Simple Summary
Endoribonuclease 2 (Dicer2) plays various physiological roles in the RNA interference (RNAi) pathway by fragmenting double-stranded RNA to generate small interfering RNA, which then mediates gene silencing. In this study, the role of Dicer2 in the regulation of molting and wing expansion in Sogatella furcifera (white-backed planthopper) was investigated. In particular, SfDicer2-mediated RNAi resulted in wing deformities and lethal modifications in S. furcifera, which are attributable to the significant inhibition of chitin synthesis and degradation and wing expansion genes. This study provides insights into the biological functions of Dicer2 in insects, which can aid in RNAi-mediated pest control.
Abstract
Endoribonuclease 2 (Dicer2) is a key nicking endonuclease involved in the small interfering RNA biosynthesis, and it plays important roles in gene regulation and antiviral immunity. The Dicer2 sequence was obtained using the transcriptomic and genomic information of Sogatella furcifera (Horváth), and the spatiotemporal characteristics and functions of molting and wing expansion regulation were studied using real-time quantitative polymerase chain reaction and RNA interference (RNAi) technology. The expression of SfDicer2 fluctuated during the nymphal stage of S. furcifera. Its expression decreased significantly over the course of molting. SfDicer2 exhibited the highest transcript level in the nymphal stage and adult fat body. After SfDicer2 was silenced, the total mortality rate was 42.69%; 18.32% of the insects died because of their inability to molt. Compared with the effects of dsGFP or water, 44.38% of the insects subjected to the silencing of SfDicer2 exhibited wing deformities after successful eclosion. After SfDicer2 RNAi, the expression of chitinase, chitin deacetylase, trehalase, chitin synthase 1, and wing expansion-related genes was significantly inhibited. These findings indicate that SfDicer2 controls molting by affecting genes associated with chitin synthesis and degradation and regulates wing expansion by altering the expression of wing expansion-related genes in S. furcifera.
1. Introduction
RNA interference (RNAi) can reduce the transcript levels of target genes and facilitate the screening of candidate genes for pest control [1,2]. Based on the mechanism of small RNA synthesis, RNAi pathways can be one of three types: small interfering RNA (siRNA), non-coding microRNA (miRNA), and PIWI-interacting RNA pathways [3,4,5,6]. Studies on insects typically employ the siRNA pathway for RNAi. After the accumulation of double-stranded RNAs (dsRNA) in insect cells, they are largely fragmented into siRNAs using endoribonuclease 2 (Dicer2) and are merged with Argonaute 2 (AGO2) to form the RNA-induced silencing complex (RISC), which is responsible for the silencing of target genes at the post-transcriptional level and the regulation of gene expression [7,8,9]. siRNAs are processed from dsRNAs encoded by viruses or introduced exogenously, whereas miRNAs are generally transcribed from endogenous genes [10]. Therefore, most studies on the biological function of small RNAs have focused on miRNAs. However, some studies have demonstrated that the endogenous siRNA-mediated RNAi pathway can regulate the activity of transposons and the expression of protein-coding genes [11].
The Dicer2 protein is an essential component of RISC, which indicates that the protein plays a central role in the initiation stage of RNAi and participates in the effect stage. A study on fruit flies (Drosophila melanogaster) revealed that Dicer2 interacted with the dsRNA-binding protein R2D2 to form RISC, identified the kinetic stability of the two ends of siRNA, and selected one of the chains as the RISC-guiding chain [12]. Although Dicer2 can fragment dsRNA into siRNA, siRNA must form a Dicer2–R2D2 complex with R2D2 (the dsRNA-binding domain of R2D2 binds to siRNA and promotes the assembly of siRISC) to mediate RNAi before it can be delivered into the RNAi pathway. The evidence provided by Kalidas et al. supports this view [13]. Adding dsRNA to the extracts of R2D2 mutants leads to the production of siRNA; however, the siRNA cannot be assembled onto siRISC. At this time, if the R2D2 mutant is transferred again, the ability of siRNA to bind to siRISC returns to normal. When dsRNA is added to the extracts of Dicer2 mutants, no siRNA is produced; R2D2 mutants are undetectable in the Dicer2 mutant extracts. Thus, Dicer2 can fragment dsRNA into siRNA and affect the stability of R2D2, and R2D2 can guide the assembly of siRNA into siRISC. Therefore, both R2D2 and Dicer2 are required for the assembly of siRNAs into siRISC [12,13]. Although the Dicer2 homozygous mutant of D. melanogaster could survive and reproduce [14,15], a gene expression profiling of the pupae of D. melanogaster Dicer2 deletion mutants uncovered 306 upregulated and 357 downregulated genes, most of which were associated with energy metabolism as well as growth and development. In addition, the pupae of Dicer2 deletion mutants had lower ATP levels than those of control insects, indicating that the Dicer2 deletion mutant of D. melanogaster had impaired mitochondrial productivity [16]. However, silencing Dicer2 in the brown rice planthopper (Nilaparvata lugens [Stål]) did not affect its growth and development [17]. To date, few studies have examined the specific effects of Dicer2 during the growth and development of other insects.
