Biology, Ecology and Management of Tephritid Fruit Flies in China: A Review
Abstract
Simple Summary
Abstract
1. Introduction
2. Economic Importance and Distribution
2.1. Economic Importance
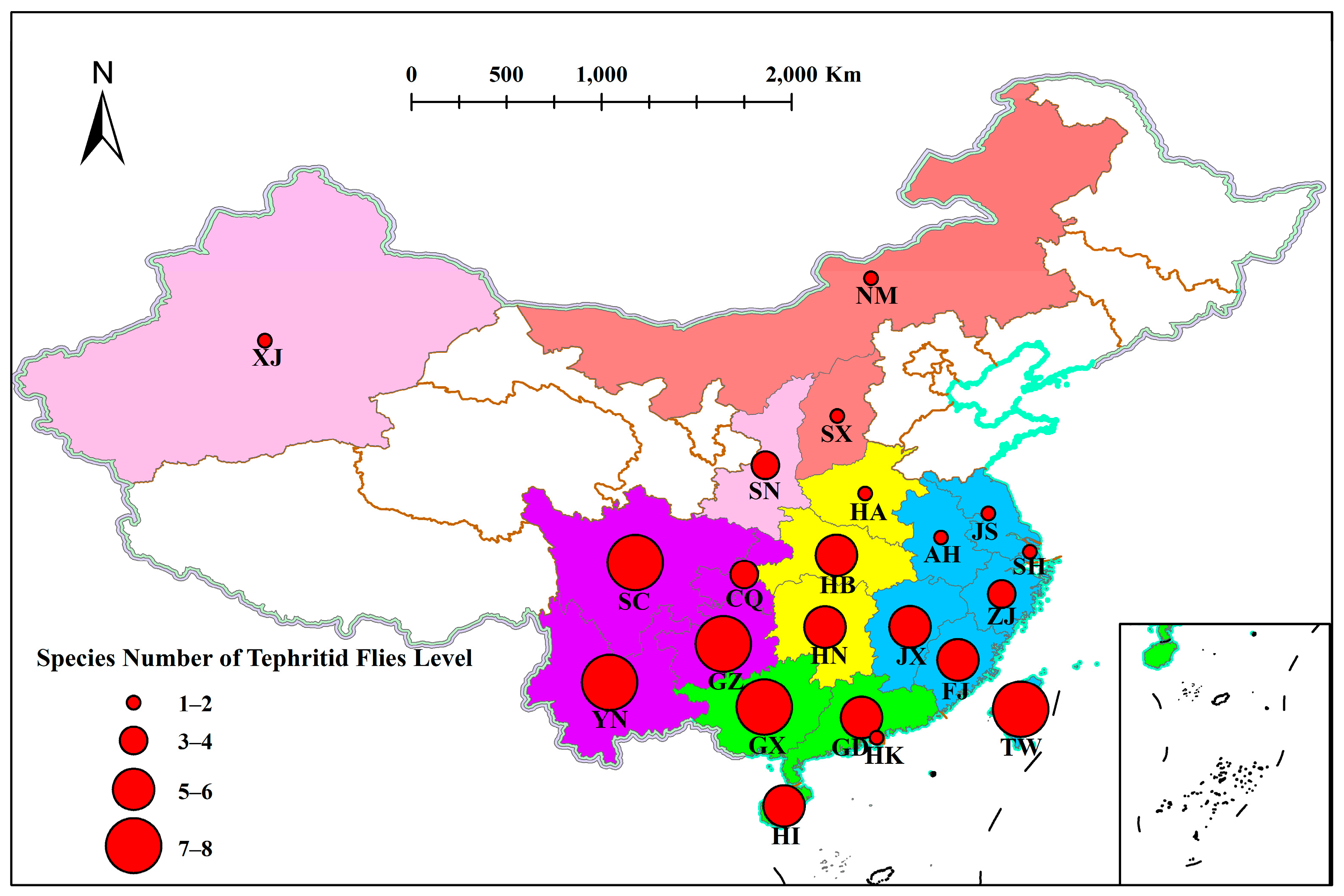
2.2. Distribution
3. Morphological Characteristics
3.1. Basic Taxonomy
3.2. Molecular Identification
4. Host Range and Damage Characteristics
4.1. Host Range
4.2. Damage Characteristics
5. Life History
6. Oviposition and Host Preference
7. Interspecific Competition
8. Integrated Management of Tephritid Fruit Flies
8.1. Monitoring Surveys
8.2. Traps
- Dry type: Cook and Cunningham, ChamP (CH), Jackson, Delta, Lynfield, OBDT, Phase IV, RS, Steiner, ST, YP, Rebell (RB);
- Wet type: McPhail, Harris;
- Dry and wet type: Easy trap (ET), MLT, Tephri (TP).
8.3. Sex Pheromones
8.4. Food Baits
8.5. Natural Enemy Utilization
8.6. Key Points of the Integrated Management System
- “Agricultural measures as the basis”
- 2.
- “Trapping and control as the main means”
- 3.
- “Chemical control as the emergency”
8.7. Sterile Insect Technique (SIT)
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scolari, F.; Valerio, F.; Benelli, G.; Papadopoulos, N.T.; Vaníčková, L. Tephritid Fruit Fly Semiochemicals: Current Knowledge and Future Perspectives. Insects 2021, 12, 408. [Google Scholar] [CrossRef] [PubMed]
- Doorenweerd, C.; Leblanc, L.; Norrbom, A.L.; San Jose, M.; Rubinoff, D. A Global Checklist of the 932 Fruit Fly Species in the Tribe Dacini (Diptera, Tephritidae). ZooKeys 2018, 730, 19–56. [Google Scholar] [CrossRef] [PubMed]
- Ismay, J. Fruit flies of economic significance: Their identification and bionomics. By Ian M. White and Marlene M. Elson-Harris. (Wallingford: CAB International, 1991). xii + 601 pp. Soft cover £30. ISBN-0-85198-790. Bull. Entomol. Res. 1992, 82, 433. [Google Scholar] [CrossRef]
- Clarke, A.R.; Measham, P.F. Competition: A Missing Component of Fruit Fly (Diptera: Tephritidae) Risk Assessment and Planning. Insects 2022, 13, 1065. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, D.; Xu, Y.; Wang, L.; Cheng, D.; Qi, Y.; Zeng, L.; Lu, Y. Invasion, Expansion, and Control of Bactrocera Dorsalis (Hendel) in China. J. Integr. Agric. 2019, 18, 771–787. [Google Scholar] [CrossRef]
- Allwood, A.J.; Leblanc, L. Losses Caused by Fruit Flies (Diptera: Tephritidae) in Seven Pacific Island Countries. In Management of Fruit Flies in the Pacific: A Regional Symposium, Nadi, Fiji, 28–31 October 1996; Allwood, A.J., Drew, R.A.I., Eds.; Australian Centre for International Agricultural Research: Canberra, Australia, 1997; pp. 208–211. [Google Scholar]
- Ning, Z.Y. Economic Loss Evaluation and Risk Assessment of Damage by Oriental Fruit Fly in Fujian. Master’s Thesis, Fujian Agriculture and Forestry University, Fuzhou, China, 2008. [Google Scholar]
- Sun, H.Y.; Qin, Y.J.; Fang, Y.; Zhao, Z.H.; Pan, X.B.; Zhao, S.Q.; Liu, H.; Lang, S.; Lu, G.C.; Li, Z.H. The potential economic loss of bitter gourd industry in China caused by Zeugodacus cucurbitae (Coquillett) based on @RISK. Plant Quar. 2018, 32, 64–69. [Google Scholar] [CrossRef]
- Pan, F.; Xiao, T.B.; Qin, S.; Chen, H.Y.; Lin, Z.F.; Xie, S.H. Study on yield of bitter gourd damaged by Bactrocera cucurbitae and its economic thresholds. China Plant Prot. 2014, 34, 12–15. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, L.; Haack, R.A.; Liu, J.; Ye, H. A noteworthy step on a vast continent: New expansion records of the guava fruit fly, Bactrocera correcta (Bezzi, 1916) (Diptera: Tephritidae), in mainland China. BioInvasions Rec. 2019, 8, 530–539. [Google Scholar] [CrossRef]
- Liu, X.; Jin, Y.; Ye, H. Recent spread and climatic ecological niche of the invasive guava fruit fly, Bactrocera correcta, in mainland China. J. Pest Sci. 2013, 86, 449–458. [Google Scholar] [CrossRef]
- Yu, J.Y.; Ren, K.L.; Xue, W.P.; Li, T.Q.; Wang, X.J.; Geng, K. The species and population dynamics of Tephritid fruit flies in the macaque peach orchard of Xiuwen County. South China Fruits 2022, 51, 117–121. [Google Scholar]
- Wei, C.H.; Zeng, L.; Lu, Y.Y. Study on ovipostion tendency and selectivity of Bactrocera correcta (Bezzi) (Diptera: Tephritidae) towards different hosts. South China Fruits 2011, 40, 67–69. [Google Scholar] [CrossRef]
- Liu, Z.S.; Ding, Y.M.; Zhou, J.; Deng, Y.L.; Bai, Y.H.; Lei, Q.W. Development and survival rate of Bactrocera correcta (Bezzi) (Diptera:Tephritidae) on mango at different temperatures. Plant Prot. 2015, 41, 113–116+156. [Google Scholar] [CrossRef]
- Wei, B.; Wei, C.M.; Li, Y.G.; Tang, J.C.; Hu, X.S.; Liu, H.; Dong, W.X. The effect of egg extracts on the behavior of gravid female Bactrocera dorsalis and Bactrocera correcta and analysis of chemicals on the egg surfaces of these species. Chin. J. Appl. Entomol. 2021, 58, 885–893. [Google Scholar] [CrossRef]
- Liang, G.Q.; Zhang, S.M.; Xu, W. Descriptions of tephritids flies in parts of southern China and two new records of China. Acta Agric. Univ. Jiangxiensis 1989, 11, 14–20. [Google Scholar]
- Qin, Y.-J.; Buahom, N.; Krosch, M.N.; Du, Y.; Wu, Y.; Malacrida, A.R.; Deng, Y.-L.; Liu, J.-Q.; Jiang, X.-L.; Li, Z.-H. Genetic Diversity and Population Structure in Bactrocera correcta (Diptera: Tephritidae) Inferred from MtDNA Cox1 and Microsatellite Markers. Sci. Rep. 2016, 6, 38476. [Google Scholar] [CrossRef]
