticks and Selected Tick-Borne Pathogens in Kentucky (USA). Ticks Tick Borne Dis. 2018, 9, 738–741. [Google Scholar] [CrossRef]
- Springer, Y.P.; Jarnevich, C.S.; Barnett, D.T.; Monaghan, A.J.; Eisen, R.J. Modeling the present and future geographic distribution of the lone star tick, amblyomma americanum (ixodida: Ixodidae), in the continental United States. Am. J. Trop. Med. Hyg. 2015, 93, 875–890. [Google Scholar] [CrossRef]
- Fowler, P.D.; Nguyentran, S.; Quatroche, L.; Porter, M.L.; Kobbekaduwa, V.; Tippin, S.; Miller, G.; Dinh, E.; Foster, E.; Tsao, J.I. Northward Expansion of Amblyomma Americanum (Acari: Ixodidae) into Southwestern Michigan. J. Med. Entomol. 2022, 59, 1646–1659. [Google Scholar] [CrossRef]
- Boorgula, G.D.Y.; Peterson, A.T.; Foley, D.H.; Ganta, R.R.; Raghavan, R.K. Assessing the Current and Future Potential Geographic Distribution of the American Dog Tick, Dermacentor Variabilis (Say) (Acari: Ixodidae) in North America. PLoS ONE 2020, 15, e0237191. [Google Scholar] [CrossRef]
- Martin, J.T.; Fischhoff, I.R.; Castellanos, A.A.; Han, B.A. Ecological Predictors of Zoonotic Vector Status Among Dermacentor Ticks (Acari: Ixodidae): A Trait-Based Approach. J. Med. Entomol. 2022, 59, 2158–2166. [Google Scholar] [CrossRef] [PubMed]
- Phillips, V.C.; Zieman, E.A.; Kim, C.-H.; Stone, C.M.; Tuten, H.C.; Jiménez, F.A. Documentation of the Expansion of the Gulf Coast Tick (Amblyomma Maculatum) and Rickettsia Parkeri: First Report in Illinois. J. Parasitol. 2020, 106, 9–13. [Google Scholar] [CrossRef]
- Alkishe, A.; Peterson, A.T. Climate Change Influences on the Geographic Distributional Potential of the Spotted Fever Vectors Amblyomma Maculatum and Dermacentor Andersoni. PeerJ 2022, 10, e13279. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.J.; Neitzel, D.F.; Moen, R.A.; Craft, M.E.; Hamilton, K.E.; Johnson, L.B.; Mulla, D.J.; Munderloh, U.G.; Redig, P.T.; Smith, K.E.; et al. Disease Risk in a Dynamic Environment: The Spread of Tick-Borne Pathogens in Minnesota, USA. Ecohealth 2015, 12, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.K.H.; Schiffman, E.K.; Davis, J.P.; Neitzel, D.F.; Sloan, L.M.; Nicholson, W.L.; Fritsche, T.R.; Steward, C.R.; Ray, J.A.; Miller, T.K.; et al. Human Infection with Ehrlichia Muris-like Pathogen, United States, 2007–2013(1). Emerg. Infect. Dis. 2015, 21, 1794–1799. [Google Scholar] [CrossRef]
- Tuten, H.C.; Burkhalter, K.L.; Noel, K.R.; Hernandez, E.J.; Yates, S.; Wojnowski, K.; Hartleb, J.; Debosik, S.; Holmes, A.; Stone, C.M. Heartland Virus in Humans and Ticks, Illinois, USA, 2018–2019. Emerg. Infect. Dis. 2020, 26, 1548–1552. [Google Scholar] [CrossRef] [PubMed]
- Sonenshine, D.E. Range Expansion of Tick Disease Vectors in North America: Implications for Spread of Tick-Borne Disease. Int. J. Environ. Res. Public Health 2018, 15, 478. [Google Scholar] [CrossRef] [PubMed]
- Gilliam, B.; Gronemeyer, P.; Chakraborty, S.; Winata, F.; Lyons, L.A.; Miller-Hunt, C.; Tuten, H.C.; Debosik, S.; Freeman, D.; O’hara-Ruiz, M.; et al. Impact of Unexplored Data Sources on the Historical Distribution of Three Vector Tick Species in Illinois. J. Med. Entomol. 2020, 57, 872–883. [Google Scholar] [CrossRef] [PubMed]
- Kopsco, H.L.; Duhaime, R.J.; Mather, T.N. Crowdsourced Tick Image-Informed Updates to U.S. County Records of Three Medically Important Tick Species. J. Med. Entomol. 2021, 58, 2412–2424. [Google Scholar] [CrossRef]
- Illinois Department of Public Health. Reportable Communicable Disease Cases, 1990–1999—Data.illinois.gov [WWW Document]. 2017a. Available online: https://data.illinois.gov/dataset/633reportable_communicable_disease_cases_1990_1999 (accessed on 22 July 2022).