Sogatella furcifera, a member of the order Hemiptera and family Delphacidae, is a major migratory pest of rice that causes significant economic losses when its infestation is severe [18]. As a paurometabolous insect, S. furcifera grows and develops in three stages: egg, nymph, and adult. S. furcifera adults have two types of wings, namely, long and short, with strong adaptability [19]. Research on the genes regulating molting and wing expansion in S. furcifera will help identify and develop new potential target genes for controlling and preventing S. furcifera infestation. Dicer2 is an essential element that serves as the “first knife” in the RNAi pathway. It is mainly involved in the synthesis of siRNA, which causes the specific degradation of homologous mRNA. Based on published transcriptome [20] and genome [21] data, SfDicer2 was explored to determine its role in the molting and wing expansion of S. furcifera using an RNAi method. The results may help devise novel strategies for pest control based on RNAi.
2. Materials and Methods
2.1. Laboratory Insects
An S. furcifera cohort was collected from a rice field in Huaxi, Guiyang, Guizhou, China (26°31.302″ N and 106°62.294″ E), in 2013 and reared on Taichung Native 1 rice plants at the tillering stage without exposure to any pesticide in the laboratory. Fresh rice seedlings were replaced every 2 days. The environmental conditions were as follows: temperature, 25 °C ± 1 °C; relative humidity, 70% ± 5%; and photoperiod, 16 h light/8 h dark. The insects were kept in an isolated area so that 1-day-old first-instar nymphs could be used as the source of test insects.
2.2. Collection of Samples from Various Developmental Stages and Tissues
We prepared S. furcifera samples to assess the expression of SfDicer2 in different developmental stages and tissues. Samples (n = 15–50) were collected at 28 different time points as follows: the first instar at days 1 and 2 (n = 50); second instar at days 1 and 2 (n = 50); third instar at days 1, 2, and 3 (n = 40); fourth instar at days 1, 2, and 3 (n = 35); fifth instar at days 1, 2, and 3 (n = 25); before molting (n = 20); during molting (n = 20); after molting (n = 20); and male and female adults at 12, 24, 36, 48, 72, and 96 h after eclosion (n = 15). The different tissue samples used in this study included the head (n = 50), integument (n = 30), fat body (n = 100), gut (n = 100), legs (n = 30), wings (n = 30), testes (n = 50), and ovaries (n = 50) from composite samples of 1- to 3-day-old fifth-instar nymphs and 1- to 4-day-old adults. All samples were stored in phosphate-buffered saline (PBS; pH = 7.4). All sampling procedures were repeated thrice to ensure the accuracy of the data. The samples were stored at −80 °C.
2.3. Total RNA Extraction and cDNA Synthesis
The total RNA was extracted from the collected samples using the HP Total RNA Kit (Omega Bio-Tek, Norcross, GA, USA) according to the manufacturer’s instructions. The concentration and purity of 1 μL of an RNA sample were tested using the NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA), and the integrity was tested using 1.2% agarose gel. Better quality RNA is generally indicated by an OD260/OD280 ratio of 1.8–2.0 and prominent rRNA bands (28S and 18S rRNA) under ultraviolet light after electrophoresis. The RNA samples that met the suitable quality standards were synthesized into first-strand cDNA using the PrimeScript RT Reagent Kit with gDNA Eraser (Takara, Dalian, China) according to the manufacturer’s instructions and stored at −20 °C for subsequent use.
2.4. Sequencing Analysis, Design, and Synthesis of Specific Primers
Based on the transcriptome data (GenBank accession number: SRP116252) [20], a portion of the SfDicer2 fragment was used as a template to search the entire genome (GenBank accession number: PRJNA331022) [21] of S. furcifera. We used the DNAMAN 7.0 software (Lynnon Biosoft, San Ramon, CA, USA) to deduce the amino acid sequence. After the Dicer2 protein encoded by SfDicer2 was verified and analyzed using the BLAST tool on the National Center for Biotechnology Information (NCBI) website (https://blast.ncbi.nlm.nih.gov/Blast.cgi, accessed on 5 June 2022), the ORF Finder tool (https://www.ncbi.nlm.nih.gov/orffinder/ accessed on 5 June 2022) on the NCBI website was employed to identify the open reading frames (ORFs) of SfDicer2. ProtParam (https://web.Expasy.org/protparam/ accessed on 6 June 2022) was used to predict the molecular composition, relative molecular mass, isoelectric point, and other physicochemical properties of the amino acids of encoded proteins. Further, the ScanProsite database (https://prosite.expasy.org/ accessed on 6 June 2022) was used to predict conserved domains. We constructed a phylogenetic tree with 1000 bootstrap replications via the neighbor-joining method using the MEGA 6.06 software [22].
The complete sequence of SfDicer2 was obtained. Primer Premier 6.0 was used to design the specific primers (Table 1). A BLAST analysis against a genomic assembly of S. furcifera showed that there was no other sequence in the assembly that was more than 18 bp completely identical between the target gene dsRNA and the genome assembly to avoid off-target effects. The specific primers were synthesized by Tsingke Biotechnology Co., Ltd. (Beijing, China).

Table 1.
Primers used for a real-time quantitative polymerase chain reaction (RT-qPCR) and dsRNA synthesis.