- Li, H.X.; Ye, H.; Lv, J. On damages and Distributions of fruit fly (Bactrocera dorsalis Hendel) in Yunnan Province, Southern China. J. Yunnan Univ. Nat. Sci. Ed. 2000, 22, 473–475. [Google Scholar] [CrossRef]
- Lei, Y.M.; Liao, D.Q.; Huang, S.C.; Xie, L.Y. Study on integrated control against Bactrocera dorsalis in guava orchard. J. Anhui Agric. Sci. 2011, 39, 6438–6440. [Google Scholar] [CrossRef]
- Xia, Z.M. Kinds of plants quarantine object and their damage in Guizhou. Guizhou Agric. Sci. 1998, 26, 22–24. [Google Scholar]
- Lin, M.G.; Zhang, Y.; Zeng, L.; Wang, X.J.; Li, F.; Shi, J. Study on population dynamics and comprehensive control of Bactrocera dorsalis (Hendel) in Guava orchard, Hainan Province. South China Fruits 2014, 43, 60–63. [Google Scholar] [CrossRef]
- He, Y.B.; Zhan, R.L.; Zhao, Y.L.; Lu, Y.Y. Occurrence and control of Bactrocera dorsalis (Hendel) on several tropical fruits. Chin. J. Trop. Crop. 2006, 27, 77–80. [Google Scholar] [CrossRef]
- Liu, N. The Biological Characteristics and the Interspecific Competition of Oriental Fruit Fly and Guava Fruit Fly. Master’s Thesis, Yunnan Agricultural University, Kunming, China, 2014. [Google Scholar]
- Zhou, G.L.; Chen, C.; Ye, J.; Hu, B.S.; Liu, F.Q. Predicting potential ecological distribution of Bactrocera dorsalis in China using GARP ecological niche modeling. Acta Ecol. Sin. 2007, 27, 3362–3369. [Google Scholar] [CrossRef]
- Lin, Z.J.; Sun, G.K.; Gao, Q.Z.; Liu, J.Y.; Zhang, Q.Y.; Chen, H.Z.; Chen, J.F.; Xu, Y.Q.; Sun, D.H.; Hong, Z.Q. Epidemic monitoring of the oriental fruit fly in Xiamen City. Entomol. J. East China 1998, 7, 72–75. [Google Scholar]
- Zhang, Y.A.; Zhao, X.Q. Study on oriental fruit fly in Sichuan Province. Southwest China J. Agric. Sci. 1994, 7, 71–75. [Google Scholar] [CrossRef]
- Zhai, Q.H.; Ni, M.; Xu, L.J. Study on the occurrence pattern and influencing factors of Bactrocera dorsalis (Hendel) in Wuxi area. Xiandai Hortic. 2018, 150–151. [Google Scholar] [CrossRef]
- Wang, Y.C.; He, Y.B.; Lei, X.T.; Zhao, Y.L. The occurrence pattern and control technology of Bactrocera dorsalis (Hendel) in Yangtze orchard. China Trop. Agric. 2005, 35–36. [Google Scholar] [CrossRef]
- Wang, Y.M.; Tan, Y.Q.; Zhang, G.A. Analysis of the suitability of Bactrocera dorsalis (Hendel) in Hubei Province. Hubei Plant Prot. 2011, 23–24. [Google Scholar] [CrossRef]
- Liu, J.H.; Xiong, X.Z.; Yang, L.Y.; Xiong, Z.P.; Pan, Y.Z. Analysis on suitable distribution area of oriental fruit fly (Bactrocera dorsalis) based on GARP ecological niche model. Plant Dis. Pests 2010, 1, 34–38. [Google Scholar] [CrossRef]
- Aketarawong, N.; BONIZZONI, M.; Thanaphum, S.; Gomulski, L.; GASPERI, G.; MALACRIDA, A.R.; Gugliemino, C. Inferences on the population structure and colonization process of the invasive oriental fruit fly, Bactrocera dorsalis (Hendel). Mol. Ecol. 2007, 16, 3522–3532. [Google Scholar] [CrossRef]
- Wan, X.; Nardi, F.; Zhang, B.; Liu, Y. The oriental fruit fly, Bactrocera dorsalis, in China: Origin and gradual inland range expansion associated with population growth. PLoS ONE 2011, 6, e25238. [Google Scholar] [CrossRef]
- Gong, X.Z.; Zhong, Y.; Huang, J.Q.; Qiu, S.; Du, Z.X.; Pan, R.W. Research Progress of Bactrocera latifons in China. North Hortic. 2016, 195–198. Available online: https://oversea.cnki.net/kns/manage/export?filename=bfyy201611050&dbname=CJFDLAST2016 (accessed on 2 January 2023).
- Liang, R.C. Survey on species and distribution of tephritids flies in Guangxi. Guangxi Plant Prot. 1988, 25–30. Available online: https://oversea.cnki.net/kns/manage/export?filename=gxzb198803007&dbname=CJFD8589 (accessed on 2 January 2023).
- Fan, Q.S. Dacus (Bactrocera) latifrons (Hendel) was collected in Wanding, Yunnan Province. Plant Quar. 1989, 3, 423. [Google Scholar] [CrossRef]
- Chen, M.Z.; Zhou, L.X. Two species of genera Dacus were trapped in Sha Tau Kok. Plant Quar. 1991, 5, 426. [Google Scholar] [CrossRef]
- Xie, Z.F.; Chen, X.F.; Chen, Z.L.; Zhou, L.X.; Wu, J.Y.; Zhu, D.W. Monitoring survey of quarantine tephritids flies in Shenzhen. Plant Quar. 1995, 9, 133–137. [Google Scholar] [CrossRef]
- Deng, Y.P.; Qiu, Q. Occurrence and monitoring surveys of tephritids flies in Guangxi. Guangxi Hortic. 2008, 19, 22–24. [Google Scholar]
- Li, W.F.; Gong, X.Z.; Huang, Y.C.; Chen, Y.L.; Huang, J.Y.; Huang, S.G.; Chen, K.S. Tephritids species and occurrence dynamics in Guangxi. J. Southwest Univ. Nat. Sci. 2008, 30, 124–128. [Google Scholar] [CrossRef]
- Chen, X.; Liu, X.F.; Ye, H. The major fruit fly (Diptera: Tephritidae) pest species and their geographic distribution in Yunnan Province. Acta Ecol. Sin. 2010, 30, 717–725. [Google Scholar]
- Lin, M.G.; Cai, B.; Zhou, H.; Wang, X.J.; Li, F.; Shi, J. Study on population dynamics and integrated control of fruit fly pests in anti-season greenhouses hami melon (Cucumis melo L.) in Hainan Province. North Hortic. 2014, 115–118. Available online: https://oversea.cnki.net/kns/manage/export?filename=bfyy201424032&dbname=CJFDLAST2015 (accessed on 2 January 2023).
