Tick Densities and Infection Prevalence on Coastal Islands in Massachusetts, USA: Establishing a Baseline
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Drag Sampling
2.3. DNA Analyses for Species Identification and Pathogen Prevalence
2.4. Vegetation Surveys
3. Results
3.1. Tick Species Other than I. scapularis and A. americanum
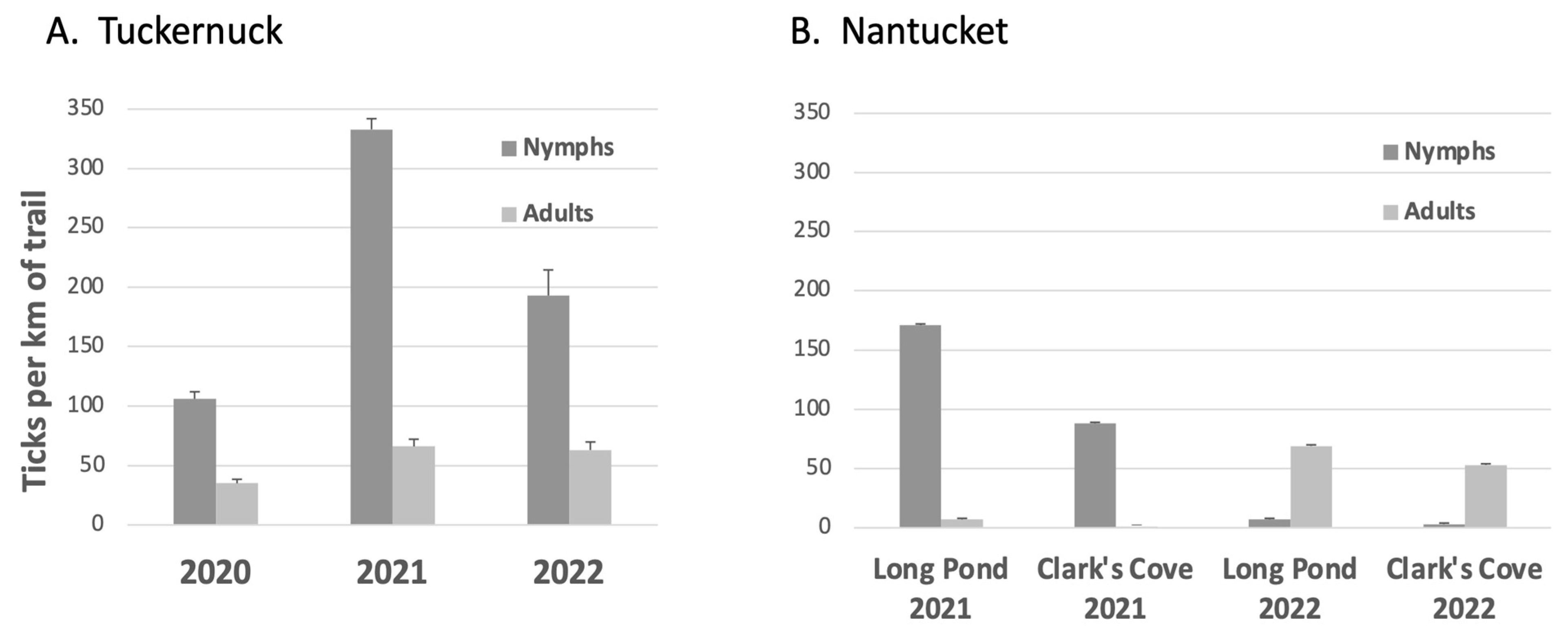
3.2. Densities of Ixodes Nymphs
3.3. Densities of Lone Star Ticks
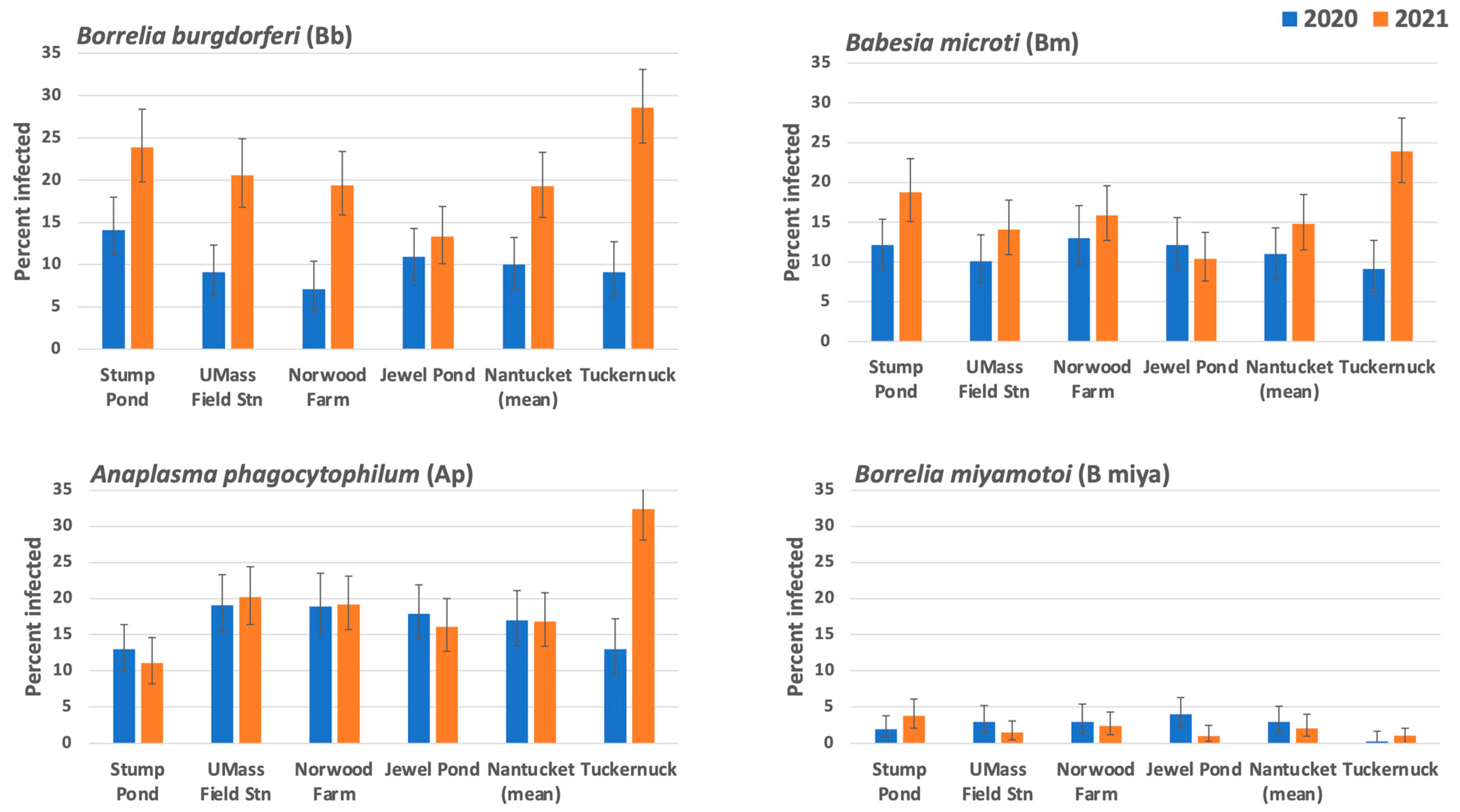
3.4. Infection Prevalence in Ixodes scapularis
3.4.1. Comparisons among Sites on Nantucket
3.4.2. Comparisons between Nantucket and Tuckernuck
4. Discussion
4.1. Densities of Ixodes Nymphs
4.2. Densities of Lone Star Ticks
4.3. Infection Prevalence in Ixodes scapularis Nymphs
4.4. Coinfections in Ixodes scapularis Nymphs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Diuk-Wasser, M.A.; Liu, Y.; Steeves, T.K.; Folsom-O’Keefe, C.; Dardick, K.R.; Lepore, T.; Bent, S.J.; Usmani-Brown, S.; Telford, S.R., III; Fish, D.; et al. Monitoring human babesiosis emergence through vector surveillance, New England, USA. Emerg. Infect. Dis. 2014, 20, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Nelson, C.A.; Saha, S.; Kugeler, K.J.; Delorey, M.J.; Shankar, M.B.; Hinckley, A.F.; Mead, P.S. Incidence of clinician-diagnosed Lyme disease, United States, 2005–2010. Emerg. Infect. Dis. 2015, 21, 1625–1631. [Google Scholar] [CrossRef]
- Russell, A.M.; Prusinski, M.; Sommer, J.; O’Connor, C.; White, J.; Falco, R.; Kokas, J.; Vinci, V.; Gall, W.; Tober, K.; et al. Epidemiology and spatial emergence of anaplasmosis, New York, USA, 2010–2018. Emerg. Infect. Dis. 2021, 27, 2154–2162. [Google Scholar] [CrossRef]
- CDC (Centers for Disease Control and Prevention). Lyme Disease. 2023. Available online: https://www.cdc.gov/lyme/index.html (accessed on 9 July 2023).
- Lane, R.H.; Piesman, J.; Burgdorfer, W. Lyme borreliosis: Relation of its causative agent to its vectors and hosts in North America and Europe. Ann. Rev. Entomol. 1991, 36, 587–609. [Google Scholar] [CrossRef]
- Eisen, R.J.; Eisen, L. The blacklegged tick, Ixodes scapularis: An increasing public health concern. Trends. Parasitol. 2018, 34, 295–309. [Google Scholar] [CrossRef]
- Schulze, T.L.; Jordan, R.A.; Healy, S.P.; Roegner, V.E.; Meddis, M.; Jahn, M.B.; Guthrie, D.L. Relative abundance and prevalence of selected Borrelia infections in Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae) from publicly owned lands in Monmouth County, New Jersey. J. Med. Entomol. 2006, 43, 1269–1275. [Google Scholar]
- Stafford, K.C., III; Molaei, G.; Little, E.A.H.; Paddock, C.D.; Karpathy, S.E.; Labonte, A.M. Distribution and ecology of the lone star tick in Connecticut and implications for range expansion and public health. J. Med. Entomol. 2018, 55, 1561–1568. [Google Scholar] [CrossRef]
- Fowler, P.D.; Nguyentran, S.; Quatroche, L.; Porter, M.L.; Kobbekaduwa, V.; Tippin, S.; Miller, G.; Dinh, E.; Foster, E.; Tsao, J.I. Northward expansion of Amblyomma americanum (Acari: Ixodidae) into southwestern Michigan. J. Med. Entomol. 2022, 59, 1646–1659. [Google Scholar] [CrossRef] [PubMed]