- Illinois Department of Public Health. Reportable Communicable Disease Cases, 2000–2009—Data.illinois.gov [WWW Document]. 2017b. Available online: https://data.illinois.gov/dataset/634reportable_communicable_disease_cases_2000_2009 (accessed on 22 July 2022).
- Illinois Department of Public Health. Reportable Communicable Disease Cases, 2010–2017—Data.illinois.gov [WWW Document]. 2018. Available online: https://data.illinois.gov/dataset/635reportable_communicable_disease_cases_2010_2012 (accessed on 22 July 2022).
- Lyons, L.A.; Brand, M.E.; Gronemeyer, P.; Mateus-Pinilla, N.; Ruiz, M.O.; Stone, C.M.; Tuten, H.C.; Smith, R.L. Comparing Contributions of Passive and Active Tick Collection Methods to Determine Establishment of Ticks of Public Health Concern Within Illinois. J. Med. Entomol. 2021, 58, 1849–1864. [Google Scholar] [CrossRef]
- National Oceanic and Atmospheric Administration/National Centers for Environmental Information, U.S. Climate Divisions. 2022. Available online: https://www.ncei.noaa.gov/access/monitoring/dyk/us-climate-divisions (accessed on 20 May 2022).
- Ogden, N.H.; Ben Beard, C.; Ginsberg, H.S.; Tsao, J.I. Possible Effects of Climate Change on Ixodid Ticks and the Pathogens They Transmit: Predictions and Observations. J. Med. Entomol. 2020, 58, 1536–1545. [Google Scholar] [CrossRef]
- Lippi, C.A.; Gaff, H.D.; White, A.L.; St John, H.K.; Richards, A.L.; Ryan, S.J. Exploring the Niche of Rickettsia Montanensis (Rickettsiales: Rickettsiaceae) Infection of the American Dog Tick (Acari: Ixodidae), Using Multiple Species Distribution Model Approaches. J. Med. Entomol. 2021, 58, 1083–1092. [Google Scholar] [CrossRef]
- Kopsco, H.L.; Smith, R.L.; Halsey, S.J. A Scoping Review of Species Distribution Modeling Methods for Tick Vectors. Front. Ecol. Evol. 2022, 10, 893016. [Google Scholar] [CrossRef]
- VectorMap—Walter Reed Biosystematics Unit. Available online: https://vectormap.si.edu/ (accessed on 8 June 2022).
- Global Biodiversity Information Facility (GBIF). gbif.org. GBIF Occurrence Download. Available online: https://doi.org/10.15468/dl.dva6ha (accessed on 9 June 2022).
- Biodiversity Information Serving Our Nation (BISON). Science Analytics and Synthesis Program of the U.S. Geological Survey (USGS), Biodiversity Information Serving Our Nation (BISON): U.S. Geological Survey (9 December 2021). Available online: https://www.sciencebase.gov/catalog/item/5138e8e5e4b02c509e50c57f (accessed on 10 June 2022).