2.5. Spatiotemporal Representation Analysis of SfDicer2
According to the specific primers designed for a real-time quantitative polymerase chain reaction (RT-qPCR) of the target gene (Table 1), the spatiotemporal expression of SfDicer2 was examined using ribosomal protein L9 (SfRPL9, GenBank accession number: KM885285) and alpha 1-tubulin (SfTUB, GenBank accession: KP735521) [23] as the internal reference genes. Using the synthesized cDNA as a template, three biological and technical repeats were generated for each sample. RT-qPCR was performed using the CFX96TM Real-time Quantitative PCR System (Bio-Rad, Hercules, CA, USA) and 20 μL of the reaction system, comprising 10 μL of FastStart Essential DNA Green Master Mix, 1 μL each of the upstream and downstream primers (10 µM), 1 μL of the S. furcifera cDNA template, and 7 μL of ddH2O. The following PCR-amplification program was used: pre-denaturation at 95 °C for 10 min; 40 cycles of denaturation at 95 °C for 30 s and annealing and extension at 60 °C for 30 s; and dissolution curve analysis at 60–95 °C.
2.6. dsRNA Synthesis
Based on the obtained sequence of SfDicer2, gene-specific primers containing the T7 polymerase promoter sequence were designed using the Primer Premier 6.0 software. The dsRNA primers (Table 1) were designed and synthesized and were verified using PCR amplification. The total reaction volume (25 μL) included 1 μL each of the upstream and downstream primers (10 µM), 12.5 μL of the PCR Mix, 3 μL of the S. furcifera cDNA template, and 7.5 μL of ddH2O. The PCR-amplification conditions were as follows: pre-denaturation at 95 °C for 3 min; 30 cycles of denaturation at 95 °C for 30 s, annealing at 55 °C for 30 s, and extension at 72 °C for 1–3 min; extension at 72 °C for 10 min; and storage at 4 °C. After the PCR products were separated using 1.2% agarose gel electrophoresis, they were subjected to purification and recycling using the E.Z.N.A. Gel Extraction Kit (Omega Bio-Tek). The recovered product was subcloned into the pMD18-T vector (Takara, Dalian, China) and transformed into Escherichia coli DH5αcompetent cells (TransGen Biotech, Beijing, China), and the colonies were selected and sent to Tsingke Biotechnology Co., Ltd. for sequencing. The correct sequence was amplified in the cultivated liquid medium, and the plasmid was extracted using the Plasmid Midi Kit (Omega Bio-Tek). The dsRNA was synthesized and purified in vitro using the TranscriptAid T7 High Yield Transcription Kit and GeneJET RNA Purification Kit (both from Thermo Fisher Scientific), according to the manufacturer’s instructions, using the plasmid as the cDNA template for PCR and the recovered high-concentration dsDNA as the template. After synthesis, the dsRNA was diluted, and the concentration was determined by the extinction coefficient RNA using a NanoDrop 2000 spectrophotometer. Subsequently, the integrity of dsRNA was determined using 1.2% agarose gel electrophoresis. The green fluorescent protein dsRNA (dsGFP) (GenBank accession number: CAA58789) was then synthesized using the same method, stored at −80 °C, and used as a non-specific negative control.
2.7. SfDicer2 RNAi Analysis
Same-sized healthy nymphs were selected to be injected with the synthesized dsRNA. The S. furcifera specimens were anesthetized for 40 s using CO2, after which the test insects were placed on a 2% agarose plate with the abdomen facing upward. The dsRNA was injected using an IM-31 microinjector (Narishige, Tokyo, Japan) at a point where the anterior and middle chests were connected. A gentle injection was required to avoid mechanical damage to the test insects. In total, 0.1 μL (approximately 100 ng) of dsRNA was injected into the 1-day-old fourth-instar specimens. The nuclease-free water and dsGFP groups were administered to the insects in the negative control groups. Each group comprised 120 nymphs, and all treatments were performed in triplicates. After injection, the test insects were transferred to a test tube containing fresh rice seedlings and placed in an artificial climate box.
Samples were collected every 24 h after injection for 3 consecutive days. Total RNA was extracted from the 10 surviving insects in each group and was reverse-transcribed into cDNA. RT-qPCR was used to detect the gene silencing’s efficiency after SfDicer2 interference. The phenotypes of S. furcifera in each group were observed every day, and mortality was examined for 8 consecutive days. All samples were collected and soaked in PBS, and the phenotype of S. furcifera was observed, compared, and photographed under a stereoscopic microscope (SMZ25 Nikon Corporation, Tokyo, Japan).
2.8. Analysis of the Effects of the Silencing of SfDicer2 on Chitin and Wing Expansion-Related Genes
To verify the effect of SfDicer2 on chitin (chitinase, chitin deacetylase, trehalase, and chitin synthase 1) and wing expansion in S. furcifera, the mRNA expression of these pathways and genes was detected using RT-qPCR. SfRPL9 and SfTUB were used as the internal references. Information regarding the fluorescence quantitative primers is presented in Table 1.
2.9. Statistical Analyses
Using the 2−∆∆CT method [24], we evaluated the transcript levels of SfDicer2, along with chitinase, chitin deacetylase, trehalase, and chitin synthase 1, and the wing expansion-related genes. All data were analyzed using SPSS 22.0 (SPSS, Chicago, IL, USA). The values are presented as the mean ± standard error (SE) of three replicates. One-way analysis of variance and Duncan’s test were used to compare the relative expression of each sample in various developmental stages, molting stages, tissues, and the three experimental conditions (p < 0.05). After silencing SfDicer2, the expression of the target genes, including chitinase, chitin deacetylase, trehalase, chitin synthase, and wing expansion-related and other related genes was evaluated and compared with that of the genes in the control(dsGFP) group. A Student’s t-test for independent samples was performed to assess statistical significance, which was indicated by p < 0.05.