- Rochlin, I.; Egizi, A.; Lindstrom, A. The original scientific description of the lone star tick (Amblyomma americanum, Acari: Ixodidae) and implications for the species’ past and future geographic distributions. J. Med. Entomol. 2022, 59, 412–420. [Google Scholar] [CrossRef]
- Molaei, G.; Little, E.A.H.; Williams, S.C.; Stafford, K.C., III. Bracing for the worst–range expansion of the lone star tick in the northeastern United States. N. Eng. J. Med. 2019, 381, 2189–2192. [Google Scholar] [CrossRef] [PubMed]
- Dupuis, A.P.; Prusinski, M.A.; O’connor, C.; Maffei, J.G.; Koetzner, C.A.; Zembsch, T.E.; Zink, S.D.; White, A.L.; Santoriello, M.P.; Romano, C.L.; et al. Bourbon virus transmission, New York, USA. Emerg. Infect. Dis. 2023, 29, 145–148. [Google Scholar] [CrossRef]
- Wellins, A. Tick associated syndrome: The alpha gal meat allergy, identification, treatment, and prevention. N. Horiz. Med. Med. Res. 2022, 2, 1–11. [Google Scholar] [CrossRef]
- LoGiudice, K.; Ostfeld, R.S.; Schmidt, K.A.; Keesing, F. The ecology of infectious disease: Effects of host diversity and community composition on Lyme disease risk. Proc. Nat. Acad. Sci. USA 2003, 100, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Ostfeld, R.S.; Taal, L.; Keesing, F.; Oggenfuss, K.; Canham, C.D. Tick-borne disease risk in a forest food web. Ecology 2018, 99, 1562–1573. [Google Scholar] [CrossRef]
- Huang, C.-I.; Kay, S.C.; Davis, S.; Tufts, D.M.; Gaffett, K.; Tefft, B.; Diuk-Wasser, M.A. High burdens of Ixodes scapularis larval ticks on white-tailed deer may limit Lyme disease risk in a low biodiversity setting. Ticks Tick Borne Dis. 2019, 10, 258–268. [Google Scholar] [CrossRef]
- Kierans, J.E.; Hutcheson, H.J.; Durden, L.A.; Klompen, J.S.H. Ixodes (Ixodes) scapularis (Acari: Ixodidae): Redescription of all active stages, distribution, hosts, geographical variation, and medical and veterinary importance. J. Med. Entomol. 1996, 33, 297–318. [Google Scholar] [CrossRef]
- Telford, S.R., III. Deer reduction is a cornerstone of integrated deer tick management. J. Integr. Pest Manag. 2017, 8, 25. [Google Scholar] [CrossRef]
- Brisson, D.D.; Dykhuizen, E.; Ostfeld, R.S. Conspicuous impacts of inconspicuous hosts on the Lyme disease epidemic. Proc. Royal Soc. B 2008, 275, 227–235. [Google Scholar] [CrossRef]
- Piesman, J.; Mather, T.N.; Dammin, G.J.; Telford, S.R., III; Lastavica, C.C.; Spielman, A. Seasonal variation of transmission risk of Lyme disease and human babesiosis. Am. J. Epidemiol. 1987, 128, 1187–1189. [Google Scholar] [CrossRef] [PubMed]
- Eisen, L.; Dolan, M.C. Evidence for personal protective measures to reduce human contact with blacklegged ticks and for environmentally based control methods to suppress host-seeking blacklegged ticks and reduce infection with Lyme disease spirochetes in tick vectors and rodent reservoirs. J. Med. Entomol. 2016, 53, 1063–1092. [Google Scholar]
- Telford, S.R., III; Buchthal, J.; Early, E.P. Early questing by lone star tick larvae, New York and Massachusetts, USA. Emerg. Infect. Dis. 2019, 25, 1592–1593. [Google Scholar] [CrossRef]
- Goethert, H.K.; Mather, T.N.; Johnson, R.W.; Telford, S.R., III. Incrimination of shrews as a reservoir for Powassan virus. Nat. Comm. Biol. 2021, 4, 1319. [Google Scholar] [CrossRef] [PubMed]