- Huang, Z.; Chen, S.P.; Xie, J.; Guo, Q.X. Rapid identification of Bactrocera latifrons (Diptera: Tephritidae) by using species-specific PCR technique. Acta Entomol. Sin. 2015, 58, 460–466. [Google Scholar] [CrossRef]
- CABI. Bactrocera latifrons (Solanum Fruit Fly); CABI Compendium: Wallingford, UK, 2022; p. 8719. [Google Scholar] [CrossRef]
- Cui, Z.; Zhou, Q.; Liu, Y.; Si, P.; Wang, Y. Molecular identification of citrus fruit flies and genetic diversity analysis of Bactrocera minax (Diptera: Tephritidae) populations in China based on mtDNA COI gene sequences. Acta Entomol. Sin. 2020, 63, 85–96. [Google Scholar]
- Yang, C.J.; Zhang, H.Y. Plant Pests Quarantine, 2nd ed.; Science Press: Beijing, China, 2009; ISBN 978-7-03-023570-1. [Google Scholar]
- Zhang, G.F.; Wang, F.L.; Lv, Z.C.; Huang, C.; Li, Y.J.; Guo, J.Y.; Li, C.R.; Wan, F.H. Research progress on the biology, ecology and the application of sterile insect technique on Bactrocera minax (Enderlein). J. Biosaf. 2015, 24, 171–176. [Google Scholar] [CrossRef]
- Xia, Y.; Ma, X.; Hou, B.; Ouyang, G. A Review of Bactrocera minax (Diptera: Tephritidae) in China for the Purpose of Safeguarding. Adv. Entomol. 2018, 6, 35–61. [Google Scholar] [CrossRef]
- Gao, L.Z. Population Genetic Structure Analysis of Bactrocera minax (Diptera:Tephritidae) in China Inferred from ND4 and Microsatellite Markers. Master’s Thesis, Southwest University, Chongqing, China, 2014. [Google Scholar]
- Li, Z.L. Studies on Spatiotemporal Distribution and Control of Bactrocera minax. Master’s Thesis, Huazhong Agricultural University, Wuhan, China, 2012. [Google Scholar]
- Yang, W.Z.; Qin, Y.J.; Wang, X.L.; Feng, X.D.; Li, Y.; Li, Z.H. Research progress on Bactrocera tsuneonis. China Plant Prot. 2022, 42, 21–28. [Google Scholar]
- Liu, M.J.; Cheng, L.L.; Zhang, X.J.; Tang, M.F.; Huang, F.; Xiao, C.; Dong, W.X. Daily activity and spatial distribution pattern of Bactrocera (Tetradacus) tsuneonis in orange orchard of Yongshan, Yunnan. Acta Agric. Jiangxi 2013, 25, 87–90. [Google Scholar] [CrossRef]
- Xia, S.W.; Song, X.P. Tetradacus tsuneonis were founded in Luodian and Bijie, Guizhou Province. Plant Quar. 1985, 47. Available online: https://oversea.cnki.net/KCMS/detail/detail.aspx?dbcode=CJFD&dbname=CJFD8589&filename=ZWJY198501019&uniplatform=OVERSEA&v=7O7qwyMKVVcROlr_scxh7otrNZYSOauhzu6neyntrw_ZIrAmw1XwOQ7a_8HK3spd (accessed on 2 January 2023).
- Zhang, Y.A. Tetradacus tsuneonis (Miyake) were founded in Pingshan, Sichuan. China Citrus 1984, 31–32. Available online: https://oversea.cnki.net/KCMS/detail/detail.aspx?dbcode=CJFD&dbname=CJFD7984&filename=FRUI198402017&uniplatform=OVERSEA&v=VqGT4wVTJTXeijknwOjlhMpWzr1bR47P0Ul7z9Rmxc2gFpA2_tqPHQGHjzn0cZGQ (accessed on 2 January 2023).
- Wan, F.H.; Zheng, X.B.; Guo, J.Y. Biology and Management of Invasive Alien Species in Agriculture and Forestry; Science Press: Beijing, China, 2005; ISBN 7-03-014409-0. [Google Scholar]
- Yue, Z.X. Monitoring results of quarantine tephritids flies in Henan Province. Plant Quar. 2002, 16, 361. [Google Scholar] [CrossRef]
- Fan, J.A. The survey of vegetable tephritids flies in Sichuan. J. Chang. Veg. 1993, 15–17+23. Available online: https://oversea.cnki.net/kns/manage/export?filename=cjsc199306011&dbname=CJFD9093 (accessed on 2 January 2023).
- Shang, M.Q.; Liu, C.H.; Zhang, D.M.; Jin, Y.X.; Xie, C.F.; Li, M.M.; Zhou, H.X. The quarantine pest, Zeugodacus scutellata (Hendel) was first time founded in Shandong. Plant Quar. 2021, 35, 38. [Google Scholar]
- Li, Y.J.; Wang, Z.; Zhang, D.M. Preliminary report on occurrence and integrated control technology of Bactrocera (Zeugodacus) scutellata (Hendel) and Dacus (Callantra) trimacula (Wang) in Zhengzhou. In Proceedings of the Symposium Proceedings; Henan Provincial Plant Protection Society, Henan Provincial Entomological Society, Henan Provincial Plant Pathology Society, Zhengzhou, China, 11 March 2022; pp. 165–170. [Google Scholar]
- Zhou, B. Bionomics of Bactrocera tau (Walker) (Diptera: Tephritidae) and the Effect of Foods on Population Dynamics. Master’s Thesis, Southwest Agricultural University, Chongqing, China, 2005. [Google Scholar]
- Lin, M.G.; Yang, Z.J.; Wang, X.J.; Li, J.Y.; Li, W.D. A taxonomic study of the subfamily Dacinae (Diptera:Tephritidae) from Hainan, China. Acta Entomol. Sin. 2006, 49, 310–314. [Google Scholar] [CrossRef]
- Li, H.P.; Lian, G.Y.; Dang, H.Y.; Ding, S.Y. Population dynamics of Dacus (Callantra) trimacula (Wang) and Bactrocera (Zeugodacus) scutellatus (Hendel) in Shanxi Province. J. Shanxi Agric. Sci. 2008, 36, 64–66. [Google Scholar] [CrossRef]
- Wu, S.Y.; Xie, G.Y.; Han, S.P.; Mao, H.Y.; Zhao, Z.H.; Yuan, G.H. Population dynamics monitoring of Bactrocera scutellata (Hendel) in Henan Province. J. Henan Agric. Univ. 2009, 43, 642–646+651. [Google Scholar] [CrossRef]
- Duan, Y.P. Dacus (Zeugodacus) scutellatus detected in Shaanxi. Plant Prot. 1989, 38–43. Available online: https://oversea.cnki.net/kns/manage/export?filename=zwbh198906020&dbname=CJFD8589 (accessed on 2 January 2023).