3. Results
3.1. Sequence Characterization and Phylogenetic Analysis of SfDicer2
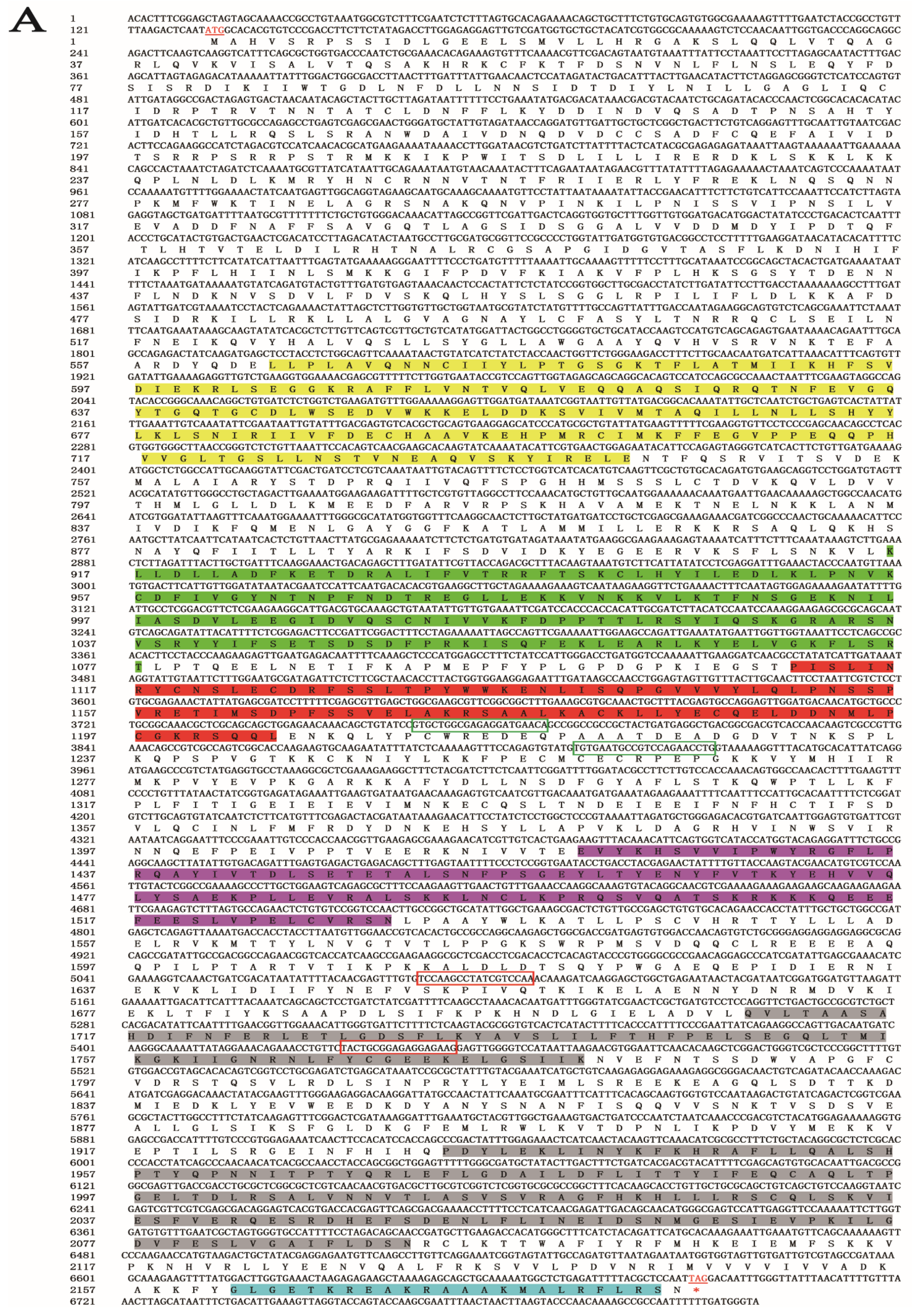
A 6818-bp gene sequence was obtained from the genomic and transcriptomic data of S. furcifera. An NCBI alignment identified the gene as SfDicer2 (GenBank accession number: ON693521). The SfDicer2 cDNA sequence contained a complete 6558-bp ORF encoding a hypothetical protein sequence of 2185 amino acids (Figure 1A). The results predicted by the online software ProtParam revealed that the molecular weight of SfDicer2 was approximately 250.15 kDa and that the theoretical isoelectric point was 7.59. The instability index was computed to be 43.68, which marked the protein as unstable.