- Barbour, A.G.; Fish, D.; Hoen, A.G.; Tsao, J.I.; Diuk-Wasser, M.A.; Bunikis, J.; Travinsky, B. Niche partitioning of Borrelia burgdorferi and Borrelia miyamotoi in the same tick vector and mammalian reservoir species. Am. J. Trop. Med. Hyg. 2009, 81, 1120–1131. [Google Scholar] [CrossRef] [PubMed]
- Diuk-Wasser, M.A.; Hoen, A.G.; Cislo, P.; Brinkerhoff, R.; Hamer, S.A.; Rowland, M.; Cortinas, R.; Vourc, G.; Melton, F.; Hickling, G.J.; et al. Human risk of infections with Borrelia burgdorferi, the Lyme disease agent, in eastern United States. Am. J. Trop. Med. Hyg. 2012, 86, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Feldman, K.A.; Connally, N.P.; Hojgaard, A.; Jones, E.H.; White, J.L.; Hinckley, A.F. Hinckley. Abundance and infection rates of Ixodes scapularis nymphs collected from residential properties in Lyme disease-endemic areas of Connecticut, Maryland, and New York. J. Vector Ecol. 2015, 40, 198–201. [Google Scholar] [CrossRef] [PubMed]
- Hayes, L.E.; Scott, J.A.; Stafford, K.C., III. Influences of weather on Ixodes scapularis nymphal densities at long-term study sites in Connecticut. Ticks Tick Borne Dis. 2015, 6, 258–266. [Google Scholar] [CrossRef]
- Johnson, T.L.; Graham, C.B.; Maes, S.E.; Hojgaard, A.; Fleshman, A.; Boegler, K.A.; Delory, M.J.; Slater, K.S.; Karpathy, S.E.; Bjork, J.K.; et al. Prevalence and distribution of seven human pathogens in host-seeking Ixodes scapularis (Acari: Ixodidae) nymphs in Minnesota, USA. Ticks Tick Borne Dis. 2018, 9, 1499–1507. [Google Scholar] [CrossRef]
- Foster, E.; Burtis, J.; Sidge, J.L.; Tsao, J.I.; Bjork, J.; Liu, G.; Neitzel, D.F.; Lee, X.; Paskewitz, S.; Caporale, D.; et al. Inter-annual variation in prevalence of Borrelia burgdorferi sensu stricto and Anaplasma phagocytophilum in host-seeking Ixodes scapularis (Acari: Ixodidae) at long-term surveillance sites in the upper midwestern United States: Implications for public health practice. Ticks Tick Borne Dis. 2022, 13, 101886. [Google Scholar] [CrossRef]
- Stafford, K.C., III; Cartter, M.L.; Magnarelli, L.A.; Ertel, S.-H.; Mshar, P.A. Temporal correlations between tick abundance and prevalence of ticks infected with Borrelia burgdorferi and increasing incidence of Lyme disease. J. Clin. Micro. 1998, 36, 1240–1244. [Google Scholar] [CrossRef]
- States, S.; Brinkerhoff, R.; Carpi, G.; Steeves, T.; Folsom-O’keefe, C.; DeVeaux, M.; Diuk-Wasser, M. Lyme disease risk not amplified in a species-poor vertebrate community: Similar Borrelia burgdorferi tick infection prevalence and OspC genotype frequencies. Infect. Genet. Evol. 2014, 27, 566–575. [Google Scholar] [CrossRef]
- Schwartz, S.; Calvente, E.; Rollinson, E.; Koon, D.S.K.; Chinnici, N. Tick-borne pathogens in questing blacklegged ticks (Acari: Ixodidae) from Pike County, Pennsylvania. J. Med. Entomol. 2022, 59, 1793–1804. [Google Scholar] [CrossRef]
- Salkeld, D.J.; Lagana, D.M.; Wachara, J.; Porter, W.T.; Nieto, N.C. Examining prevalence and diversity of tick-borne pathogens in questing Ixodes pacificus ticks in California. Appl. Environ. Microbiol. 2021, 87, e00319-21. [Google Scholar] [CrossRef] [PubMed]
- Spielman, A. Human babesiosis on Nantucket Island—Transmission by nymphal Ixodes ticks. Am. J. Trop. Med. Hyg. 1976, 25, 784–787. [Google Scholar] [CrossRef] [PubMed]
- Piesman, J.; Spielman, A. Host-associations and seasonal abundance of immature Ixodes dammini in southeastern Massachusetts. Ann. Entomol. Soc. Amer. 1979, 72, 829–832. [Google Scholar] [CrossRef]
