Parthenogenetic Reproduction in Strumigenys Ants: An Update
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
3. Results
3.1. Reproduction Biology
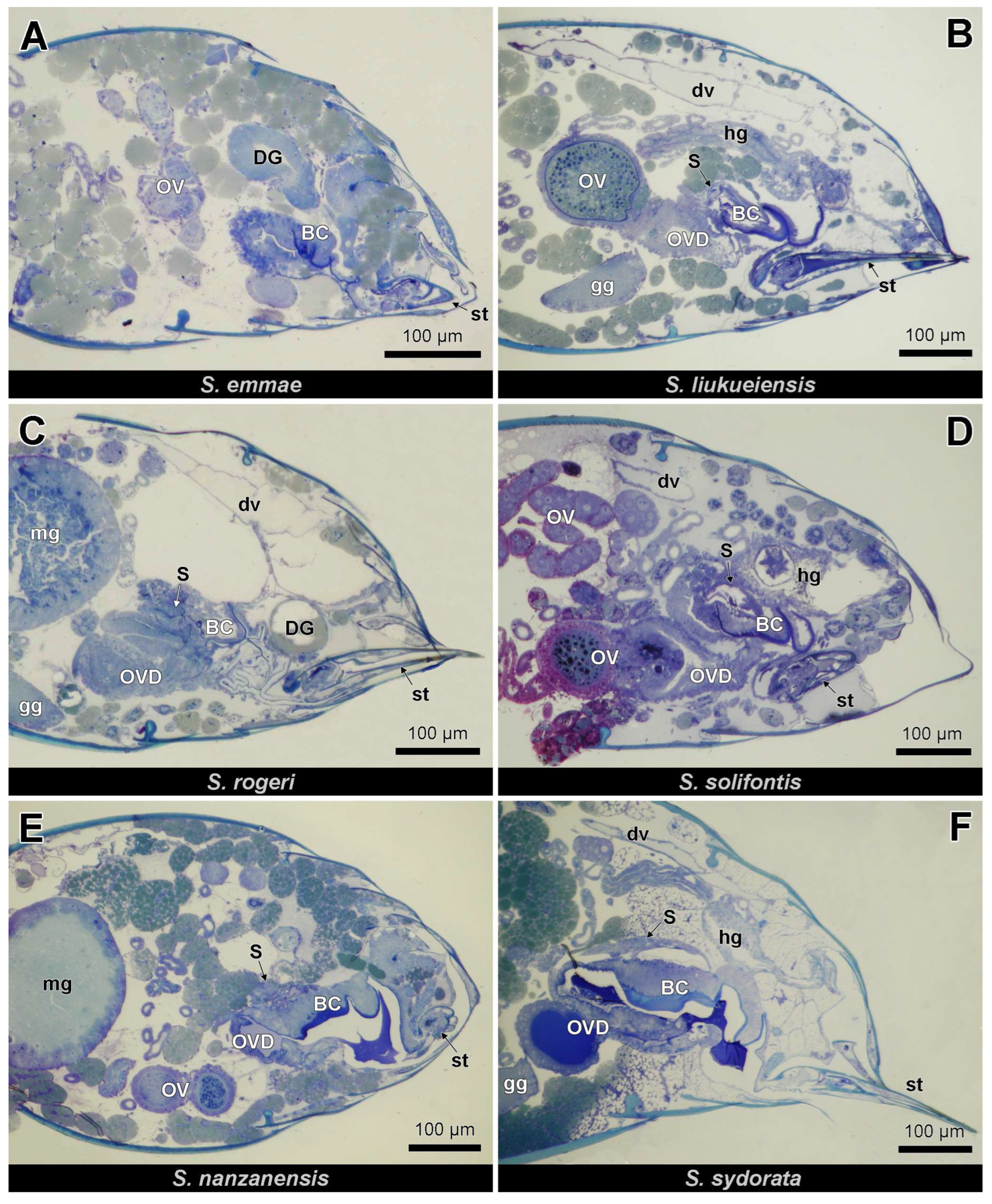
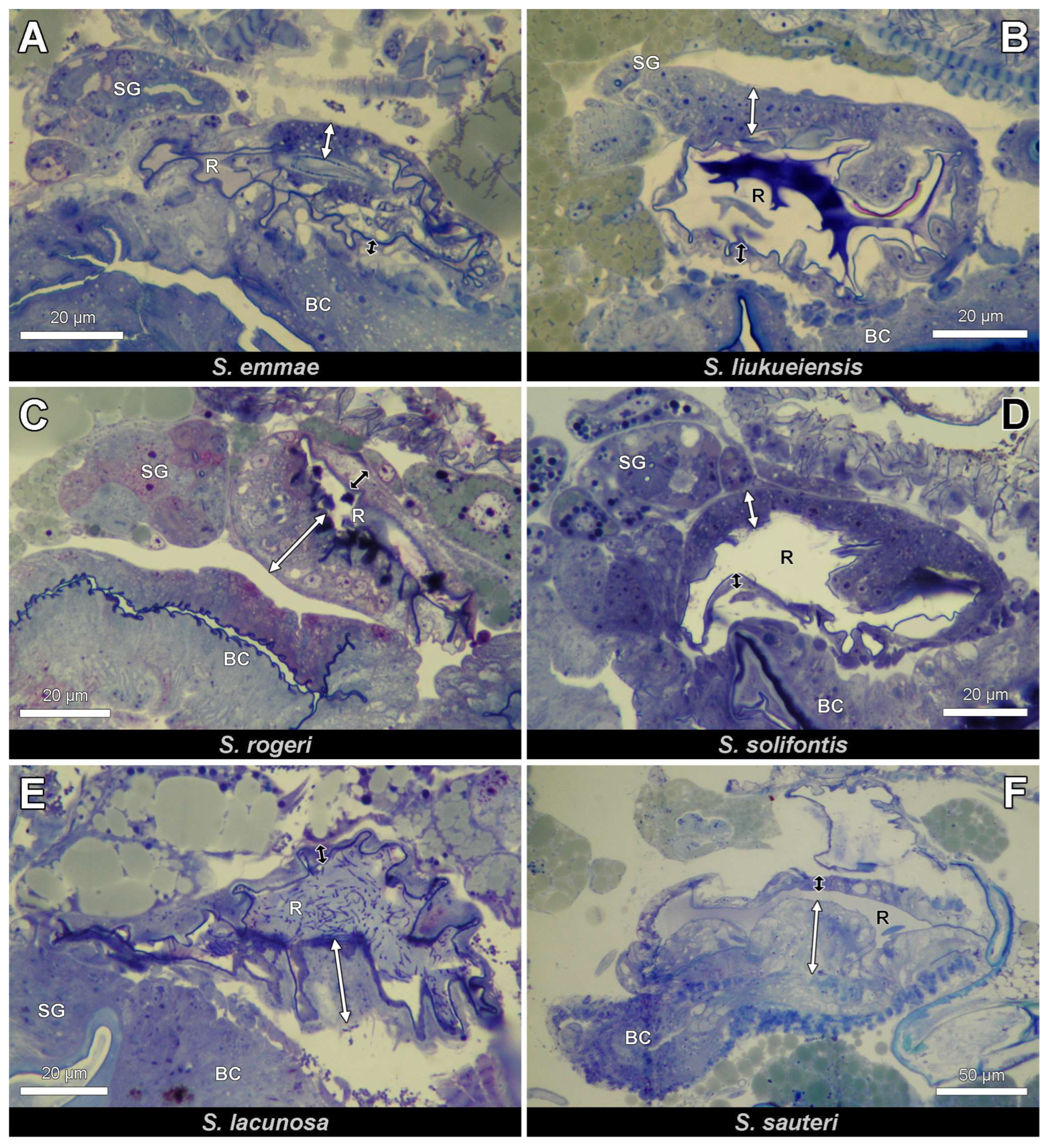
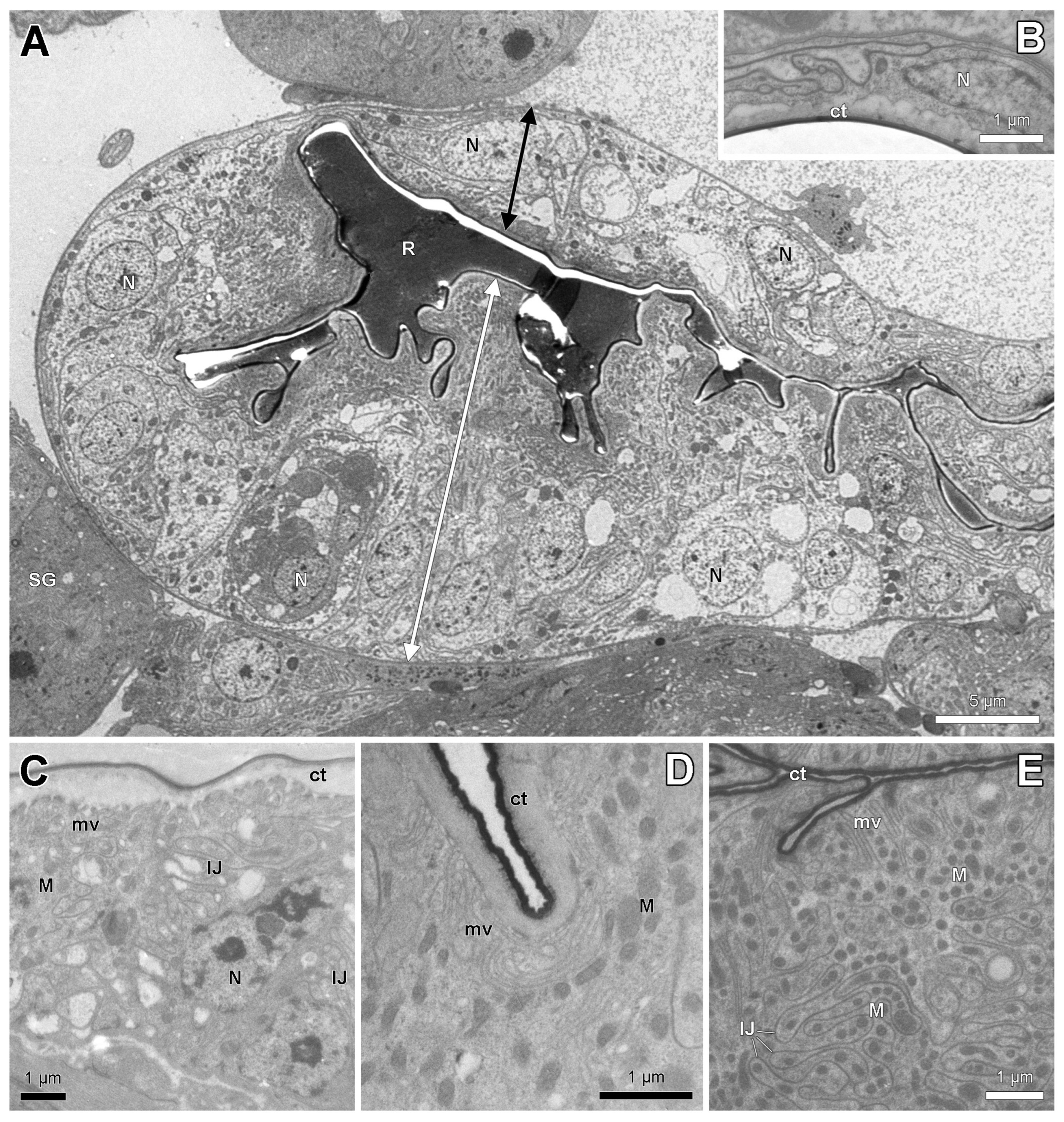
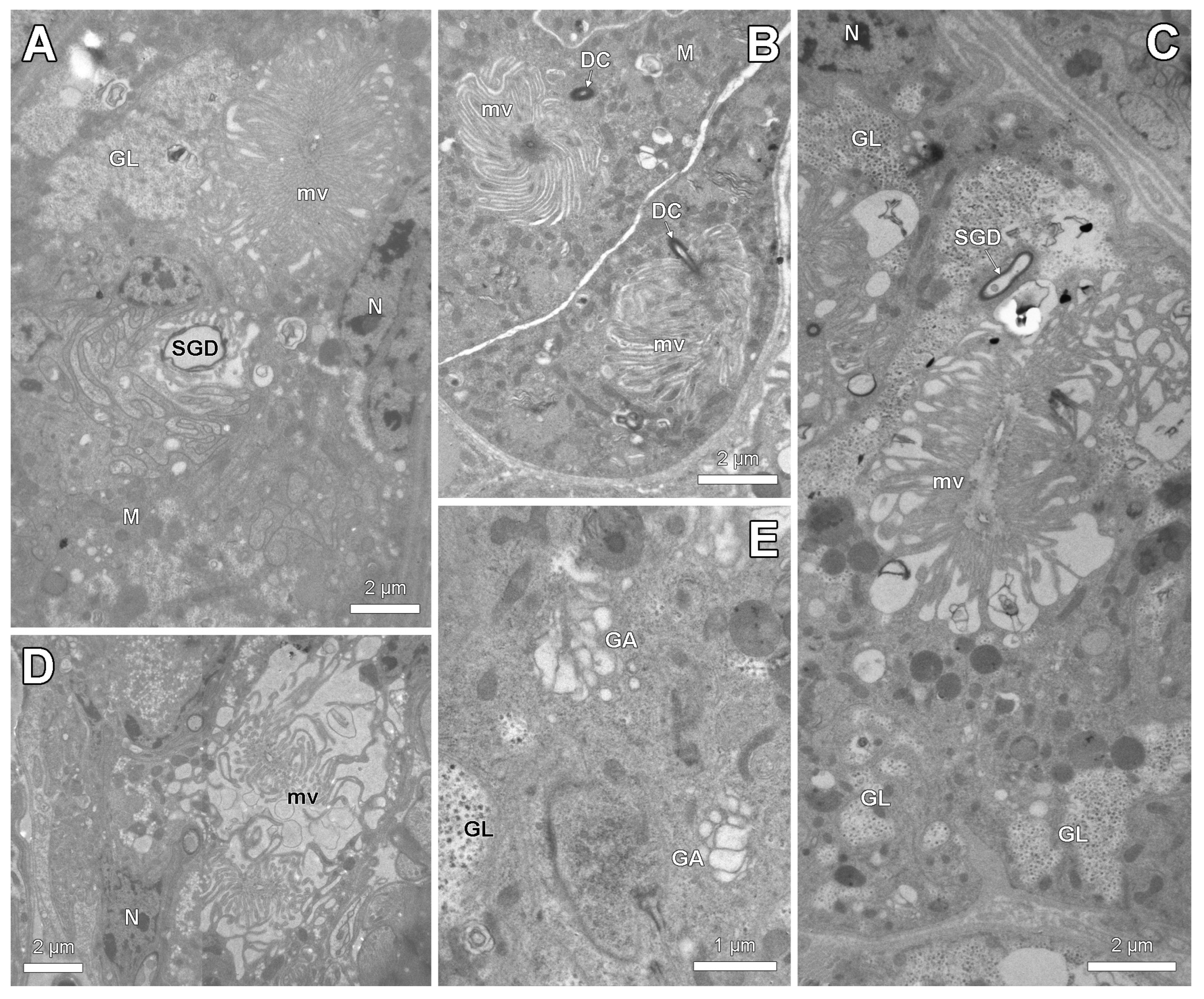
3.2. Histology and Ultrastructure
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trivers, R.L.; Hare, H. Haplodiploidy and the evolution of the social insect. Science 1976, 191, 249–263. [Google Scholar] [CrossRef] [PubMed]
- Rabeling, C.; Kronauer, D.J.C. Thelytokous parthenogenesis in eusocial Hymenoptera. Annu. Rev. Entomol. 2013, 58, 92. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, K.; Yamauchi, K. Production of females by parthenogenesis in the ant, Cerapachys biroi. Insect. Soc. 1995, 42, 333–336. [Google Scholar] [CrossRef]
- Heinze, J.; Hölldobler, B. Thelytokous parthenogenesis and dominance hierarchies in the ponerine ant, Platythyrea punctata. Naturwissenschaften 1995, 82, 40–41. [Google Scholar]
- Cagniant, M.H. Apparition d’ouvrières à partir d’oeufs pondus par des ouvrières chez la fourmi Cataglyphis cursor Fonscolombe (Hyménoptères, Formicidae). C.R. Acad. Sci. Paris 1973, 277, 2197–2198. [Google Scholar]
- Leniaud, L.; Darras, H.; Boulay, R.; Aron, S. Social hybridogenesis in the clonal ant Cataglyphis hispanica. Curr. Biol. 2012, 22, 1188–1193. [Google Scholar] [CrossRef]