- Aiello-Lammens, M.E.; Boria, R.A.; Radosavljevic, A.; Vilela, B.; Anderson, R.P. spThin: An R Package for Spatial Thinning of Species Occurrence Records for Use in Ecological Niche Models. Ecography 2015, 38, 541–545. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km Spatial Resolution Climate Surfaces for Global Land Areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Ghosh, A.; Mandel, A. _geodata: Download Geographic Data_. R Package Version 0.5-3. 2022. Available online: https://CRAN.R-project.org/package=geodata (accessed on 1 June 2022).
- EC-Earth Consortium (EC-Earth). EC-Earth-Consortium EC-Earth3-Veg Model Output Prepared for CMIP6 ScenarioMIP ssp585; Version 20221223; Earth System Grid Federation: Seattle, WA, USA, 2022. [Google Scholar] [CrossRef]
- Döscher, R.; Acosta, M.; Alessandri, A.; Anthoni, P.; Arsouze, T.; Bergman, T.; Bernardello, R.; Boussetta, S.; Caron, L.-P.; Carver, G.; et al. The EC-Earth3 Earth System Model for the Coupled Model Intercomparison Project 6. Geosci. Model Dev. 2022, 15, 2973–3020. [Google Scholar] [CrossRef]
- Ashfaq, M.; Rastogi, D.; Kitson, J.; Abid, M.A.; Kao, S.-C. Evaluation of CMIP6 GCMs over the CONUS for Downscaling Studies. J. Geophys. Res. 2022, 127, e2022JD036659. [Google Scholar] [CrossRef]
- Meinshausen, M.; Nicholls, Z.R.J.; Lewis, J.; Gidden, M.J.; Vogel, E.; Freund, M.; Beyerle, U.; Gessner, C.; Nauels, A.; Bauer, N.; et al. The Shared Socio-Economic Pathway (SSP) Greenhouse Gas Concentrations and Their Extensions to 2500. Geosci. Model Dev. 2020, 13, 3571–3605. [Google Scholar] [CrossRef]
- U.S. Geological Survey (USGS)—Gap Analysis Project (GAP). White-tailed Deer (Odocoileus virginianus) mWTDEx_CONUS_2001v1 Habitat Map: U.S. Geological Survey Data Release. 2018. Available online: https://doi.org/10.5066/F7SF2TM0 (accessed on 1 June 2022).
- Dewitz, J.; U.S. Geological Survey. National Land Cover Database (NLCD) 2019 Products (ver. 2.0, June 2021): U.S. Geological Survey Data Release. 2021. Available online: https://doi.org/10.5066/P9KZCM54 (accessed on 1 June 2022).
- Hijmans, R.J. Raster: Geographic Data Analysis and Modeling. R Package Version 3.5-15. 2022. Available online: https://CRAN.R-project.org/package=raster (accessed on 1 June 2022).
- USGS EROS Archive—Digital Elevation—Shuttle Radar Topography Mission (SRTM) Void Filled. 2018. Available online: https://www.usgs.gov/centers/eros/science/usgs-eros-archive-digital-elevation-shuttle-radar-topography-mission-srtm-void?qt-science_center_objects=0 (accessed on 10 June 2022).
- Naimi, B.; Araújo, M.B. Sdm: A Reproducible and Extensible R Platform for Species Distribution Modelling. Ecography 2016, 39, 368–375. [Google Scholar] [CrossRef]
- The R Development Core Team. R: A Language and Environment for Statistical Computing, R Found. Stat. Comput. 2022. Available online: http://www.r-project.org (accessed on 1 June 2022).