- Spielman, A.; Etkind, P.; Piesman, J.; Ruebush, T.K.; Juranek, D.D.; Jacobs, M.S.; Ii, T.K.R. Reservoir hosts of human babesiosis on Nantucket Island. Am. J. Trop. Med. Hyg. 1981, 30, 560–565. [Google Scholar] [CrossRef]
- Telford, S.R., III; Dawson, J.E.; Katavolos, P.; Warner, C.K.; Kolbert, C.P.; Persing, D.H. Perpetuation of the agent of human granulitic ehrlichiosis in a deer tick-rodent cycle. Proc. Natl. Acad. Sci. USA 1996, 93, 6209–6214. [Google Scholar] [CrossRef]
- Goethert, H.K.; Mather, T.N.; Buchthal, J.; Telford, S.R., III. Retrotransposon-based bloodmeal analysis of nymphal deer ticks demonstrates spatiotemporal diversity of Borrelia burgdorferi and Babesia microti reservoirs. Appl. Env. Microbiol. 2021, 87, e02370-20. [Google Scholar] [CrossRef]
- Goethert, H.K.; Telford, S.R., III. Host contributions to the force of Borrelia burgdorferi and Babesia microti transmission differ at edges of and within a small habitat patch. Appl. Env. Microbiol. 2022, 88, e02391-21. [Google Scholar] [CrossRef]
- Goethert, H.K.; Telford, S.R., III. Limited capacity of deer to serve as zooprophylactic hosts for Borrelia burgdorferi in the northeastern United States. Appl. Env. Microbiol. 2022, 88, e00042-22. [Google Scholar] [CrossRef]
- Ginsberg, H.S.; Ewing, C.P.; Bosler, A.F.O.E.M.; Daley, J.G.; Sayre, M.W. Increased population densities of Amblyomma americanum (Acari: Ixodidae) on Long Island, New York. J. Parasitol. 1991, 77, 493–495. [Google Scholar] [CrossRef]
- Tufts, M.; Diuk, M.A. First hemispheric report of invasive tick species Haemaphysalis punctata, first state report of Haemaphysalis longicornis, and range expansion of native tick species in Rhode Island, USA. Parasites Vectors 2021, 14, 394. [Google Scholar] [CrossRef] [PubMed]
- Rand, P.W.; Lubelczyk, C.; Holman, M.A.; Lacombe, E.H.; Smith, R.P., Jr. Abundance of Ixodes scapularis (Acari: Ixodidae) after the complete removal of deer from an isolated offshore island, endemic for Lyme disease. J. Med. Entomol. 2004, 41, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.C.; Stafford, K.C., III; Linske, M.A.; Brackney, D.E.; LaBonte, A.M.; Stuber, H.R.; Cozens, D.W. Effective control of the motile stages of Amblyomma americanum and reduced Ehrlichia spp. prevalence in adults via permethrin treatment of white-tailed deer in coastal Connecticut, USA. Ticks Tick Borne Dis. 2021, 12, 101675. [Google Scholar] [CrossRef] [PubMed]
- Buchthal, J.; Evans, S.W.; Lunshof, J.; Telford, S.R., III; Esvelt, K.M. Mice Against Ticks: An experimental community-guided effort to prevent tick-borne disease by altering the shared environment. Phil. Trans. Royal Soc. B 2019, 374, 20180105. [Google Scholar] [CrossRef] [PubMed]
- Snow, A.A. Genetically engineering wild mice to combat Lyme disease: An ecological perspective. BioScience 2019, 69, 746–756. [Google Scholar] [CrossRef]
- Nantucket Current. 2022. Available online: https://www.nantucketcurrent.com/new-data-shows-explosive-growth-of-nantucket-s-summer-population (accessed on 9 July 2023).
- Massachusetts Division of Fisheries and Wildlife. Available online: https://www.mass.gov/service-details/deer-harvest-data (accessed on 9 July 2023).