- Pearcy, M.; Goodisman, M.A.D.; Keller, L. Sib mating without inbreeding in the longhorn crazy ant. Proc. R. Soc. B Biol. Sci. 2011, 278, 2677–2681. [Google Scholar] [CrossRef]
- Grasso, D.A.; Wenseleers, T.; Mori, A.; Le Moli, F.; Billen, J. Thelytokous worker reproduction and lack of Wolbachia infection in the harvesting ant Messor capitatus. Ethol. Ecol. Evol. 2000, 12, 309–314. [Google Scholar] [CrossRef]
- Ito, F.; Makita, S.; Nakao, H.; Hosokawa, R.; Kikuchi, T.; Yamane, S. Thelytokous parthenogenesis by dealate queens in the myrmicine ant Monomorium hiten distributed in Nansei Islands, western Japan, with description of the male. Asian Myrmecol. 2021, 14, e014001. [Google Scholar]
- Idogawa, N.; Sasaki, T.; Tsuji, K.; Dobata, S. Comprehensive analysis of male-free reproduction in Monomorium triviale (Formicidae: Myrmicinae). PLoS ONE 2021, 16, e0246710. [Google Scholar] [CrossRef]
- Fernández-Marín, H.; Zimmerman, J.K.; Wcislo, W.T.; Rehner, S.A. Colony foundation, nest architecture and demography of a basal fungus-growing ant, Mycocepurus smithii (Hymenoptera, Formicidae). J. Nat. Hist. 2005, 39, 1735–1743. [Google Scholar] [CrossRef]
- Masuko, K. Thelytokous parthenogenesis in the ant Myrmecina nipponica (Hymenoptera: Formicidae). Zool. Soc. Japan 2014, 31, 582–586. [Google Scholar]
- Itow, T.; Kobayashi, K.; Kubota, M.; Ogata, K.; Imai, H.T.; Crozier, R.H. The reproductive cycle of the queenless ant Pristomyrmex pungens. Insect. Soc. 1984, 31, 87–102. [Google Scholar] [CrossRef]