- Barbet-Massin, M.; Jiguet, F.; Albert, C.H.; Thuiller, W. Selecting Pseudo-Absences for Species Distribution Models: How, Where and How Many? Methods Ecol. Evol. 2012, 3, 327–338. [Google Scholar] [CrossRef]
- Grimmett, L.; Whitsed, R.; Horta, A. Presence-Only Species Distribution Models Are Sensitive to Sample Prevalence: Evaluating Models Using Spatial Prediction Stability and Accuracy Metrics. Ecol. Model. 2020, 431, 109194. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the Accuracy of Species Distribution Models: Prevalence, Kappa and the True Skill Statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Jobe, D.A.; Nelson, J.A.; Adam, M.D.; Martin, S.A., Jr. Lyme Disease in Urban Areas, Chicago. Emerg. Infect. Dis. 2007, 13, 1799–1800. [Google Scholar] [CrossRef]
- Rydzewski, J.; Mateus-Pinilla, N.; Warner, R.E.; Nelson, J.A.; Velat, T.C. Ixodes Scapularis (Acari: Ixodidae) Distribution Surveys in the Chicago Metropolitan Region. J. Med. Entomol. 2012, 49, 955–959. [Google Scholar] [CrossRef]
- Guerra, M.A.; Walker, E.D.; Kitron, U. Canine Surveillance System for Lyme Borreliosis in Wisconsin and Northern Illinois: Geographic Distribution and Risk Factor Analysis. Am. J. Trop. Med. Hyg. 2001, 65, 546–552. [Google Scholar] [CrossRef]
- Guerra, M.; Walker, E.; Jones, C.; Paskewitz, S.; Cortinas, M.R.; Stancil, A.; Beck, L.; Bobo, M.; Kitron, U. Predicting the Risk of Lyme Disease: Habitat Suitability for Ixodes Scapularis in the North Central United States. Emerg. Infect. Dis. 2002, 8, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Lippi, C.A.; Gaff, H.D.; White, A.L.; Ryan, S.J. Scoping Review of Distribution Models for Selected Amblyomma Ticks and Rickettsial Group Pathogens. PeerJ 2021, 9, e10596. [Google Scholar] [CrossRef]
- Alkishe, A.; Raghavan, R.K.; Peterson, A.T. Likely Geographic Distributional Shifts among Medically Important Tick Species and Tick-Associated Diseases under Climate Change in North America: A Review. Insects 2021, 12, 225. [Google Scholar] [CrossRef] [PubMed]
- Strobl, C.; Malley, J.; Tutz, G. An Introduction to Recursive Partitioning: Rationale, Application, and Characteristics of Classification and Regression Trees, Bagging, and Random Forests. Psychol. Methods 2009, 14, 323–348. [Google Scholar] [CrossRef] [PubMed]
- Valavi, R.; Elith, J.; Lahoz-Monfort, J.J.; Guillera-Arroita, G. Modelling Species Presence-only Data with Random Forests. Ecography 2021, 44, 1731–1742. [Google Scholar] [CrossRef]
- Drake, J.M.; Randin, C.; Guisan, A. Modelling Ecological Niches with Support Vector Machines. J. Appl. Ecol. 2006, 43, 424–432. [Google Scholar] [CrossRef]
- Valavi, R.; Guillera-Arroita, G.; Lahoz-Monfort, J.J.; Elith, J. Predictive Performance of Presence-only Species Distribution Models: A Benchmark Study with Reproducible Code. Ecol. Monogr. 2022, 92, e01486. [Google Scholar] [CrossRef]
- Hahn, M.B.; Jarnevich, C.S.; Monaghan, A.J.; Eisen, R.J. Modeling the Geographic Distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the Contiguous United States. J. Med. Entomol. 2016, 53, 1176–1191. [Google Scholar] [CrossRef] [PubMed]
- Eisen, R.J.; Eisen, L.; Beard, C.B. County-Scale Distribution of Ixodes Scapularis and Ixodes Pacificus (Acari: Ixodidae) in the Continental United States. J. Med. Entomol. 2016, 53, 349–386. [Google Scholar] [CrossRef]
- Wikel, S.K. Changing Geographic Ranges of Human Biting Ticks and Implications for Tick-Borne Zoonoses in North America. Zoonotic Diseases 2022, 2, 126–146. [Google Scholar] [CrossRef]
- Illinois Department of Public Health. Reported Tickborne Cases 2011–2021 [WWW Document]. 2022. Available online: https://dph.illinois.gov/topics-services/diseases-and-conditions/diseases-a-z-list/lyme-disease/data/tickborne-cases-2011-2021.html (accessed on 8 November 2022).