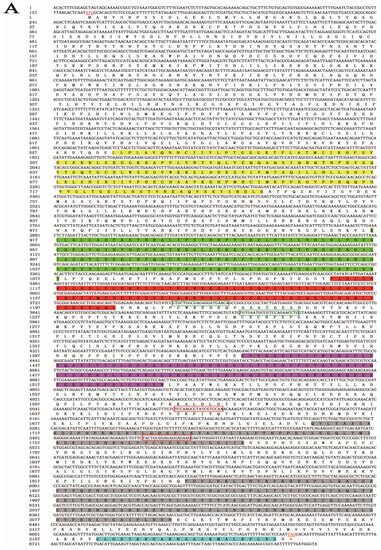
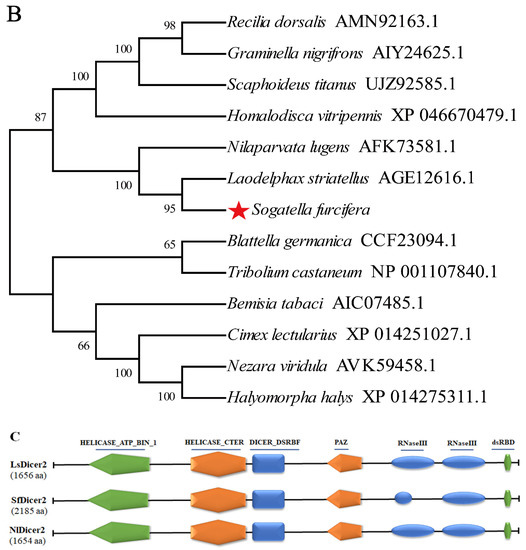
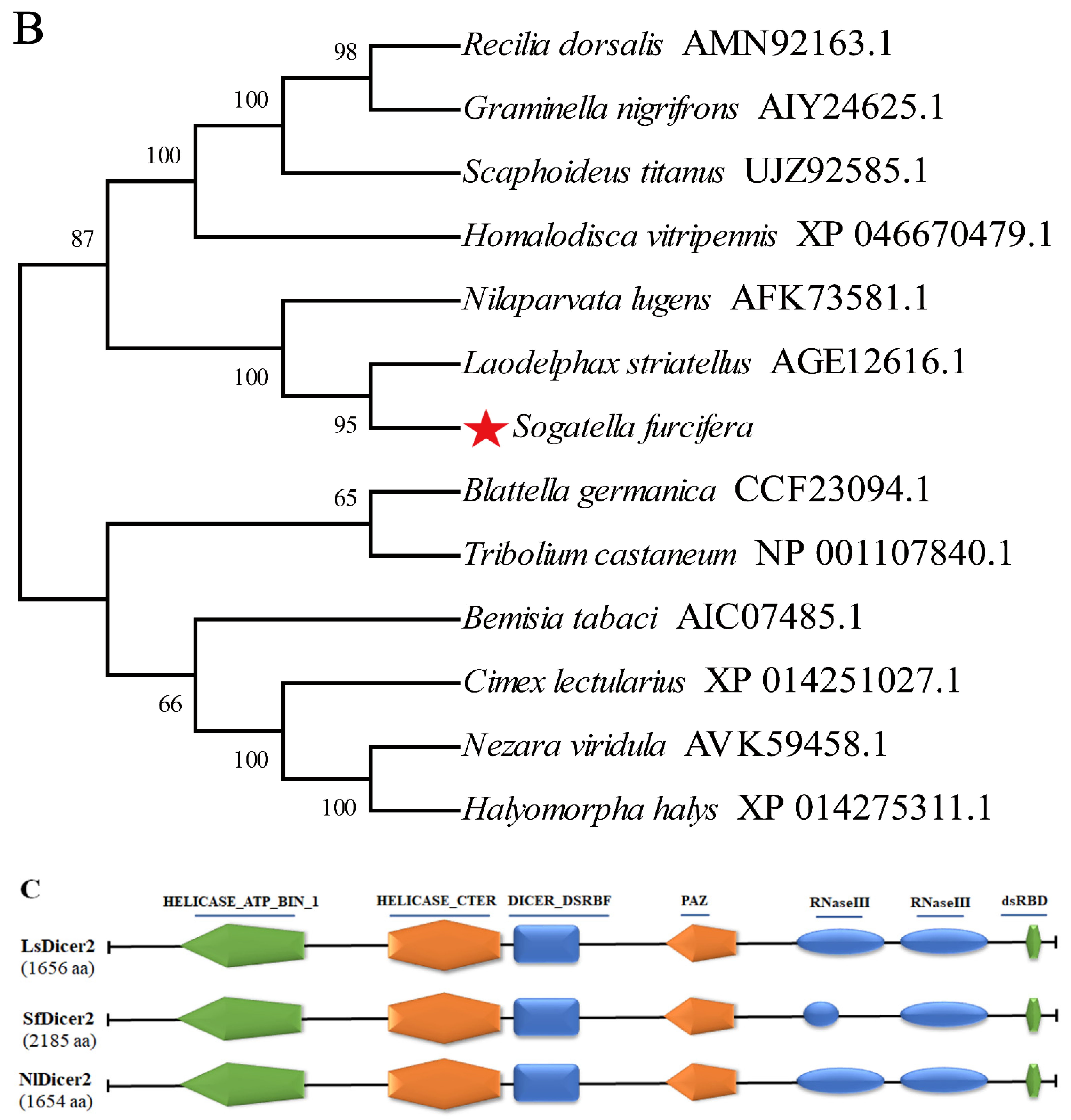
Figure 1.
Bioinformatic analysis of SfDicer2 in S. furcifera. (A) Analysis of nucleotide sequence of SfDicer2 and amino acid sequence of SfDicer2. Underlined and red font ATG, start codon; underlined and red font TGA with an asterisk, stop codon; yellow background, HELICASE_ATP_BIND_1; green background, HELICASE_CTER; red background, DICER_DSRBF; purple background, PAZ; gray background, RNase III; and blue background, dsRBD. The green boxes represent the primers for RT-qPCR, and the red boxes represent the primers for dsRNA synthesis. (B) Phylogenetic analysis of SfDicer2 homologs obtained from insect species based on amino acid sequences. The sequences were downloaded from the GenBank protein database. The red star indicates the Dicer2 protein of S. furcifera. (C) Conserved domain analysis of Dicer2 proteins. The different shapes and colors represent different protein domains (green pentagon, HELICASE_ATP_BIND_1 domain; orange hexagon, HELICASE_CTER; dark blue rectangle, DICER_DSRBF domain; orange pentagon, PAZ domain; dark blue oval, RNase III domain; and green hexagon, dsRBD). A conserved domain analysis was performed using the ScanProsite online server.