- Masuko, K. Thelytokous parthenogenesis in the ant Strumigenys hexamera (Hymenoptera: Formicidae). Annls Entomol. Soc. Amer. 2013, 106, 479–484. [Google Scholar] [CrossRef]
- Ito, F.; Touyama, Y.; Gotoh, A.; Kitahiro, S.; Billen, J. Thelytokous parthenogenesis by queens in the dacetine ant Pyramica membranifera (Hymenoptera: Formicidae). Naturwissenschaften 2010, 97, 725–728. [Google Scholar] [CrossRef]
- Lee, C.-C.; Hsu, S.-F.; Yang, C.-C.; Lin, C.-C. Thelytokous parthenogenesis in the exotic dacetine ant Strumigenys rogeri (Hymenoptera: Formicidae) in Taiwan. Entomol. Sci. 2018, 21, 28–33. [Google Scholar] [CrossRef]
- Ohkawara, K.; Nakayama, M.; Satoh, A.; Trindl, A.; Heinze, J. Clonal reproduction and genetic caste differences in a queen-polymorphic ant, Vollenhovia emeryi. Biol. Lett. 2006, 2, 359–363. [Google Scholar] [CrossRef]
- Fournier, D.; Estoup, A.; Orivel, J.; Foucaud, J.; Jourdan, H.; Le Breton, J.; Keller, L. Clonal reproduction by males and females in the little fire ant. Nature 2005, 435, 1230–1234. [Google Scholar] [CrossRef]
- Gotoh, A.; Billen, J.; Tsuji, K.; Sasaki, T.; Ito, F. Histological study of the spermatheca in three thelytokous parthenogenetic ant species, Pristomyrmex punctatus, Pyramica membranifera and Monomorium triviale (Hymenoptera: Formicidae). Acta Zool. 2012, 93, 200–207. [Google Scholar] [CrossRef]
- Tseng, S.P.; Darras, H.; Hsu, P.W.; Yoshimura, T.; Lee, C.Y.; Wetterer, J.K.; Keller, L.; Yang, C.C.S. Genetic analysis reveals the putative native range and widespread double-clonal reproduction in the invasive longhorn crazy ant. Mol. Ecol. 2022. [Google Scholar] [CrossRef]