- Kilpatrick, A.M.; Dobson, A.D.M.; Levi, T.; Salkeld, D.J.; Swei, A.; Ginsberg, H.S.; Kjemtrup, A.; Padgett, K.A.; Jensen, P.M.; Fish, D.; et al. Lyme Disease Ecology in a Changing World: Consensus, Uncertainty and Critical Gaps for Improving Control. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017, 372, 20160117. [Google Scholar] [CrossRef] [PubMed]
- McBride, S.E.; Lieberthal, B.A.; Buttke, D.E.; Cronk, B.D.; De Urioste-Stone, S.M.; Goodman, L.B.; Guarnieri, L.D.; Rounsville, T.F.; Gardner, A.M. Patterns and Ecological Mechanisms of Tick-Borne Disease Exposure Risk in Acadia National Park, Mount Desert Island, Maine, United States. J. Med. Entomol. 2022, 60, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Levi, T.; Keesing, F.; Oggenfuss, K.; Ostfeld, R.S. Accelerated Phenology of Blacklegged Ticks under Climate Warming. Philos. Trans. R. Soc. B: Biol. Sci. 2015, 370, 20130556. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Lun, X.; Li, C.; Zhou, R.; Zhao, Z.; Wang, J.; Zhang, Q.; Liu, Q. Predicting the Potential Global Distribution of Amblyomma Americanum (Acari: Ixodidae) under Near Current and Future Climatic Conditions, Using the Maximum Entropy Model. Biology 2021, 10, 1057. [Google Scholar] [CrossRef] [PubMed]
- Rochlin, I.; Egizi, A.; Lindström, A. The Original Scientific Description of the Lone Star Tick (Amblyomma Americanum, Acari: Ixodidae) and Implications for the Species’ Past and Future Geographic Distributions. J. Med. Entomol. 2022, 59, 412–420. [Google Scholar] [CrossRef]
- Goddard, J.; Varela-Stokes, A.S. Role of the Lone Star Tick, Amblyomma americanum (L.), in Human and Animal Diseases. Vet. Parasitol. 2009, 160, 1–12. [Google Scholar] [CrossRef]
- Paddock, C.D.; Yabsley, M.J. Ecological Havoc, the Rise of White-Tailed Deer, and the Emergence of Amblyomma Americanum-Associated Zoonoses in the United States. Curr. Top. Microbiol. Immunol. 2007, 315, 289–324. [Google Scholar] [CrossRef]
- Rochlin, I.; Egizi, A.; Ginsberg, H.S. Modeling of Historical and Current Distributions of Lone Star Tick, Amblyomma Americanum (Acari: Ixodidae), Is Consistent with Ancestral Range Recovery. Exp. Appl. Acarol. 2022, 89, 85–103. [Google Scholar] [CrossRef]
- de Oliveira, S.V.; Romero-Alvarez, D.; Martins, T.F.; dos Santos, J.P.; Labruna, M.B.; Gazeta, G.S.; Escobar, L.E.; Gurgel-Gonçalves, R. Amblyomma Ticks and Future Climate: Range Contraction due to Climate Warming. Acta Trop. 2017, 176, 340–348. [Google Scholar] [CrossRef]
- Carson, D.A.; Kopsco, H.; Gronemeyer, P.; Mateus-Pinilla, N.; Smith, G.S.; Sandstrom, E.N.; Smith, R.L. Knowledge, Attitudes, and Practices of Illinois Medical Professionals Related to Ticks and Tick-Borne Disease. One Health 2022, 15, 100424. [Google Scholar] [CrossRef]
- Bayles, B.R.; Evans, G.; Allan, B.F. Knowledge and Prevention of Tick-Borne Diseases Vary across an Urban-to-Rural Human Land-Use Gradient. Ticks Tick Borne Dis. 2013, 4, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Soucy, A.; de Urioste-Stone, S. Tourist Behaviour and Tick-Borne Disease Risk. In WIT Transactions on Ecology and the Environment; WIT Press: Southampton, UK, 2020; Volume 248, pp. 77–88. ISBN 9781784664077. [Google Scholar]
- 6th National Risk Assessment of Hazardous Heat [WWW Document]. FirstStreet. Available online: https://firststreet.org/research-lab/published-research/article-highlights-from-hazardous-heat/ (accessed on 11 December 2022).