We investigated the degree of similarity between the Dicer2 protein of S. furcifera and that of other species using a BLAST homology search and comparison analysis. The Dicer2 protein of S. furcifera showed high similarities of 81.49% and 75.81% with that of Laodelphax striatellus (GenBank accession number: AGE12616.1) and N. lugens (GenBank accession number: AFK73581.1), respectively, although S. furcifera is distantly related to other insects. The phylogenetic tree revealed that the Dicer2 protein of S. furcifera was most closely related to that of N. lugens and L. striatellus (Figure 1B). A domain analysis revealed that the Dicer2 protein had seven typical conservative domains in three rice planthoppers: a superfamilies one and two helicase ATP-binding type-1 domain profile (HELICASE_ATP_BIND_1), a superfamilies one and two helicase C-terminal domain profile (HELICASE_CTER), a Dicer double-stranded RNA-binding fold domain profile (DICER_DSRBF), a PAZ domain profile (PAZ), two ribonuclease III family domain profiles (RNase III), and a double-stranded RNA-binding domain profile (dsRBD). In addition, seven conserved domains that were key to the catalytic activity of Dicer2 enzymes were identified (Figure 1C).
3.2. SfDicer2 Expression in Various Developmental Stages and Tissues
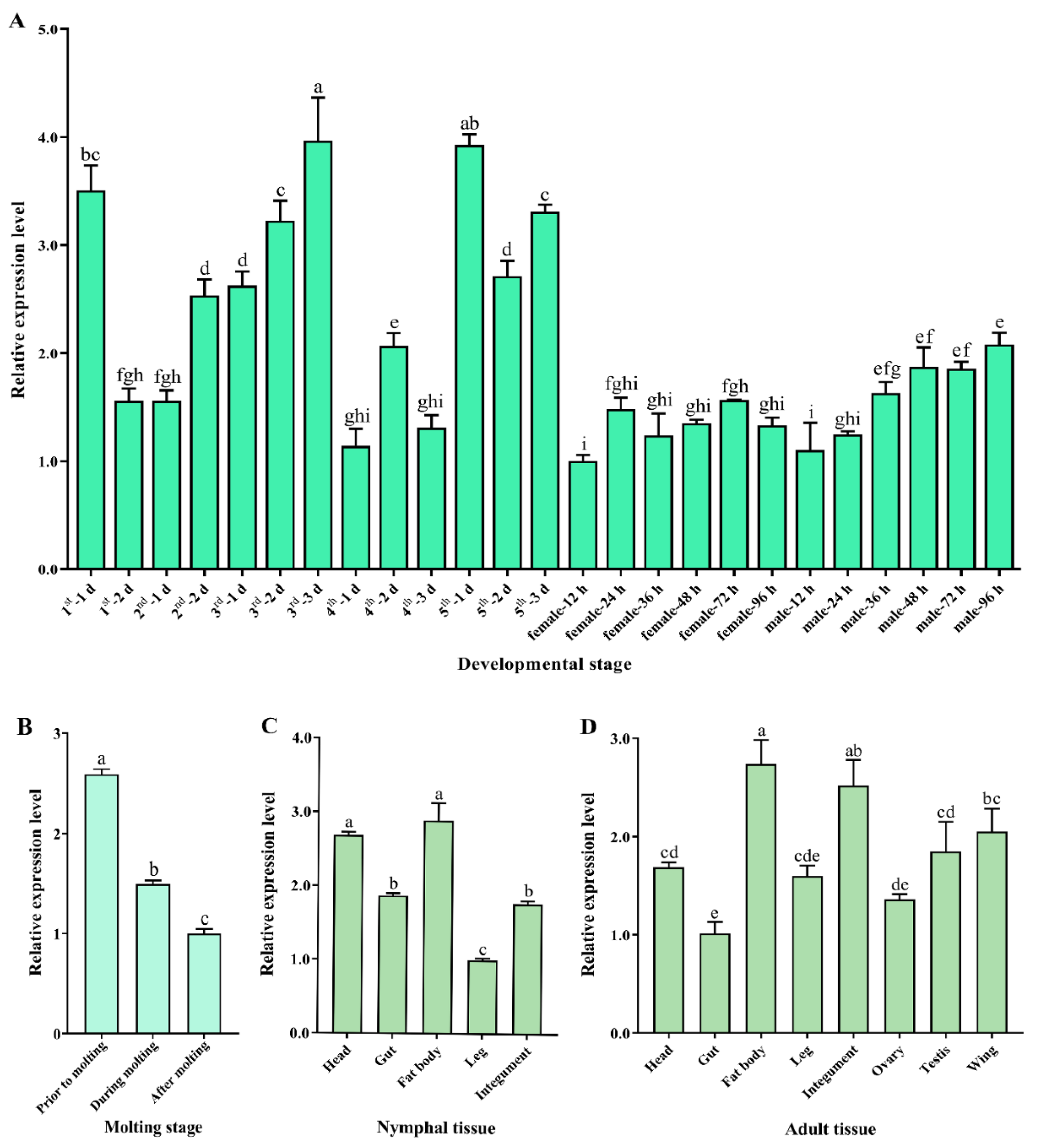
SfDicer2 was expressed in all developmental stages of S. furcifera. In particular, the expression of SfDicer2 was high in 1-day-old first-instar nymphs, 2- and 3-day-old third-instar nymphs, and 1- and 3-day-old fifth-instar nymphs, indicating that SfDicer2’s expression fluctuated in the nymphal stage (Figure 2A, Table S1). Based on the findings before, during, and after molting, the expression of SfDicer2 decreased with increasing molting time from the nymphal stage to the adult stage (p < 0.05; Figure 2B, Table S1). SfDicer2’s expression in different tissues of the fifth-instar nymphs decreased in the order of fat body = head > gut = integument > legs (Figure 2C, Table S1). Moreover, its expression in the fat body of adults was significantly higher than that of the head, wing, testis, gut, and ovary, but there was no significant difference compared with the integument (p > 0.05; Figure 2D, Table S1).