- Noirot, C.; Quennedey, A. Fine structure of insect epidermal glands. Annu. Rev. Entomol. 1974, 19, 61–80. [Google Scholar] [CrossRef]
- Wetterer, J.K. Worldwide spread of Emma’s dacetine ant, Strumigenys emmae (Hymenoptera: Formicidae). Myrmecol. News 2012, 16, 69–74. [Google Scholar]
- Michlewicz, M. Strumigenys emmae (Emery, 1890) (Hymenoptera: Formicidae) in Poland—First record of this pantropic ant species from Europe with remarks on its biology in greenhouse. Ann. Up. Sil. Mus. Bytom Entomol. 2022, 31, 1–5. [Google Scholar]
- Wetterer, J.K. Worldwide spread of the membraniferous dacetine ant, Strumigenys membranifera (Hymenoptera: Formicidae). Myrmecol. News 2011, 14, 129–135. [Google Scholar]
- Wetterer, J.K. Worldwide spread of Roger’s dacetine ant, Strumigenys rogeri (Hymenoptera: Formicidae). Myrmecol. News 2012, 16, 1–6. [Google Scholar]
- Baer, B. The copulation biology of ants (Hymenoptera: Formicidae). Myrmecol. News 2011, 14, 55–68. [Google Scholar]
- Liberti, J.; Baer, B.; Boomsma, J.J. Queen reproductive tract secretions enhance sperm motility in ants. Biol. Lett. 2016, 12, 20160722. [Google Scholar] [CrossRef]
- Wheeler, D.E.; Krutzsch, P.H. Ultrastructure of the spermatheca and its associated gland in the ant Crematogaster opuntiae (Hymenoptera, Formicidae). Zoomorphology 1994, 114, 203–212. [Google Scholar] [CrossRef]