- Raghavan, R.K.; Goodin, D.G.; Hanzlicek, G.A.; Zolnerowich, G.; Dryden, M.W.; Anderson, G.A.; Ganta, R.R. Maximum Entropy-Based Ecological Niche Model and Bio-Climatic Determinants of Lone Star Tick (Amblyomma americanum) Niche. Vector Borne Zoonotic Dis. 2016, 16, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, R.K.; Peterson, A.T.; Cobos, M.E.; Ganta, R.; Foley, D. Current and Future Distribution of the Lone Star Tick, Amblyomma americanum (L.) (Acari: Ixodidae) in North America. PLoS ONE 2019, 14, e0209082. [Google Scholar] [CrossRef] [PubMed]
- Gandy, S.; Kilbride, E.; Biek, R.; Millins, C.; Gilbert, L. No Net Effect of Host Density on Tick-borne Disease Hazard due to Opposing Roles of Vector Amplification and Pathogen Dilution. Ecol. Evol. 2022, 12, e9253. [Google Scholar] [CrossRef] [PubMed]
- Esri Land Cover 2050. Available online: https://livingatlas.arcgis.com/landcover-2050/ (accessed on 8 August 2022).
- Booth, T.H. Checking Bioclimatic Variables That Combine Temperature and Precipitation Data before Their Use in Species Distribution Models. Austral Ecol. 2022, 47, 1506–1514. [Google Scholar] [CrossRef]




| VARIABLE | DESCRIPTION | Unit | Source * |
|---|---|---|---|
| BIO1 | Annual Mean Temperature | °C | WorldClim 2.1 |
| BIO2 | Mean Diurnal Range (Mean of monthly (max temp—min temp)) | °C | WorldClim 2.1 |
| BIO3 | Isothermality (BIO2/BIO7) (×100) | % | WorldClim 2.1 |
| BIO4 | Temperature Seasonality (standard deviation ×100) | °C | WorldClim 2.1 |
| BIO5 | Max Temperature of Warmest Month | °C | WorldClim 2.1 |
| BIO6 | Min Temperature of Coldest Month | °C | WorldClim 2.1 |
| BIO7 | Temperature Annual Range (BIO5-BIO6) | °C | WorldClim 2.1 |
| BIO8 | Mean Temperature of Wettest Quarter | °C | WorldClim 2.1 |
| BIO9 | Mean Temperature of Driest Quarter | °C | WorldClim 2.1 |
| BIO10 | Mean Temperature of Warmest Quarter | °C | WorldClim 2.1 |
| BIO11 | Mean Temperature of Coldest Quarter | °C | WorldClim 2.1 |
| BIO12 | Annual Precipitation | mm | WorldClim 2.1 |
| BIO13 | Precipitation of Wettest Month | mm | WorldClim 2.1 |
| BIO14 | Precipitation of Driest Month | mm | WorldClim 2.1 |
| BIO15 | Precipitation Seasonality | % | WorldClim 2.1 |
| BIO16 | Precipitation of Wettest Quarter | mm | WorldClim 2.1 |
| BIO17 | Precipitation of Driest Quarter | mm | WorldClim 2.1 |