- Lin, H.F.; Gao, Y.B.; Yang, X.J.; Tao, W.P.; Yu, L.F. The monitoring survey of quarantine tephritids flies in Hefei. Plant Quar. 2007, 21, 111–112. [Google Scholar]
- Satar, A.; He, S.Y.; Tian, C.M.; Luo, Y.Q.; Yu, F.; Feng, X.F. Occurrence and distribution pattern of Carpomya vesuviana (Costa) pupae in the Turpan region. Plant Quar. 2008, 22, 295–297. [Google Scholar]
- He, S.Y.; Zhu, Y.F.; Satar, A.; Weng, J.B.; Chen, M.; Tian, C.M. Pest risk assessment of Carpomya vesuviana in China. Sci. Silvae Sin. 2011, 47, 107–116+197. [Google Scholar]
- Zhao, B. Primary Research of Biological Characteristics and Attractants of Rhagoletis batava obseuriosa (Diptera:Tephritidae) in the West of Inner Mongolia. Master’s Thesis, Hebei University, Baoding, China, 2017. [Google Scholar]
- Zhang, N. Research on Biology and Ecology of Rhagoletis batava (Diptera: Tephritidae) in Altay. Master’s Thesis, Xinjiang Agricultural University, Ürümqi, China, 2018. [Google Scholar]
- Zhu, J.M. Research on Genetic Diversity and Monitoring of Technology Different Geographical Populations of Rhagoletis batava Hering. Master’s Thesis, Xinjiang Agricultural University, Ürümqi, China, 2020. [Google Scholar]
- Han, B.B. Statistical analysis of Chinese literature about Bactrocera cucurbitae. J. Zhejiang Agric. Sci. 2021, 62, 983–986+991. [Google Scholar] [CrossRef]
- McQuate, G.T.; Liquido, N.J.; Nakamichi, K.A.A. Annotated World Bibliography of Host Plants of the Melon Fly, Bactrocera cucurbitae (Coquillett) (Diptera: Tephritidae). In Insecta Mundi; Center for Systematic Entomology: Gainesville, FL, USA, 2017. [Google Scholar]
- Chen, Q.H.; Chen, R.; Chen, S.G. Investigation of the spatial distribution of Zeugodacus cucurbitae on bitter melons. Mod. Agric. Sci. Technol. 2011, 143–144. [Google Scholar] [CrossRef]
- Jiang, X.L.; Ren, L.Q.; Xiao, S.; He, W.Z.; Sun, B.Z. Study on the biology of Bactrocera cucurbitae. Plant Quar. 2003, 17, 74–76. [Google Scholar] [CrossRef]
- Zhang, Q.S. Biological characteristics and control of Zeugodacus cucurbitae. China Veg. 2002, 37–38. [Google Scholar] [CrossRef]
- Chen, H.D.; Zhou, C.Q.; Yang, P.J.; Liang, G.Q. On the seasonal population dynamics of Bactrocera cucurbitae, Bactrocera dorsalis and Bactrocera tau in Guangzhou. Acta Phytophylacica Sin. 1995, 22, 348–354. [Google Scholar]
- Deng, J.Q.; Zhu, X.M.; Han, P.; Yang, G.P.; Li, Z.C. Research progress of the melon fly, Bactrocera cucurbitae (Diptera: Tephritidae) in China. Plant Quar. 2021, 35, 1–7. [Google Scholar] [CrossRef]
- Chen, J.H.; Xu, Z.H.; Chen, W.M.; Jin, G.L.; Wang, Z.L. Notes on five species of tephritid fruit flies in Zhejiang Province. Acta Agric. Zhejiangensis 2006, 18, 28–31. [Google Scholar] [CrossRef]
- Kong, L.B.; Lin, W.; Li, Z.H.; Wan, F.H.; Wang, Z.L.; Huang, G.S. A predication of potential georaphic distribution of melon fruit fly based on CLIMEX and DIVA-GIS. Acta Phytophylacica Sin. 2008, 35, 148–154. [Google Scholar]
- Xu, H.G.; Qiang, S. China’s Invasive Alien Species; Science Press: Beijing, China, 2011; ISBN 978-7-03-030388-2. [Google Scholar]
- Zhang, Y.N. Genetic Diversity Analysis of the Melon Fly, Bactrocera cucuribitae (Diptera:Tephritidae) and Screening Olfactory Receptor Genes. Master’s Thesis, Hainan University, Haikou, China, 2017. [Google Scholar]
- Wang, Y.T.; Bai, Q.; Chen, H.S.; Tian, Z.Y.; Gao, X.Y.; Zhou, Z.S. Distribution differences between Zeugodacus cucurbitae and Zeugodacus tau in China. J. Environ. 2022, 44, 1170–1175. [Google Scholar]
- Zhao, X.Q.; Fan, J.A. A Study on the ecogeographical distribution of fruit flies infecting fruits and vegetables in Sichuan. J. Southwest Agric. Univ. 1996, 18, 515–518. [Google Scholar] [CrossRef]
- Jiang, S.F.; Zhang, Y.A.; Zhao, Y.X.; Ming, Y. Investigation of fruit and vegetable tephritid flies in Jinsha River Valley. Sichuan J. Agric. Sci. 1987, 2, 26–31. [Google Scholar] [CrossRef]
- Wang, J.C.; Yuan, Z.L.; Yang, J.Y. A dangerous pest was found in Gansu, Zeugodacus tau. Gansu Agric. Sci. Technol. 1994, 42. Available online: https://oversea.cnki.net/kns/manage/export?filename=gsnk408.024&dbname=CJFD9495 (accessed on 2 January 2023).
- Li, X.Z. The Population Character of Bactrocera tau and Its Physiological Modulation Mechanism to Diets and Heat Shocks. Ph.D. Thesis, Southwest University, Chongqing, China, 2007. [Google Scholar]
- Liang, R.C.; Huang, S.G.; Huang, J.Y.; Zhao, Y.X.; Ming, Y. The investigation of fruit and vegetable tephritid flies in Guangxi. Guangxi Agric. Sci. 1985, 36–40+55. [Google Scholar]
- Zhang, Z.M.; Fang, B.Q. Preliminary investigation report on fruit and vegetable tephritid in Guiyang. Guizhou Agric. Sci. 1988, 14. Available online: https://oversea.cnki.net/kns/manage/export?filename=gate198804002&dbname=CJFD8589 (accessed on 2 January 2023).Li, X.Z. The Population Character of Bactrocera tau and Its Physiological Modulation Mechanism to Diets and Heat Shocks. Ph.D. Thesis, Southwest University, Chongqing, China, 2007. [Google Scholar]
- Zhang, Y.; Chen, J.Y. Recent advances in research of Bactrocera (Zeugodacus) tau (Walker) in China. Chin. J. Trop. Agric. 2018, 38, 70–77. [Google Scholar] [CrossRef]
- Zhou, S.K.; Li, G.X.; Qiu, Z.H.; Li, Z.; Li, X.R. Observation of biological characteristics and control of Zeugodacus tau (Walker). Acta Phytophylacica Sin. 1993, 11–12. Available online: https://oversea.cnki.net/kns/manage/export?filename=zwbh199305004&dbname=CJFD9093 (accessed on 2 January 2023).
- White, I.M. Taxonomy of the Dacina (Diptera: Tephritidae) of Africa and the Middle East. Afr. Entomol. 2006, 1–156. [Google Scholar]
- Guo, T.D.; Gong, Q.T.; Ye, B.H.; Wu, H.B.; Sun, R.H. Progress in domestic research on Bactrocera dorsalis (Hendel). Deciduous Fruits 2019, 51, 43–46. [Google Scholar] [CrossRef]
- Wang, Z.H.; Feng, H.L.; Lv, Z.Z.; Zhao, H.; Guo, M.X.; Zhu, T.M.; Zeng, X.D.; Chen, J.J. Research progress in detection technology of tephritids insects. Hubei Plant Prot. 2009, 28–30. [Google Scholar] [CrossRef]
- Jiang, F. Technique System for Molecular Identification of Quarantine Fruit Flies in China. Ph.D. Thesis, China Agricultural University, Beijing, China, 2015. [Google Scholar]
- Liu, X.F.; Wang, D.M.; Ye, H. Overview on Research of Bactrocera correcta (Bezzi). Trop. Agric. Sci. Technol. 2005, 28, 30–33. [Google Scholar]
- Yan, Z.H.; Liu, N.; Tang, G.W.; Wang, W.X.; Li, L.L.; Xiao, M.; Tao, M.; Chen, G.H. Daily activity and their host species survey of guava fruit fly. Southwest J. Agric. Sci. 2016, 29, 1864–1868. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, Y.H.; Zhao, L.L.; Zhou, X. Research progress of oriental fruit fly Bactrocera dorsalis (Hendel) (Diptera: Tetriphitidae). Chin. Agric. Sci. Bull. 2008, 24, 391–397. [Google Scholar]