- Gobin, B.; Ito, F.; Peeters, C.; Billen, J. Queen-worker differences in spermatheca reservoir of phylogenetically basal ants. Cell Tissue Res. 2006, 326, 169–178. [Google Scholar] [CrossRef]
- Gobin, B.; Ito, F.; Billen, J.; Peeters, C. Degeneration of sperm reservoir and the loss of mating ability in worker ants. Naturwissenschaften 2008, 95, 1041–1048. [Google Scholar] [CrossRef]
- Kinomura, K.; Yamauchi, K. Frequent occurrence of gynandromorphs in the natural population of the ant Vollenhovia emeryi (Hymenoptera: Formicidae). Insect. Soc. 1994, 41, 273–278. [Google Scholar] [CrossRef]
- AntWeb. Version 8.86.1. California Academy of Science. Available online: https://www.antweb.org (accessed on 6 February 2023).

| Species (Collection Locality) | Colony | Experimental Period | Workers | Queens | Males |
|---|---|---|---|---|---|
| S. emmae (Baihe Reservoir, Tainan City, Taiwan) | 7 | 367 | 18 | 0 | |
| I | 10 December 2017–30 June 2018 | 65 | 0 | 0 | |
| II | 10 December 2017–30 June 2018 | 38 | 6 | 0 | |
| III | 10 December 2017–30 June 2018 | 53 | 3 | 0 | |
| IV | 10 December 2017–30 June 2018 | 60 | 6 | 0 | |
| V | 10 December 2017–30 June 2018 | 42 | 3 | 0 | |
| VI | 10 December 2017–30 June 2018 | 45 | 0 | 0 | |
| VII | 10 December 2017–30 June 2018 | 64 | 0 | 0 | |
| S. lacunosa (Lugu Township, Nantou County, Taiwan) | 3 | 0 | 0 | 7 | |
| I | 10 December 2017–30 June 2018 | 0 | 0 | 1 | |
| II | 10 December 2017–30 June 2018 | 0 | 0 | 2 | |
| III | 10 December 2017–30 June 2018 | 0 | 0 | 4 | |
| S. liukueiensis (Jiji Township, Nantou County, Taiwan) | 5 | 225 | 12 | 0 | |