| BIO18 | Precipitation of Warmest Quarter | mm | WorldClim 2.1 |
| BIO19 | Precipitation of Coldest Quarter | mm | WorldClim 2.1 |
| Elevation | Height above sea level | m | USGS SRTM |
| Deer habitat | Suitable white-tailed deer habitat | Presence/absence | USGS GAP Analysis |
| Landcover CLASS | Water, developed, impervious, barren, forest, grassland, cropland, wetland | % | NLCD 2019 |
| Species | Ixodes scapularis | Amblyomma americanum | Dermacentor variabilis | Amblyomma maculatum | Ixodes scapularis | Amblyomma americanum | Dermacentor variabilis | Amblyomma maculatum | Ixodes scapularis | Amblyomma americanum | Dermacentor variabilis | Amblyomma maculatum | Ixodes scapularis | Amblyomma americanum | Dermacentor variabilis | Amblyomma maculatum | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of occurrence (presence) points | 62 | 99 | 290 | 15 | 62 | 99 | 290 | 15 | 62 | 99 | 290 | 15 | 62 | 99 | 290 | 15 | |
| Number of pseudo-absence points | 70 | 100 | 300 | 20 | 70 | 100 | 300 | 20 | 70 | 100 | 300 | 20 | 70 | 100 | 300 | 20 | |
| Evaluation Metric | AUC | Correlation | TSS | Deviance | |||||||||||||
| Algorithm * | GLM | 0.88 | 0.86 | 0.84 | 0.60 | 0.67 | 0.64 | 0.61 | 0.21 | 0.71 | 0.67 | 0.71 | 0.45 | 1.85 | 1.13 | 1.02 | 7.26 |
| BRT | 0.89 | 0.89 | 0.85 | - | 0.68 | 0.68 | 0.63 | - | 0.72 | 0.72 | 0.63 | - | 0.99 | 1.00 | 1.04 | - | |
| CART | 0.81 | - | 0.81 | 0.70 | 0.57 | - | 0.56 | 0.36 | 0.59 | - | 0.57 | 0.47 | 1.46 | - | 1.21 | 2.68 | |
| MaxEnt | 0.88 | 0.87 | 0.87 | 0.75 | 0.66 | 0.64 | 0.65 | 0.43 | 0.71 | 0.68 | 0.65 | 0.64 | 0.96 | 0.97 | 0.93 | 1.38 | |
| RF | 0.89 | 0.89 | 0.87 | 0.76 | 0.68 | 0.69 | 0.65 | 0.43 | 0.72 | 0.71 | 0.65 | 0.61 | 0.87 | 0.85 | 0.90 | 1.29 | |
| MARS | 0.74 | 0.81 | 0.84 | 0.66 | 0.44 | 0.55 | 0.60 | 0.28 | 0.49 | 0.59 | 0.59 | 0.41 | 13.0 | 3.32 | 1.09 | 21.8 | |
| SVM | 0.89 | 0.86 | 0.86 | 0.80 | 0.68 | 0.64 | 0.64 | 0.54 | 0.72 | 0.66 | 0.63 | 0.71 | 0.88 | 0.94 | 0.94 | 1.32 | |
| Environmental Variable * | Tick Species | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ixodes scapularis | Amblyomma americanum | Dermacentor variabilis | Amblyomma maculatum | |||||||||
| Climate Scenario (SSP 8.5) | ||||||||||||
| Hist. | 2050 | 2070 | Hist. | 2050 | 2070 | Hist. | 2050 | 2070 | Hist. | 2050 | 2070 | |
| Barren | 2.5 | 1.3 | 1.5 | 1.7 | 2.8 | 1.4 | 1.0 | 1.7 | 0.5 | 2.6 | 2.3 | 9.8 |