- Oldale, R.N. Cape Cod, Martha’s Vineyard, and Nantucket: The Geologic Story; Cape Cod Publications: Dennis, MA, USA, 2001; 224p. [Google Scholar]
- CDC (Centers for Disease Control and Prevention). Surveillance for Ixodes scapularis and Pathogens Found in This Tick Species in the United States. 2023. Available online: https://www.cdc.gov/ticks/resources/TickSurveillance_Iscapularis-P.pdf (accessed on 9 July 2023).
- Richer, L.M.; Brisson, D.; Melo, R.; Ostfeld, R.; Zeidner, N.; Gomes-Solecki, M. Reservoir targeted vaccine against Borrelia burgdorferi: A new strategy to prevent Lyme disease transmission. J. Infect. Dis. 2014, 209, 1972–1980. [Google Scholar] [CrossRef]
- Allen, D.; Borgmann-Winter, B.; Bashor, L.; Ward, J. The density of the Lyme disease vector Ixodes scapularis (blacklegged tick) differs between the Champlain Valley and Green Mountains, Vermont. Northeast. Nat. 2019, 26, 545–560. [Google Scholar] [CrossRef]
- Ginsberg, H.S.; Ewing, C.P. Habitat distribution of Ixodes dammini (Acari: Ixodidae) and Lyme disease spirochetes on Fire Island, NY. J. Med. Entomol. 1989, 26, 183–189. [Google Scholar] [CrossRef]
- Falco, R.C.; Fish, D. A comparison of methods for sampling the deer tick, Ixodes dammini, in a Lyme disease endemic area. Exp. Applied Entomol. 1992, 14, 165–173. [Google Scholar] [CrossRef]
- Simmons, T.W.; Welch, E.N.; Manges, A.B.; Peters, N.A.; Duchamp, J.E. Relative efficiency of drag fabrics for collection of blacklegged tick (Acari: Ixodidae) larvae, nymphs, and adults. J. Med. Entomol. 2021, 58, 1248–1255. [Google Scholar] [CrossRef]
- Nyrhilä, S.; Sormunen, J.J.; Mäkelä, S.; Sippola, E.; Vesterinen, E.J.; Klemola, T. One out of ten: Low sampling efficiency of cloth dragging challenges abundance estimates of questing ticks. Exp. Appl. Acarol. 2020, 82, 571–585. [Google Scholar] [CrossRef]
- Daniels, T.J.; Falco, R.C.; Fish, D. Estimating population size and drag sampling efficiency for the blacklegged tick (Acari: Ixodidae). J. Med. Entomol. 2000, 37, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Borgmann-Winter, B.; Allen, D. How the distance between drag-cloth checks affects the estimate of adult and nymphal Ixodes scapularis (Acari: Ixodidae) density. J. Med. Entomol. 2019, 57, 623–626. [Google Scholar] [CrossRef] [PubMed]
- Schulze, T.L.; Jordan, R.A. Daily variation in sampled densities of Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae) nymphs at a single site—Implications for assessing acarological risk. J. Med. Entomol. 2022, 59, 741–751. [Google Scholar] [CrossRef]
- Telford, S.R., III; Urioste, S.S.; Spielman, A. Clustering of host-seeking nymphal deer ticks (Ixodes dammini) infected by Lyme disease spirochetes (Borrelia burgdorferi). Am. J. Trop. Med. Hyg. 1992, 47, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Schulze, T.L.; Jordan, R.A. Meteorologically mediated diurnal questing of Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae) nymphs. J. Med. Entomol. 2003, 40, 395–402. [Google Scholar] [CrossRef]
- Xu, G.; Pearson, P.; Rich, S.M. Ehrlichia muris in Ixodes cookei ticks, northeastern United States, 2016–2017. Emerg. Infect. Dis. 2018, 24, 1143–1144. [Google Scholar] [CrossRef]
- Xu, G.; Pearson, P.; Dykstra, E.; Andrews, E.S.; Rich, S.M. Human-biting Ixodes ticks and pathogen prevalence from California, Oregon, and Washington. Vector Borne Zoonotic Dis. 2019, 19, 106–114. [Google Scholar] [CrossRef]
- Kohn, M.A.; Senyak, J. UCSF CTSI. 2021. Available online: https://sample-size.net/confidence-interval-proportion/ (accessed on 9 July 2023).
- Telford, S.R., III; Goethert, H.K.; Lepore, T.J. Semicentennial of human babesiosis, Nantucket Island. Pathogens 2021, 10, 1159. [Google Scholar] [CrossRef]
- Mathisson, D.C.; Kross, S.M.; Palmer, M.I.; Diuk-Wasser, M.A. Effect of vegetation on the abundance of tick vectors in the northeastern United States: A review of the literature. J. Med. Entomol. 2021, 58, 2030–2037. [Google Scholar] [CrossRef]
- Ginsberg, H.S.; Zhioua, E.; Mitra, S.; Fischer, J.; Buckley, P.A.; Verret, F.; Underwood, H.B.; Buckley, F.G. Woodland type and spatial distribution of nymphal Ixodes scapularis (Acari: Ixodidae). Environ. Entomol. 2004, 33, 1266–1273. [Google Scholar] [CrossRef]
- Schulze, T.L.; Jordan, R.A. Influence of meso- and microscale habitat structure on focal distribution of sympatric Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae). J. Med. Entomol. 2005, 42, 285–294. [Google Scholar] [CrossRef]