- Yan, Z.H.; Zhang, J.L.; Fang, X.J.; Zhu, W.L.; Zhang, W.H.; Wu, G.Y.; Zhang, L.; Tao, M.; Chen, G.H. Host species of the insect pest Bactrocera dorsalis in Mengzi, Yunnan, and population dynamics of Bactrocera dorsalis and its related environmental factors in loquat garden. Chin. J. Ecol. 2016, 35, 3037–3044. [Google Scholar] [CrossRef]
- Fang, S.M.; Luo, X.F.; Gao, H.L.; Zhu, L.L.; Li, J.B. Investigation on wild host plants of Bactrocera dorsalis in Huizhou Area. J. Anhui Agric. Sci. 2019, 47, 146–147+163. [Google Scholar]
- Ma, X.L.; Li, Z.H.; Ni, W.L.; Qu, W.W.; Wu, J.J.; Wan, F.H.; Hu, X.N. The Current and Future Potential Geographical Distribution of the Solanum Fruit Fly, Bactrocera latifrons (Diptera: Tephritidae) in China. In Proceedings of the International Conference on Computer and Computing Technologies in Agriculture, Beijing, China, 29–31 October 2011; Springer: Berlin/Heidelberg, Germany, 2012; pp. 236–246. [Google Scholar]
- Liquido, N.J.; Harris, E.J.; Dekker, L.A. Ecology of Bactrocera latifrons (Diptera: Tephritidae) Populations: Host Plants, Natural Enemies, Distribution, and Abundance. Ann. Entomol. Soc. Am. 1994, 87, 71–84. [Google Scholar] [CrossRef]
- Xiong, H.L.; Xiang, Z.J.; Liu, H.; Zhou, H.Z.; Feng, X.D.; Wang, Y.M.; Sun, J.S.; Qin, X.J.; Wang, F.X. Detection and Identification Method for Bactrocera tsuneonis (Miyake); Ministry of Agriculture and Rural Affairs of the People’s Republic of China (Former: Ministry of Agriculture of the People’s Republic of China): Beijing, China, 2011. [Google Scholar]
- Wang, J.W.; Li, Z.H.; Chen, H.J.; Geng, J.; Wang, Z.L.; Wan, F.H. The potential geographic distribution of Bactrocera (Tetradacus) tsuneonis (Diptera: Tephritidae). Plant Quar. 2009, 23, 1–4. [Google Scholar] [CrossRef]
- Xu, Z. Studies about the Selective Preference of Bactrocera cucuribitae (Coquillett) to Cucurbitaceae Plants. Master’s Thesis, Fujian Agriculture and Forestry University, Fuzhou, China, 2018. [Google Scholar]
- Al Baki, M.A.; Keum, E.; Kim, H.; Song, Y.; Kim, Y.; Kwon, G.; Park, Y. Age grading and gene flow of overwintered Bactrocera scutellata populations. J. Asia-Pac. Entomol. 2017, 20, 1402–1409. [Google Scholar] [CrossRef]
- Li, X.Z.; Yang, H.Y.; Hu, K.; Wang, J.G. Temporal Dynamics of Bactrocera (Zeugodacus) tau (Diptera: Tephritidae) Adults in North Jiangxi, a Subtropical Area of China Revealed by Eight Years of Trapping with Cuelure. J. Asia-Pac. Entomol. 2020, 23, 1–6. [Google Scholar] [CrossRef]
- Liu, L.H.; Liu, Y.H.; Zhou, B.; Zhang, Y.Q. Studies on damage and quantity dynamics of Bactrocera tau Walker (Trypetidae: Bactrocera) in different host fields. J. Southwest Univ. Nat. Sci. 2005, 27, 176–179. [Google Scholar] [CrossRef]
- Xu, Y.J.; Zeng, L.; Lu, Y.Y.; Lin, J.T. Ovipositional selection of the Bactrocera dorsalis (Hendel) to the different fruit. J. Huazhong Agric. Univ. 2005, 24, 25–26. [Google Scholar] [CrossRef]
- Huang, Z.; Guo, Q.X.; Wu, Q.M.; Huang, K.H. Morphology, hazards and China-invading risk of Bactrocera correcta. Acta Agric. Jiangxi 2014, 26, 61–63+67. [Google Scholar] [CrossRef]
- Weng, W.S.; Deng, X.R.; He, C.R.; Xu, B.H. Larvae of tephritid flies caused diarrhea 1 case. Chin. J. Parasit. Dis. Control 2004, 17, 25. [Google Scholar] [CrossRef]
- Yin, Z.H. Biological Characteristics and Selection to Host Fruit of Neoceratitis asiatica (Becker). Master’s Thesis, Peking Union Medical College, Beijing, China, 2021. [Google Scholar]
- Zhang, Q.Y.; Lin, Z.J.; Liu, J.Y.; Chen, H.Z.; Gao, Q.Z.; Sun, G.K.; Hong, Z.Q.; Sun, D.H.; Chen, J.F. Study on the biology of Bactrocera dorsalis. Entomol. J. East China 1998, 7, 68–71. [Google Scholar]
- Xiao, S.; Jiang, X.L.; Zhang, C.L.; Ying, X.S.; Yang, Y.X. Observation of biological characteristics of Bactrocera dorsalis and Zeugodacus cucurbitae in Ruili. Plant Quar. 2001, 15, 332–336. [Google Scholar]
- He, S.Y.; Zhu, Y.F.; Satar, A.; Yu, F.; Weng, J.B.; Tian, C.M. Occurrence of Carpomya vesuviana in Turpan region. Chin. Bull. Entomol. 2009, 46, 930–934+822. [Google Scholar]
- Xie, Q.; Zhang, R.J. Study advance on biology and ecology of Bactrocera dorsalis (Hendel) and its control. Ecol. Sci. Ecol. Sci. 2005, 24, 52–56. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Lin, Z.J.; Liu, J.Y.; Lin, X.Y. Biological characteristics of Dacus tau (Walker). Plant Quar. 1991, 5, 164–167. [Google Scholar] [CrossRef]
- Yuan, S.Y.; Kong, Q.; Li, Z.Y.; Xiao, C.; Chen, B.; Zhang, D.G. Study on biology of Bactrocera (Zeugodacus) cucurbitae. Acta Agric. Boreali-Occident. Sin. 2005, 14, 43–45+67. [Google Scholar] [CrossRef]
- He, W.Z.; Sun, B.Z.; Li, C.J.; Long, Z.B. Bionomics of Bactrocera dorsalis and its control in Hekou County of Yunnan Province. Entomol. Knowl. 2002, 39, 50–52. [Google Scholar] [CrossRef]
- Lin, W.W.; Wang, F.L.; Huang, C.; Wang, X.J.; Li, C.R. Biological characteristics of the Bactrocera minax. Xiandai Hortic. 2012, 79–81. [Google Scholar] [CrossRef]
- Chen, Z.Z. Pupal Diapause Termination of Bactrocera minax and its Parasitoid Biology. Ph.D. Thesis, Huazhong Agricultural University, Wuhan, China, 2018. [Google Scholar]
- Zhao, B.; Su, Z.; Li, S.S.; Men, J.; Cheng, T.M.; Wei, J.R. Biological characteristics of Rhagoletis batava obseuriosa (Diptera: Tephritidae). For. Res. 2017, 30, 576–581. [Google Scholar] [CrossRef]
- Liang, G.Q.; Liang, G.Z.; Lin, M.; Liang, F. Tephritids and Its Prevention; Guangdong Science and Technology Press: Guangzhou, China, 1993; ISBN 7-5359-1197-8. [Google Scholar]
- Ma, X.Y.; Wang, M.; Zu, D.; Zhang, C.N.; Yang, Y. Biological characteristics and toxicity determination of Bactrocera cucurbitae. Guangdong Agric. Sci. 2013, 40, 77–79+82. [Google Scholar] [CrossRef]
- Lu, H.X.; He, K.P.; Ruan, H.F.; Mu, B.Z. The biological features of Chinese citrus fly, Dacus citri (Chen). J. Hubei Agric. Coll. 1997, 17, 11–15. [Google Scholar]
- Yuan, R.L.; Yang, S.; Wang, X.W.; Chen, P. Test on flight ability of Bactrocera dorsalis. J. West China For. Sci. 2014, 43, 66–71. [Google Scholar] [CrossRef]
- Chen, P.; Ye, H.; Mu, Q.A. Migration and dispersal of the Oriental fruit fly, Bactrocera dorsalis in regions of Nujiang River based on fluorescence mark. Acta Ecol. Sin. 2007, 27, 2468–2476. [Google Scholar] [CrossRef]
- Li, W.F.; Yang, L.; Tang, K.; Zeng, L.; Liang, G.W. Microsatellite polymorphism of Bactrocera dorsalis (Hendel) populations in China. Acta Ecol. Sin. 2007, 50, 1255–1262. [Google Scholar] [CrossRef]