| Cropland | 1.0 | 1.2 | 1.5 | 1.9 | 1.3 | 4.5 | 0.7 | 0.6 | 0.8 | 10.0 | 3.1 | 8.6 |
| Developed | 0.7 | 1.5 | 0.9 | - | - | - | 1.6 | 1.2 | 0.8 | 10.3 | 6.2 | 7.6 |
| Deer Habitat | - | - | - | 3.9 | 2.1 | 2.4 | - | - | - | - | - | - |
| Elevation | 1.8 | 2.1 | 0.6 | 3.1 | 3.1 | 6.2 | 1.0 | 3.8 | 1.2 | - | - | - |
| Forest | 16.5 | 27.2 | 28.0 | 11.8 | 21.8 | 11.8 | 30.4 | 26.5 | 30.0 | 29.1 | 50.0 | 45.3 |
| Grass/Shrub | 3.9 | 2.8 | 1.7 | 0.5 | 1.0 | 0.7 | 0.8 | 0.5 | 0.4 | 2.3 | 1.6 | 7.7 |
| Water | 1.0 | 0.8 | 0.7 | 3.6 | 0.3 | 0.2 | 0.5 | 0.5 | 0.4 | 1.7 | 0.6 | 0.6 |
| Wetland | 10.8 | 6.3 | 13.6 | 17.9 | 4.1 | 7.3 | 12.7 | 12.3 | 11.1 | 10.1 | 0.9 | 13.1 |
| BIO2 | - | - | - | 1.8 | 4.2 | 3.1 | 2.5 | 3.4 | 4.2 | - | - | - |
| BIO5 | 1.5 | 1.7 | 2.1 | - | - | - | - | - | - | - | ||
| BIO7 | 0.7 | 0.6 | 2.1 | - | - | - | 0.7 | 1.4 | 1.7 | - | - | - |
| BIO8 | 1.5 | 0.5 | 0.9 | 0.9 | 1.1 | 0.4 | 1.1 | 1.1 | 0.6 | - | - | - |
| BIO9 | 0.6 | 1.2 | 2.5 | 0.9 | 0.9 | 0.8 | 0.7 | 0.8 | 0.5 | - | - | - |
| BIO10 | - | - | - | - | - | - | 0.8 | 0.7 | 0.6 | - | - | - |
| BIO13 | - | 1.7 | 0.9 | 1.3 | 0.7 | 0.8 | 0.7 | 0.9 | 0.3 | - | - | - |
| BIO15 | - | - | - | 2.4 | 2.9 | 1.2 | - | - | - | 2.8 | 3.9 | 6.8 |
| BIO18 | 3.0 | 2.0 | 3.0 | 0.5 | 0.3 | 0.8 | 0.9 | 1.2 | 1.2 | 2.8 | 7.8 | 10.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kopsco, H.L.; Gronemeyer, P.; Mateus-Pinilla, N.; Smith, R.L. Current and Future Habitat Suitability Models for Four Ticks of Medical Concern in Illinois, USA. Insects 2023, 14, 213. https://doi.org/10.3390/insects14030213
Kopsco HL, Gronemeyer P, Mateus-Pinilla N, Smith RL. Current and Future Habitat Suitability Models for Four Ticks of Medical Concern in Illinois, USA. Insects. 2023; 14(3):213. https://doi.org/10.3390/insects14030213
Chicago/Turabian StyleKopsco, Heather L., Peg Gronemeyer, Nohra Mateus-Pinilla, and Rebecca L. Smith. 2023. "Current and Future Habitat Suitability Models for Four Ticks of Medical Concern in Illinois, USA" Insects 14, no. 3: 213. https://doi.org/10.3390/insects14030213
APA StyleKopsco, H. L., Gronemeyer, P., Mateus-Pinilla, N., & Smith, R. L. (2023). Current and Future Habitat Suitability Models for Four Ticks of Medical Concern in Illinois, USA. Insects, 14(3), 213. https://doi.org/10.3390/insects14030213