- Stafford, K.C., III; Williams, S.C.; Molaei, G. Integrated pest management in controlling ticks and tick-associated diseases. J. Integr. Pest Manag. 2017, 8, 28. [Google Scholar] [CrossRef]
- Ostfeld, R.S.; Hazler, K.R.; Cepeda, O.M. Temporal and spatial dynamics of Ixodes scapularis (Acari: Ixodidae) in a rural landscape. J. Med. Entomol. 1996, 33, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Schulze, T.L.; Jordan, R.A.; Schulze, C.J.; Hung, R.W. Precipitation and temperature as predictors of the local abundance of Ixodes scapularis (Acari: Ixodidae) nymphs. J. Med. Entomol. 2009, 46, 1025–1029. [Google Scholar] [CrossRef]
- Linske, M.A.; Stafford, K.C., III; Williams, S.C.; Lubelczyk, C.B.; Welch, M.; Henderson, E.F. Impacts of deciduous leaf litter and snow presence on nymphal Ixodes scapularis (Acari: Ixodidae) overwintering survival in coastal New England, USA. Insects 2019, 10, 227. [Google Scholar] [CrossRef] [PubMed]
- Burtis, J.C.; Sullivan, P.; Levi, T.; Oggenfuss, K.; Fahey, T.J.; Ostfeld, R.S. The impact of temperature and precipitation on blacklegged tick activity and Lyme disease incidence in endemic and emerging regions. Parasites Vectors 2016, 9, 606. [Google Scholar] [CrossRef]
- Ostfeld, R.S.; Schauber, E.M.; Canham, C.D.; Keesing, F.; Jones, C.G.; Wolff, J.O.; Tsao, J.; Barbour, A.G.; Luke, C.J.; Fikrig, E.; et al. Effects of acorn production and mouse abundance on abundance and Borrelia burgdorferi infection prevalence of nymphal Ixodes scapularis ticks. Vector Borne Zoo. Dis. 2001, 1, 55–63. [Google Scholar] [CrossRef]
- Ginsberg, H.S.; Zhioua, E. Nymphal survival and habitat distribution of Ixodes scapularis and Amblyomma americanum. Exp. Applied Entomol. 1996, 20, 533–544. [Google Scholar]
- Durden, L.A.; Oliver, J.H., Jr.; Kinsey, A.A. Ticks (Acari: Ixodidae) and spirochetes (Spirochaetaceae: Spirochaetales) recovered from birds on a Georgia Barrier Island. J. Med. Entomol. 2001, 38, 213–236. [Google Scholar] [CrossRef]
- Telford, S.R., III. Personal Communication to A. Snow; Tufts University: North Grafton, MA, USA, 2022. [Google Scholar]
- TickReport. 2023. Available online: https://www.tickreport.com/stats (accessed on 9 July 2023).
- Telford, S.R., III; Spielman, A. Enzootic transmission of the agent of Lyme disease in rabbits. Am. J. Trop. Med. Hyg. 1989, 41, 482–490. [Google Scholar] [CrossRef]
- Hersh, M.H.; Tibbetts, M.; Strauss, M.; Ostfeld, R.S.; Keesing, F. Reservoir competence of wildlife host species for Babesia microti. Emerg. Infect. Dis. 2012, 18, 1951–1957. [Google Scholar] [CrossRef]
- Piesman, J.; Spielman, A.; Etkind, P.; Ruebush, T.K.; Juranek, D.D. Role of deer in the epizootiology of Babesia microti in Massachusetts, USA. J. Med. Entomol. 1979, 15, 537–540. [Google Scholar] [CrossRef]
- Telford, S.R., III; Mather, T.N.; Wilson, S.I.M.M.L.; Spielman, A. Incompetence of deer as reservoirs of the Lyme disease spirochete. Am. J. Trop. Med. Hyg. 1988, 39, 105–109. [Google Scholar] [CrossRef]
- Pearson, P.; Rich, C.; Feehan, M.J.; Ditchkoff, S.S.; Rich, S.M. White-tailed deer serum kills the Lyme disease spirochete, Borrelia burgdorferi. Vector-Borne Zoo. Dis. 2023, 23, 303–305. [Google Scholar] [CrossRef] [PubMed]
- Keesing, F.; McHenry, D.J.; Killilea, M.; Brunner, J.L.; Ostfeld, R.; Hersh, M.; Logiudice, K.; Tibbetts, M.; Schmidt, K.A. Prevalence of human-active and variant 1 strains of the tick-borne pathogen Anaplasma phagocytophilum in hosts and forest of Eastern North America. Am. J. Trop. Med. Hyg. 2014, 91, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Trost, C.N.; Lindsay, L.R.; Dibernardo, A.; Chilton, N.B. Three genetically distinct clades of Anaplasma phagocytophilum in Ixodes scapularis. Ticks Tick Borne Dis. 2008, 9, 1518–1527. [Google Scholar] [CrossRef] [PubMed]
- Massung, R.F.; Courtney, J.W.; Hiratzka, S.L.; Pitzer, V.E.; Smith, G.; Dryden, R.I. Anaplasma phagocytophilum in white-tailed deer. Emerg. Infect. Dis. 2005, 11, 1604–1606. [Google Scholar] [CrossRef] [PubMed]
- Hojgaard, A.; Osikowicz, L.M.; Rizzo, M.F.; Ayres, B.N.; Nicholson, W.L.; Eisen, R.J. Using next generation sequencing for molecular detection and differentiation of Anaplasma phagocytophilum variants from host seeking Ixodes scapularis ticks in the United States. Ticks Tick Borne Dis. 2022, 13, 10204. [Google Scholar] [CrossRef]