- Shi, W.; Kerdelhué, C.; Ye, H. Population Genetic Structure of the Oriental Fruit Fly, Bactrocera dorsalis (Hendel) (Diptera: Tephritidae) from Yunnan Province (China) and Nearby Sites across the Border. Genetica 2010, 138, 377–385. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Huang, S.S.; Zeng, D.Q.; Li, Z.L.; Lin, Y. Research progress in host selection of fruit fly. J. South. Agric. 2012, 43, 621–625. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Zhou, X.M.; Huang, S.Z.; Han, Q.H.; Lei, C.L. The oviposition preference of Bactrocera (Tetradacus) minax. Entomol. Knowl. 2007, 44, 867–870. [Google Scholar] [CrossRef]
- Ren, L.L.; Qi, L.Y.; Jiang, Q.G.; Zhou, S.D.; Dai, H.G. Oviposition preference of oriental fruit fly, Bactrocera dorsalis. Entomol. Knowl. 2008, 45, 593–597. [Google Scholar]
- Tai, H.K.; Li, Z.Y.; Luo, H.Y.; Li, B.; Zhao, X.H.; Sun, W.; Yang, S.S.; Xiao, C. Effect of the color and the solution of sweet bait on the common guava fruit fly. J. Anhui Agric. Sci. 2009, 37, 6481–6482. [Google Scholar] [CrossRef]
- Zheng, X.P.; Liu, Q.S.; Xie, Q.; Zhang, R.J. Influence of larval feeding experience on adult oviposition selectivity of Bactrocera dorsalis (Hendel). Acta Sci. Nat. Univ. Sunyatseni 2007, 46, 84–87+102. [Google Scholar] [CrossRef]
- Yuan, S.Y.; Xiao, C.; Kong, Q.; Chen, B.; Li, Z.Y.; Gao, Y.H. Oviposition prefernce of Bactrocera dorsalis Hendel. Acta Agric. Univ. Jiangxiensis 2005, 27, 81–84. [Google Scholar] [CrossRef]
- Denno, R.F.; McClure, M.S.; Ott, J.R. Interspecific Interactions in Phytophagous Insects: Competition Reexamined and Resurrected. Annu. Rev. Entomol. 1995, 40, 297–331. [Google Scholar] [CrossRef]
- Reitz, S.R.; Trumble, J.T. Competitive Displacement among Insects and Arachnids. Annu. Rev. Entomol. 2002, 47, 435–465. [Google Scholar] [CrossRef]
- Ma, J.; Lin, J.T.; Chen, H.J.; Hu, X.N. The potential role of interspecific competition in fruit fly invasions. J. Environ. Entomol. 2009, 31, 361–364. [Google Scholar] [CrossRef]
- Liu, H. Study of Interspecific Interaction of Bactrocera dorsalis Hendel and Bectrocera correcta Bezzi (Diptera:Tephritidae). Ph.D. Thesis, Southwest Agricultural University, Guangzhou, China, 2016. [Google Scholar]
- Christenson, L.D.; Foote, R.H. Biology of Fruit Flies. Annu. Rev. Entomol. 1960, 5, 171–192. [Google Scholar] [CrossRef]
- Ma, J.; Hu, X.N.; Liu, H.J. Interspecific competition in tephritids flies and their role in invasion. In Proceedings of the Abstracts of the Second National Conference on Biological Invasion, Guangzhou, China, 21–24 November 2008; p. 64. [Google Scholar]
- Zhou, C.Q.; Mei, L.Z. Studies of intraspecific competition between Bactrocera cucurbitae (Coquillett) and Bactrocera tau (Walk) (Diptera: Tephritidae). Acta Sci. Nat. Univ. Sunyatseni 1999, 38, 60–64. [Google Scholar] [CrossRef]
- Zhang, J.L.; Yan, Z.H.; Fang, X.J.; Wu, H.; Liu, H.P.; Zhu, W.L.; Li, A.Q.; Du, Y.; Chen, G.H.; Tao, M. Oviposition selectivity and competitive research on Bactrocera cucurbitae (Coquillett) and Bactrocera tau (Walker). J. Yunnan Agric. Univ. Nat. Sci. 2017, 32, 427–431. [Google Scholar] [CrossRef]
- Wu, J.J.; Liu, H.J.; Gu, Y.J.; Hu, X.N.; Li, C.Y. Technical elements of tephritids flies monitoring I. Plant Quar. 2009, 23, 49–51. [Google Scholar]
- Kong, D.Y.; Teng, S.N.; Sun, T.; Ye, J. Development of the technique for fruit fly survey. Plant Quar. 2021, 35, 16–20. [Google Scholar] [CrossRef]
- ISPM 26. Establishment of Pest Free Areas for Fruit Flies (Tephritidae); Food and Agricultural Organization of the United Nations, Secretariat of the International Plant Protection Convention: Rome, Italy, 2018; p. 60. [Google Scholar]
- Li, Z.; Hong, T.S.; Wen, T.; Zhu, Z.C.; Song, S.R.; Sun, D.Z. Design and development of orchard fruit fly monitoring system based on the internet-of-things. J. Hunan Agric. Univ. Nat. Sci. 2015, 41, 89–93. [Google Scholar] [CrossRef]
- Wang, L.Y.; Yang, C.X.; Guo, B.B.; She, D.M.; Mei, X.D.; Yang, X.L.; Ning, J. Research progress and application prospects on insect sex pheromone. Chin. J. Pestic. Sci. 2022, 24, 997–1016. [Google Scholar] [CrossRef]
- Metcalf, R.L.; Metcalf, E.R. Plant Kairomones in Insect Ecology and Control. In Contemporary Topics in Entomology; Springer: New York, NY, USA, 1992. [Google Scholar]
- Tan, K.H.; Nishida, R. Zingerone in the Floral Synomone of Bulbophyllum baileyi (Orchidaceae) Attracts Bactrocera Fruit Flies during Pollination. Biochem. Syst. Ecol. 2007, 35, 334–341. [Google Scholar] [CrossRef]
- Tan, K.H.; Nishida, R. Synomone or Kairomone?-Bulbophyllum apertum Flower Releases Raspberry Ketone to Attract Bactrocera Fruit Flies. J. Chem. Ecol. 2005, 31, 497–507. [Google Scholar] [CrossRef]
- Tan, K.H.; Tan, L.T.; Nishida, R. Floral Phenylpropanoid Cocktail and Architecture of Bulbophyllum vinaceum Orchid in Attracting Fruit Flies for Pollination. J. Chem. Ecol. 2006, 32, 2429–2441. [Google Scholar] [CrossRef]
- Tan, K.H.; Nishida, R.; Toong, Y.C. Floral Synomone of a Wild Orchid, Bulbophyllum cheiri, Lures Bactrocera Fruit Flies for Pollination. J. Chem. Ecol. 2002, 28, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- Nishida, R.; Tan, K.H.; Wee, S.L.; Hee, A.K.W.; Toong, Y.C. Phenylpropanoids in the Fragrance of the Fruit Fly Orchid, Bulbophyllum cheiri, and Their Relationship to the Pollinator, Bactrocera papayae. Biochem. Syst. Ecol. 2004, 32, 245–252. [Google Scholar] [CrossRef]
- Park, S.J.; Faveri, S.G.D.; Cheesman, J.; Hanssen, B.L.; Cameron, D.N.S.; Jamie, I.M.; Taylor, P.W. Zingerone in the Flower of Passiflora maliformis Attracts an Australian Fruit Fly, Bactrocera jarvisi (Tryon). Molecules 2020, 25, 2877. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.W.; Zhou, P.; Zhang, J.H.; Yang, D.; Li, Z.H.; Zhang, X.L.; Zhu, S.F.; Yu, Y.X.; Chen, N.Z. Identification of Odorant Binding Proteins in Carpomya vesuviana and Their Binding Affinity to the Male-Borne Semiochemicals and Host Plant Volatiles. J. Insect Physiol. 2017, 100, 100–107. [Google Scholar] [CrossRef]
- Zhou, Q.; Liu, L.; You, K.X.; Jing, Q. A Kind of Sex Pheromone and Its Extraction Method and Application for Citrus Fruit Fly. Invention Patent CN103766336A; Hunan Normal University, Changsha, China, 7 May 2014. [Google Scholar]
- Du, Y.G.; Chen, J.H.; Ji, Q.E. The attraction of one new protein for Bactrocera dorsalis. J. Fujian Coll. For. 2007, 27, 259–262. [Google Scholar] [CrossRef]
- Liu, J.M.; Dong, M.H.; Du, Y.G.; Dai, H.J.; Han, J.H. Killing effect of beer waste yeast hydrolysate protein combined with borax on Bactrocera dorsalis. Shandong Agric. Sci. 2013, 45, 110–112. [Google Scholar] [CrossRef]
- Yang, Q.Y. Research on the Behavioral Responses of Bactrocera dorsalis to the Volatile Compounds from Protein Bait. Master’s Thesis, Fujian Agriculture and Forestry University, Fuzhou, China, 2016. [Google Scholar]