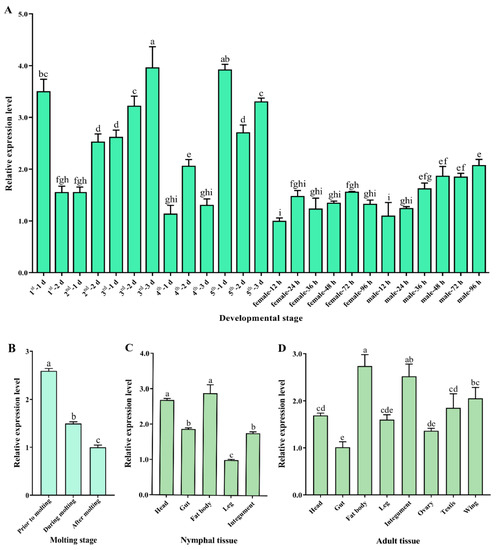
Figure 2.
Relative expression of SfDicer2 in various developmental stages and tissues of S. furcifera. SfRPL9 and SfTUB were used as internal control genes. (A) Relative expression of SfDicer2 from day 1 in first-instar insects to adulthood in males and females at 96 h after eclosion, as determined using RT-qPCR. (B) Relative expression of SfDicer2 before, during, and after molting, as determined using RT-qPCR. (C) Relative expression of SfDicer2 in different tissues of the fifth-instar nymphs, as determined using RT-qPCR. (D) Relative expression of SfDicer2 in different tissues of 1-day-old male and female adults, as determined using RT-qPCR. The expression levels of SfDicer2 in 12-hours-old female adults, after molting, in nymphal legs and adult gut were used as the references. Data in the figure are presented as the means ± SEs of three replicates. Different letters above the bars indicate significant differences in gene expression among the different developmental stages or different tissues (Duncan’s multiple range test, p < 0.05).
3.3. SfDicer2 RNAi Causes Wing Expansion Failure and Death in S. furcifera
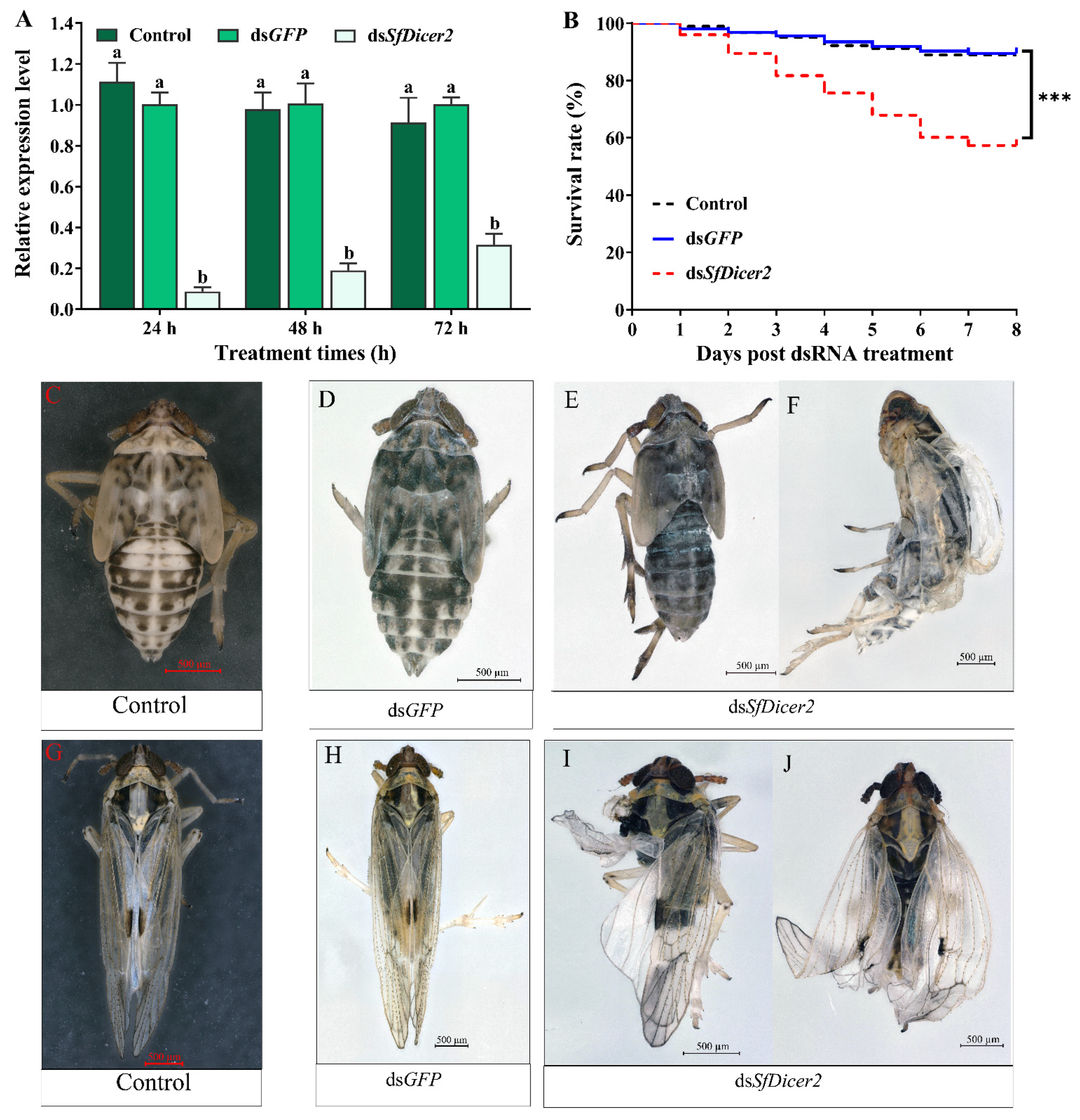
To explore whether the decrease in mRNA expression of the target gene affects the survival rate, molting, and wing expansion, healthy 1-day-old fourth-instar nymphs of S. furcifera were selected for RNA silencing, and each nymph was injected with 0.1 μL (approximately 100 ng) of dsRNA or water. The RT-qPCR results revealed that, compared with the dsGFP-injected insects, the expression of SfDicer2 in the dsSfDicer2 treatment group decreased by 90.50% after 24 h and by 82.14% after 48 h; the interference efficiency after 72 h was 68.37% (Figure 3A, Table S2). Compared with the expression in the two control (dsGFP or water) groups, the target gene expression in the experimental group (dsSfDicer2) was significantly downregulated (p < 0.05). After 8 days, the survival rate of the experimental group was significantly lower than that of the two control (dsGFP or water) groups (p < 0.001; Figure 