| I | 10 December 2017–30 June 2018 | 54 | 1 | 0 | |
| II | 10 December 2017–30 June 2018 | 52 | 3 | 0 | |
| III | 10 December 2017–30 June 2018 | 42 | 4 | 0 | |
| IV | 10 December 2017–30 June 2018 | 37 | 3 | 0 | |
| V | 10 December 2017–30 June 2018 | 40 | 1 | 0 | |
| S. membranifera (Huisun Forest, Nantou County, Taiwan) | 3 | 325 | 1 * | 1 ** | |
| I | 16 July 2017–1 June 2021 | 120 | 0 | 0 | |
| II | 16 July 2017–1 June 2021 | 130 | 0 | 0 | |
| III | 16 July 2017–1 June 2021 | 75 | 1 * | 1 ** | |
| S. minutula (Hengchun Township, Pingtung County, Taiwan) | 2 | 0 | 0 | 3 | |
| I | 10 December 2017–30 June 2018 | 0 | 0 | 2 | |
| II | 10 December 2017–30 June 2018 | 0 | 0 | 1 | |
| S. rogeri (Jiji Township, Nantou County, Taiwan) | 6 | 301 | 12 | 1 | |
| I | 20 December 2017–30 June 2018 | 45 | 2 | 0 | |
| II | 20 December 2017–30 June 2018 | 81 | 3 | 0 | |
| III | 20 December 2017–30 June 2018 | 57 | 1 | 0 | |
| IV | 20 December 2017–30 June 2018 | 41 | 1 | 0 | |
| V | 20 December 2017–30 June 2018 | 40 | 4 | 0 | |
| VI | 20 December 2017–30 June 2018 | 37 | 1 | 1 | |
| S. solifontis (Sun Moon Lake, Nantou County, Taiwan) | 3 | 153 | 7 | 0 | |
| I | 5 July 2018–15 January 2019 | 38 | 2 | 0 | |
| II | 5 July 2018–15 January 2019 | 59 | 2 | 0 | |
| III | 5 July 2018–15 January 2019 | 56 | 3 | 0 |
| Species | Collection Locality and Date | N | Dissected Queens | Mated Queens | Virgin Queens |
|---|---|---|---|---|---|