- Yang, Q.Y.; Idrees, A.; Du, Y.G.; Ji, Q.E.; Cai, P.M.; Gu, X.H.; Wang, B.; Chen, J.H. Attraction of 7 volatile compounds from spent brewer’s yeast enzymatic hydrolysate to adult Bactrocera dorsalis (Hendel). J. Fujian Agric. For. Univ. Nat. Sci. Ed. 2016, 45, 505–509. [Google Scholar] [CrossRef]
- Xiao, C.; Jiang, X.L.; Ye, W.F.; Zhou, L.B. Effect of sugar on the effect of hydrolyzed protein on the trapping of tephritids flies. In Proceedings of the Abstracts of the Third National Congress on Biological Invasions, Haikou, China, 26 November 2010; p. 384. [Google Scholar]
- Zhou, X.W.; Niu, C.Y.; Han, P.; Desneux, N. Field Evaluation of Attractive Lures for the Fruit Fly Bactrocera minax (Diptera: Tephritidae) and Their Potential Use in Spot Sprays in Hubei Province (China). J. Econ. Entomol. 2012, 105, 1277–1284. [Google Scholar] [CrossRef]
- Su, R.R.; Yi, X.L.; Liu, J.M.; Zheng, X.L.; Lu, W.; Wang, X.Y. Progress in research on natural enemies of the oriental fruit fly, Bactrocera dorsalis and the application of these in the biological control of this pest. Chin. J. Appl. Entomol. 2021, 58, 1026–1037. [Google Scholar] [CrossRef]
- Lin, L.; Huang, J.C.; Chen, J.H.; Ji, Q.E.; Yang, J.Q. Parasitical efficiency of Diachasmimorpha longicaudata on Bactrocera dorsalis. Entomol. J. East China 2006, 15, 288–290. [Google Scholar]
- Geng, J.L.; Yang, J.Q.; Zheng, M.L.; Huang, J.C.; Ji, Q.E.; Chen, J.H. Effects of agar to water ration on the parasite efficiency of Fopius sriasnus. J. Fujian Coll. For. 2009, 29, 166–168. [Google Scholar] [CrossRef]
- Liu, J.M.; Huang, Q.C.; Deng, T.J.; Yang, Z.Q.; Lu, W. Field Control of Bactrocera dorsalis by artificial release with Diachasmimorpha longicaudata (Ashmead). Guangdong Agric. Sci. 2021, 48, 118–123. [Google Scholar] [CrossRef]
- Huang, J.C.; Ji, Q.E.; Yang, J.Q.; Chen, J.H.; Guo, Q.L.; Geng, J.L. Successful introduction of the dominant species of the parasitic wasp for Bactrocera dorsalis, Fopius arisanus (Sonan) in China. In Proceedings of the Abstracts of the First National Conference on Biological Invasion, Fuzhou, China, 7 December 2007; p. 251. [Google Scholar]
- Guo, Q.L.; Chen, J.H.; Lan, H.B.; Ji, Q.E.; Lin, W.C.; Pan, Y.H.; Zhuang, M.S. Parasitical efficiency of Fopius arisanus on eggs of Bactrocera dorsalis. Chin. J. Trop. Agric. 2015, 35, 51–52+59. [Google Scholar]
- Sun, G.K.; Chen, J.F.; Zhang, Q.Y.; Lin, Z.J. Preliminary report on integrated control trials of Bactrocera dorsalis. Entomol. J. East China 2000, 9, 115–118. [Google Scholar]
- Bai, Q.; Song, F.M. Preliminary report on the occurrence and control of mango damage of Bactrocera dorsalis. Guangxi Trop. Agric. 1997, 19–22. Available online: https://oversea.cnki.net/kns/manage/export?filename=rdnk199704007&dbname=CJFD9697 (accessed on 2 January 2023).
- McGraw, E.A.; O’Neill, S.L. Beyond Insecticides: New Thinking on an Ancient Problem. Nat. Rev. Microbiol. 2013, 11, 181–193. [Google Scholar] [CrossRef]
- Lees, R.S.; Gilles, J.R.L.; Hendrichs, J.; Vreysen, M.J.B.; Bourtzis, K. Back to the Future: The Sterile Insect Technique against Mosquito Disease Vectors. Curr. Opin. Insect Sci. 2015, 10, 156–162. [Google Scholar] [CrossRef]
- Bouyer, J.; Culbert, N.J.; Dicko, A.H.; Pacheco, M.G.; Virginio, J.; Pedrosa, M.C.; Garziera, L.; Pinto, A.T.M.; Klaptocz, A.; Germann, J.; et al. Field Performance of Sterile Male Mosquitoes Released from an Uncrewed Aerial Vehicle. Sci. Robot. 2020, 5, eaba6251. [Google Scholar] [CrossRef]
- Bouyer, J.; Yamada, H.; Pereira, R.; Bourtzis, K.; Vreysen, M.J.B. Phased Conditional Approach for Mosquito Management Using Sterile Insect Technique. Trends Parasitol. 2020, 36, 325–336. [Google Scholar] [CrossRef]
- Cai, C.H. The Effects of Gut Microbiota on the Growth and the Repair of Irradiated Damage in Bactrocera dorsalis. Ph.D. Thesis, Huazhong Agricultural University, Wuhan, China, 2020. [Google Scholar]
- Wang, H.S.; Zhao, C.D.; Li, H.X.; Lou, H.Z.; Liu, Q.R.; Kang, W.; Hu, J.G.; Zhang, H.Q.; Chu, J.M.; Xia, D.R.; et al. Effectiveness of radiation sterile technology for the control of citrus flies, Dacus citris. J. Nucl. Agric. Sci. 1990, 4, 135–138. [Google Scholar]
- Wang, H.S.; Hu, J.G.; Lu, D.G.; Kang, W.; Zhang, H.Q. Demonstration trial of using radiation sterile technology to control citrus flies, Dacus citris. Chin. J. Biol. Control. 1995, 11, 13–16. [Google Scholar] [CrossRef]
- Hu, J.F.; Yang, J.Q.; Chen, J.H.; Huang, J.C.; Ji, Q.E.; He, R.B. Effect of releasing sterile male of oriental fruit fly in the field. J. Fujian Agric. For. Univ. Nat. Sci. Ed. 2011, 40, 582–584. [Google Scholar] [CrossRef]
- Ye, W.D.; Li, F.; Zou, Y.X.; Wu, S.Y.; Wu, X.X. Origin and development of the Integrated Pest Management (IPM) concept. South China Agric. 2022, 16, 30–32+42. [Google Scholar] [CrossRef]
- Lv, J.Y.; Meng, Z.J. The olfactory recognition mechanism of herbivore insects on plant volatiles: A review. Chin. Agric. Sci. Bull. 2022, 38, 122–129. [Google Scholar]
| Specific Name | Regions | Provinces | Native Range | First Reported | References |
|---|---|---|---|---|---|
| Bactrocera correcta | East | Taiwan | India and South-East Asia | 1982, Yunnan | [10,11,12,13,14,15,16,17] |
| South | Guangxi Zhuangzu Zizhiqu | ||||
| Southwest | Sichuan (only detected in Miyi County) and Yunnan | ||||
| Bactrocera dorsalis | Central | Hubei and Hunan | South-East China | 1911, Taiwan | [16,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32] |
| East | Anhui, Jiangsu, Zhejiang, Shanghai Shi ⊙, Jiangxi, Fujian and Taiwan | ||||
| South | Guangdong, Guangxi Zhuangzu Zizhiqu, Hainan and Hong Kong ● | ||||
| Southwest | Guizhou, Sichuan, Chongqing Shi ⊙ and Yunnan | ||||
| Bactrocera latifrons (only captured by bait traps) | East | Fujian and Taiwan | South-East Asia | - | [16,33,34,35,36,37,38,39,40,41,42,43] |
| South | Hainan, Guangdong and Guangxi Zhuangzu Zizhiqu | ||||
| Southwest | Guizhou and Yunnan | ||||
| Bactrocera minax | Northwest | Shaanxi | China | - | [44,45,46,47,48,49] |
| East | Jiangxi and Taiwan | ||||
| Central | Hubei and Hunan | ||||
| South | Guangxi Zhuangzu Zizhiqu | ||||
| Southwest | Guizhou, Sichuan, and Yunnan | ||||
| Bactrocera tsuneonis | East | Taiwan | East Asia | 1940, Sichuan | [16,20,50,51,52,53,54] |
| Central | Hunan | ||||
| Southwest | Guizhou, Sichuan, and Yunnan | ||||
| Zeugodacus scutellatus (only captured by bait traps) | North | Shanxi | East Asia | 1912, Taiwan | [16,52,55,56,57,58,59,60,61,62,63,64] |
| Northwest | Shaanxi (only 6 adults captured by bait traps in 1984) | ||||
| East | Anhui, Jiangxi, Fujian and Taiwan | ||||
| Central | Henan, Hubei and Hunan | ||||
| South | Guangdong, Guangxi Zhuangzu Zizhiqu and Hainan | ||||