- Schulze, T.L.; Jordan, R.A.; Healy, S.P.; Roegner, V.E. Detection of Babesia microti and Borrelia burgdorferi in host-seeking Ixodes scapularis (Acari: Ixodidae) in Monmouth County, New Jersey. J. Med. Entomol. 2013, 50, 379–383. [Google Scholar] [CrossRef]
- Prusinski, M.A.; Kokas, J.E.; Hukey, K.T.; Kogut, S.J.; Lee, J.; Backenson, P.B. Prevalence of Borrelia burgdorferi (Spirochaetales: Spirochaetaceae), Anaplasma phagocytophilum (Rickettsiales: Anaplasmataceae), and Babesia microti (Piroplasmida: Babesiidae) in Ixodes scapularis (Acari: Ixodidae) collected from recreational lands in the Hudson Valley Region, New York State. J. Med. Entomol. 2014, 51, 226–236. [Google Scholar] [PubMed]
- Van Buskirk, J.; Ostfeld, R.S. Habitat heterogeneity, dispersal, and local risk of exposure to Lyme disease. Ecol. Appl. 1988, 8, 365–378. [Google Scholar] [CrossRef]
- Jordan, R.A.; Gable, S.; Egizi, A. Relevance of spatial and temporal trends in nymphal tick density and infection prevalence for public health and surveillance practice in long-term endemic areas: A case study of Monmouth County, NJ. J. Med. Entomol. 2022, 58, 1451–1466. [Google Scholar] [CrossRef]
- Hersh, M.H.; Ostfeld, R.S.; McHenry, D.J.; Tibbetts, M.; Brunner, J.L.; Killilea, M.E.; Logiudice, K.; Schmidt, K.A.; Keesing, F. Co-infection of blacklegged ticks with Babesia microti and Borrelia burgdorferi is higher than expected and acquired from small mammal hosts. PLoS ONE 2014, 9, e99348. [Google Scholar] [CrossRef]
- Edwards, M.J.; Russell, J.C.; Davidson, E.N.; Yanushefski, T.J.; Fleischman, B.L.; Heist, R.O.; Leep-Lazar, J.G.; Stuppi, S.L.; Esposito, R.A.; Suppan, L.M. A 4-yr survey of the range of ticks and tick-borne pathogens in the Lehigh Valley region of eastern Pennsylvania. J. Med. Entomol. 2019, 56, 1122–1134. [Google Scholar] [CrossRef] [PubMed]
- Dunn, J.M.; Krause, P.J.; Davis, S.; Vannier, E.G.; Fitzpatrick, M.C.; Rollend, L.; Belperron, A.A.; States, S.L.; Stacey, A.; Bockenstedt, L.K.; et al. Borrelia burgdorferi promotes the establishment of Babesia microti in the northeastern United States. PLoS ONE 2014, 9, e115494. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, M.; Quilliam, D.N.; Bandy, U.; Fulton, J.P.; Marak, T.P.; Berns, A. Rhode Island tick-borne disease surveillance summary 2012–2013. Rhode Isl. Med. J. 2014, 97, 46. [Google Scholar]
- Troppy, S.; Haney, G.; Cocoros, N.; Cranston, K.; DeMaria, J.A. Infectious disease surveillance in the 21st century: An integrated web-based surveillance and case management system. Public Health Rep. 2014, 129, 132–138. [Google Scholar] [CrossRef]
- Lee, X.; Coyle, D.R.; Johnson, D.K.H.; Murphy, M.W.; McGeehin, M.A.; Murphy, R.J.; Raffa, K.F.; Paskewitz, S.M. Prevalence of Borrelia burgdorferi and Anaplasma phagocytophilum in Ixodes scapularis (Acari: Ixodidae) nymphs collected in managed red pine forests in Wisconsin. J. Med. Entomol. 2004, 51, 694–701. [Google Scholar] [CrossRef]
- Langenwalder, D.B.; Schmidt, S.; Silaghi, C.; Skuballa, J.; Pantchev, N.; Matei, I.A.; Mihalca, A.; Gilli, U.; Zajkowska, J.; Ganter, M.; et al. The absence of the drhm gene is not a marker for human-pathogenicity in European Anaplasma phagocytophilum strains. Parasites Vectors 2020, 13, 238. [Google Scholar] [CrossRef]
- Krause, P.J.; Telford, S.R., III; Spielman, A.; Sikand, V.; Ryan, R.; Christianson, D.; Persing, D.H. Concurrent Lyme disease and babesiosis—Evidence for increased severity and duration of illness. JAMA 1996, 275, 1657–1660. [Google Scholar] [CrossRef] [PubMed]
- Eisen, L.; Eisen, R.J. Critical evaluation of the linkage between tick-based risk measures and the occurrence of Lyme disease cases. J. Med. Entomol. 2016, 53, 1050–1062. [Google Scholar] [CrossRef] [PubMed]
- González, J.; Fonseca, D.M.; Toledo, A. Seasonal dynamics of tick species in the ecotone of parks and recreational areas in Middlesex County (New Jersey, USA). Insects 2023, 14, 258. [Google Scholar] [CrossRef] [PubMed]
- Rochlin, I.; Benach, J.L.; Furie, M.B.; Thanassi, D.G.; Kim, H.K. Rapid invasion and expansion of the Asian longhorned tick (Haemaphysalis longicornis) into a new area on Long Island, New York, USA. Ticks Tick-Borne Dis. 2023, 14, 102088. [Google Scholar] [CrossRef]
- Nantucket Current. Available online: https://nantucketcurrent.com/news/new-invasive-tick-discovered-on-nantucket#:~:text=An%20invasive%20species%20of%20tick,its%20way%20to%20the%20island (accessed on 9 July 2023).