| S. formosensis | Ren’ai Township, Nantou County, Taiwan (16 September 2022) | 3 | 3 | 3 | 0 |
| S. hispida | Yuchi Township, Nantou County, Taiwan (12 December 2016) | 1 | 8 | 7 | 1 |
| S. lacunosa | Lugu Township, Nantou County, Taiwan (20 October 2022) | 2 | 2 | 2 | 0 |
| S. liukueiensis | Jiji Township, Nantou County, Taiwan (20 October 2022) | 3 | 9 | 0 | 9 |
| S. minutula | Huisun Forest, Nantou County, Taiwan (11 December 2016) | 1 | 2 | 2 | 0 |
| S. rogeri | Jiji Township, Nantou County, Taiwan (16 September 2022) | 2 | 11 | 0 | 11 |
| S. solifontis | Huisun Forest, Nantou County, Taiwan (12 December 2016) | 1 | 1 | 0 | 1 |
| S. solifontis | Sun Moon Lake, Nantou County, Taiwan (13 December 2016) | 7 | 21 | 0 | 21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Sung, P.-J.; Lin, C.-C.; Ito, F.; Billen, J. Parthenogenetic Reproduction in Strumigenys Ants: An Update. Insects 2023, 14, 195. https://doi.org/10.3390/insects14020195
Wang C, Sung P-J, Lin C-C, Ito F, Billen J. Parthenogenetic Reproduction in Strumigenys Ants: An Update. Insects. 2023; 14(2):195. https://doi.org/10.3390/insects14020195
Chicago/Turabian StyleWang, Chu, Ping-Jui Sung, Chung-Chi Lin, Fuminori Ito, and Johan Billen. 2023. "Parthenogenetic Reproduction in Strumigenys Ants: An Update" Insects 14, no. 2: 195. https://doi.org/10.3390/insects14020195
APA StyleWang, C., Sung, P.-J., Lin, C.-C., Ito, F., & Billen, J. (2023). Parthenogenetic Reproduction in Strumigenys Ants: An Update. Insects, 14(2), 195. https://doi.org/10.3390/insects14020195