| Southwest | Guizhou, Sichuan, Chongqing Shi ⊙ and Yunnan | ||||
| Carpomya vesuviana | Northwest | Xinjiang Uygur Zizhiqu (currently limited in Turpan region and under official control) | India | 2007, Xinjiang (Turpan) | [65,66] |
| Rhagoletis batava obseuriosa | North | Nei Mongol Zizhiqu | Russia | 1985, Liaoning | [67,68,69] |
| Northwest | Shaanxi and Xinjiang Uygur Zizhiqu | ||||
| Zeugodacus cucurbitae | East | Zhejiang, Jiangxi, Fujian and Taiwan | India | 1960, Taiwan | [16,39,70,71,72,73,74,75,76,77,78,79,80] |
| Central | Hubei and Hunan | ||||
| South | Guangdong, Guangxi Zhuangzu Zizhiqu, Hainan, and Hong Kong ● | ||||
| Southwest | Guizhou, Sichuan Chongqing Shi ⊙ and Yunnan | ||||
| Zeugodacus tau | East | Zhejiang, Jiangxi, Fujian and Taiwan | Asia | 1912, Guangdong and Yunnan | [16,59,77,81,82,83,84,85,86,87,88,89] |
| Central | Henan, Hubei and Hunan | ||||
| South | Guangdong, Guangxi Zhuangzu Zizhiqu and Hainan | ||||
| Southwest | Guizhou, Sichuan, Chongqing Shi ⊙ and Yunnan |
| Tephritid Species | Plant Type | Plant Family | Plant Species | Degree of Damage | References |
|---|---|---|---|---|---|
| Bactrocera correcta | Fruit | Anacardiaceae | Anacardium occidentale | nd | [3,94,95] |
| Mangifera indica | +++ | ||||
| Annonaceae | Annona squamosa | ++ | |||
| Combretaceae | Terminalia catappa | nd | |||
| Musaceae | Musa nana | ++ | |||
| Myrtaceae | Psidium guajava | +++ | |||
| Syzygium samarangense | nd | ||||
| Oxalidaceae | Averrhoa carambola | +++ | |||
| Rhamnaceae | Ziziphus jujuba | nd | |||
| Ziziphus mauritiana | ++ | ||||
| Rosaceae | Prunus salicina | + | |||
| Prunus spp. | nd | ||||
| Pseudocydonia sinensis | ++ | ||||
| Pyrus pyrifolia | + | ||||
| Rutaceae | Citrus maxima | + | |||
| Citrus reticulata | ++ | ||||
| Citrus sinensis | + | ||||
| Sapotaceae | Manilkara zapota | nd | |||
| Vegetable | Cucurbitaceae | Cucumis sativus | + | ||
| Momordica charantia | ++ | ||||
| Solanaceae | Capsicum annuum | + | |||
| Solanum lycopersicum | + | ||||
| Solanum melongena | + | ||||
| Bactrocera dorsalis | Fruit | Actinidiaceae | Actinidia fulvicoma | + | [96,97,98] |
| Anacardiaceae | Mangifera indica | +/++++ | |||
| Annonaceae | Desmos chinensis | + | |||
| Ebenaceae | Diospyros kaki | + | |||
| Diospyros morrisiana | ++ | ||||
| Diospyros tutcheri | + | ||||
| Euphorbiaceae | Phyllanthus emblica | + | |||
| Melastomataceae | Melastoma dodecandrum | + | |||
| Moraceae | Broussonetia kaempferi | + | |||
| Broussonetia papyrifera | + | ||||
| Ficus hirta | + | ||||
| Ficus sagittata | + | ||||
| Musaceae | Musa nana | nd | |||
| Myricaceae | Myrica rubra | ++ | |||
| Myrtaceae | Acmena acuminatissima | + | |||
| Cleistocalyx operculatus | ++ | ||||
| Psidium guajava | +++/++++ | ||||
| Rhodomyrtus tomentosa | ++ | ||||
| Syzygium jambos | ++++ | ||||
| Syzygium levinei | + | ||||
| Syzygium samarangense | ++++ | ||||
| Oxalidaceae | Averrhoa carambola | +++ | |||
| Punicaceae | Punica granatum | +++ | |||
| Rhamnaceae | Ziziphus jujuba | ++++ | |||
| Ziziphus spp. | nd | ||||
| Rhizophoraceae | Carallia brachiata | ++ | |||
| Rosaceae | Amygdalus davidiana | ++ | |||
| Duchesnea indica | + | ||||
| Eriobotrya fragrans | + | ||||
| Eriobotrya japonica | ++/++++ | ||||
| Malus pumila | + | ||||
| Prunus mume | + | ||||
| Prunus persica | +/++++ | ||||
| Prunus phaeosticta | + | ||||
| Prunus salicina | + | ||||
| Pseudocydonia sinensis | + | ||||
| Pyrus calleryana | + | ||||
| Pyrus pyrifolia | + | ||||
| Rubus leucanthus | + | ||||
| Rubus reflexus | + | ||||
| Rubus rosifolius | + | ||||
| Rubus sumatranus | + | ||||
| Rutaceae | Citrus limon | + | |||
| Citrus maxima | + | ||||
| Citrus reticulata | +++ | ||||
| Clausena lansium | ++ | ||||
| Fortunella hindsii | ++ | ||||
| Sapotaceae | Manilkara zapota | + | |||
| Vitaceae | Cayratia japonica | + | |||
| Vitis amurensis | + | ||||
| Vitis vinifera | + | ||||
| Vegetable | Cucurbitaceae | Cucumis melo | + | ||
| Cucumis sativus | ++ | ||||
| Cucurbita moschata | + | ||||
| Luffa aegyptiaca | ++++ | ||||
| Momordica charantia | + | ||||
| Sechium edule | + | ||||
| Solanaceae | Capsicum annuum | + | |||
| Solanum lycopersicum | ++ | ||||
| Solanum melongena | + | ||||
| Bactrocera latifrons | Vegetable | Solanaceae | Capsicum annuum | + | [33,99,100] |
| Solanum melongena | + | ||||
| Bactrocera minax | Fruit | Rutaceae | Citrus aurantium | nd | [47] |
| Citrus erythrosa | nd | ||||
| Citrus junos | nd | ||||
| Citrus limon | nd | ||||
| Citrus maxima | +++/++++ | ||||
| Citrus medica | +++/++++ | ||||
| Citrus paradisi | nd | ||||
| Citrus poonensis | +/++++ | ||||
| Citrus reticulata | nd | ||||
| Citrus sinensis | +/++/+++/++++ | ||||
| Citrus tangerina | +/+++ | ||||
| Citrus unshiu | +/++/+++/++++ | ||||
| Fortunella margarita | nd | ||||
| Poncirus trifoliata | nd | ||||
| Bactrocera tsuneonis | Fruit | Rutaceae | Citrus aurantium | nd | [101,102] |
| Citrus reticulata | nd | ||||
| Citrus sinensis | nd | ||||
| Fortunella japonica | nd | ||||
| Carpomya vesuviana | Fruit | Rhamnaceae | Ziziphus spp. | nd | [65] |
| Rhagoletis batava obseuriosa | Fruit | Elaeagnaceae | Hippophae spp. | nd | [68] |
| Zeugodacus cucurbitae | Vegetable | Cucurbitaceae | Benincasa hispida | nd | [97,103] |
| Citrullus lanatus | nd | ||||
| Cucumis sativus | ++++ | ||||
| Cucurbita moschata | nd | ||||
| Cucurbita pepo | nd | ||||
| Luffa aegyptiaca | ++++ | ||||
| Momordica charantia | ++ | ||||
| Sechium edule | ++ | ||||
| Zeugodacus scutellatus | Vegetable | Cucurbitaceae | Cucurbitaceae flowers | nd | [16,104] |
| Zeugodacus tau | Vegetable | Cucurbitaceae | Benincasa hispida | nd | [97,105,106] |
| Citrullus lanatus | ++ | ||||
| Cucumis sativus | +/++ | ||||
| Cucurbita moschata | ++/+++/++++ | ||||
| Cucurbita pepo | nd | ||||
| Luffa aegyptiaca | +/++ | ||||
| Momordica charantia | + | ||||
| Sechium edule | ++ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Xu, Y.; Chen, X. Biology, Ecology and Management of Tephritid Fruit Flies in China: A Review. Insects 2023, 14, 196. https://doi.org/10.3390/insects14020196
He Y, Xu Y, Chen X. Biology, Ecology and Management of Tephritid Fruit Flies in China: A Review. Insects. 2023; 14(2):196. https://doi.org/10.3390/insects14020196
Chicago/Turabian StyleHe, Yuxin, Yijuan Xu, and Xiao Chen. 2023. "Biology, Ecology and Management of Tephritid Fruit Flies in China: A Review" Insects 14, no. 2: 196. https://doi.org/10.3390/insects14020196
APA StyleHe, Y., Xu, Y., & Chen, X. (2023). Biology, Ecology and Management of Tephritid Fruit Flies in China: A Review. Insects, 14(2), 196. https://doi.org/10.3390/insects14020196