- Xu, G.; Foster, E.; Ribbe, F.; Hojgaard, A.; Eisen, R.J.; Paull, S.; Rich, S.M. Detection of Ehrlichia muris eauclairensis in blacklegged ticks (Ixodes scapularis) and white-footed mice (Peromyscus leucopus) in Massachusetts. Vector-Borne Zoo. Dis. 2023, 23, 311–315. [Google Scholar] [CrossRef]


| Site Name | Year Sampled | Vegetation | Trail Distance (km) | Lat/Long at 0 km | Property Owner |
|---|---|---|---|---|---|
| Tuckernuck Island * (1 site) | 2020–2022 | Mature oak woods, high mesic shrubs | 1.65 | 41°18′13.827″ N, 70°15′23.997″ W | Tuckernuck Land Trust, private property |
| Nantucket Island (11 sites) | |||||
| 2020–2022 | Mixed woods, high mesic shrubs | 0.96 | 41°17′13.184″ N, 69°59′43.139″ W | Nantucket Islands Land Bank, Nantucket Conservation Foundation |
| 2020–2022 | Successional shrubs and grass | 0.54 | 41°17′33.409″ N, 69°59′43.139″ W | Nantucket Conservation Foundation |
| 2020–2022 | Mixed woods, high mesic shrubs | 0.94 | 41°17′25.567″ N, 70°1′26.811″ W | Nantucket Conservation Foundation |
| 2020–2022 | Mixed woods and scrub oak | 0.94 | 41°17′19.029″ N, 69°59′27.947″ W | Mass Audubon |
| 2020–2022 | Mixed conifer forest, shrub border | 0.50 | 41°15′38.205″ N, 70°4′47.223″ W | Commonwealth of MA |
| 2020–2022 | Open/disturbed pitch pine woods | 0.64 | 41°16′37.853″ N, 70°4′15.578″ W | Commonwealth of MA |
| 2020–2022 | Low and medium-height scrub oak | 1.10 | 41°15′6.790″ N, 70°0′42.788″ W | Nantucket Conservation Foundation |
| 2020–2022 | Grassland adjacent to high mesic shrubs | 0.78 | 41°16′51.682″ N, 70°8′42.164″ W | Nantucket Islands Land Bank |
| 2020–2022 | Grassland with low heath shrubs | 1.50 | 41°17′32.323″ N, 70°10′11.418″ W | Linda Loring Nature Foundation |
| 2021, 2022 | Grassy path through high mesic shrubs | 1.40 | 41°16′19.079″ N, 70°10′53.758″ W | Nantucket Islands Land Bank |
| 2021, 2022 | Grassland with low heath shrubs | 0.40 | 41°15′54.623″ N, 70°9′51.466″ W | Nantucket Conservation Foundation |
| Island | Site | Year | Total Nymphs | Percent Infected | Co-Infected | Bb+Bm | Bb+Ap | Bm+Ap | Bb+Bm+Ap |
|---|---|---|---|---|---|---|---|---|---|
| Nantucket | All sites | 2020 | 1614 | 35 | 7 | 3 * | 1 | 1 | 0.6 |
| (562) | (108) | (51) | (19) | (18) | (9) | ||||
| Stump Pond | 2020 | 448 | 31 | 8 | 3 * | 2 | 2 | 1 | |
| (141) | (35) | (14) | (8) | (10) | (3) | ||||
| UMass Field Stn | 2020 | 398 | 34 | 6 | 3 * | 1 | 1 | 1 | |
| (135) | (25) | (13) | (3) | (4) | (2) | ||||
| Norwood Farm | 2020 | 338 | 38 | 5 | 2 * | 1 | 1 | 1 | |
| (128) | (17) | (8) | (3) | (2) | (3) | ||||
| Jewel Pond | 2020 | 430 | 37 | 7 | 4 * | 1 | 0.5 | 0.2 | |
| (158) | (31) | (16) | (5) | (2) | (1) | ||||
| Nantucket | All sites | 2021 | 1683 | 41 | 11 | 7 ** | 2 | 1 | 1 |
| (685) | (187) | (116) | (29) | (17) | (15) | ||||
| Stump Pond | 2021 | 398 | 42 | 13 | 9 ** | 0 | 2 | 2 | |
| (169) | (52) | (34) | (1) | (7) | (6) | ||||
| UMass Field Stn | 2021 | 412 | 42 | 13 | 7 ** | 3 | 1 | 1 | |
| (175) | (52) | (27) | (14) | (4) | (5) | ||||
| Norwood Farm | 2021 | 458 | 46 | 10 | 6 ** | 2 | 1 | 0.4 | |
| (210) | (48) | (29) | (8) | (6) | (2) | ||||
| Jewel Pond | 2021 | 415 | 32 | 8 | 6 ** | 1 | 0 | 0.5 | |
| (131) | (35) | (26) | (6) | (0) | (2) | ||||
| Tuckernuck | Tuckernuck | 2020 | 330 | 25 | 6 | 5 * | 1 | 1 | 0 |
| (82) | (21) | (15) | (2) | (4) | (0) | ||||
| Tuckernuck | 2021 | 444 | 54 | 25 | 8 ** | 7 * | 2 | 7 ** | |
| (241) | (109) | (37) | (29) | (10) | (31) |
| Location | Year | DON | Bb DIN | Bm DIN | Ap DIN | B miya DIN |
|---|---|---|---|---|---|---|
| Nantucket | 2020 | 108 | 11 | 12 | 18 | 3 |
| 2021 | 138 | 27 | 20 | 23 | 3 | |
| Tuckernuck | 2020 | 62 | 6 | 6 | 8 | 0 |
| 2021 | 140 | 40 | 33 | 45 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Snow, A.A.; Pearson, P.; Xu, G.; Allen, D.N.; Santamaria, R.; Rich, S.M. Tick Densities and Infection Prevalence on Coastal Islands in Massachusetts, USA: Establishing a Baseline. Insects 2023, 14, 628. https://doi.org/10.3390/insects14070628
Snow AA, Pearson P, Xu G, Allen DN, Santamaria R, Rich SM. Tick Densities and Infection Prevalence on Coastal Islands in Massachusetts, USA: Establishing a Baseline. Insects. 2023; 14(7):628. https://doi.org/10.3390/insects14070628
Chicago/Turabian StyleSnow, Allison A., Patrick Pearson, Guang Xu, David N. Allen, Roberto Santamaria, and Stephen M. Rich. 2023. "Tick Densities and Infection Prevalence on Coastal Islands in Massachusetts, USA: Establishing a Baseline" Insects 14, no. 7: 628. https://doi.org/10.3390/insects14070628
APA StyleSnow, A. A., Pearson, P., Xu, G., Allen, D. N., Santamaria, R., & Rich, S. M. (2023). Tick Densities and Infection Prevalence on Coastal Islands in Massachusetts, USA: Establishing a Baseline. Insects, 14(7), 628. https://doi.org/10.3390/insects14070628