3B, Table S3). Compared with the results in the two control (dsGFP or water) groups (Figure 3C,F, Table S3), the mortality rate in the dsSfDicer2 treatment group was 42.69% (Figure 3D,E, Table S3). In particular, 35.62% of the deaths were attributable to the failure of epidermal dehiscence and molting. Furthermore, the mortality rate associated with wing deformity was 7.30%. After SfDicer2 RNAi, the wings of some S. furcifera insects curled or folded (Figure 3G,H); the wing deformity rate of the surviving insects was 37.08% (Table 2).
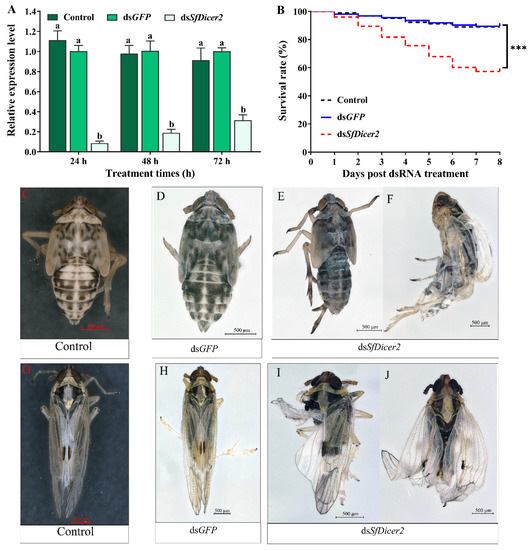
Figure 3.
Comparison between the S. furcifera treatment (dsSfDicer2) and the two control (dsGFP or water) groups. (A) Effects of SfDicer2 silencing at the transcriptional level. Data in the figure are presented as the means ± SEs of three replicates. The significance of the differences between the treatment (dsSfDicer2) and control (dsGFP or water) groups was determined using Student’s t-test for independent samples. (B) Survival rates after eight consecutive days. (C,D) Normal phenotypes of 3-day-old fifth-instar nymphs of S. furcifera (before molting) in the two control (dsGFP or water) groups. (E) Abnormal phenotype (lengthening body, ventral retraction, and inability to molt) of 3-day-old fifth-instar nymphs of S. furcifera (before molting) in the treatment group (dsSfDicer2). (F) S. furcifera failed to fully molt and died in the treatment group (dsSfDicer2). (G,H) Normal wings of adults in the two control (dsGFP or water) groups. (I,J) Curled or folded wings in the treatment group (dsSfDicer2). Different letters above the bars indicate significant differences in gene expression among the three groups of experimental conditions (Duncan’s multiple range test, p < 0.05); Significant differences between the treatment and control (dsGFP) groups are indicated using asterisks (***, p < 0.001).

Table 2.
Effects of SfDicer2 RNAi on the molting and wing expansion of S. furcifera.
3.4. Effects of SfDicer2 Silencing on the Gene Expression of Chitinase, Chitin deacetylase, Trehalase, and Chitin synthase in S. furcifera
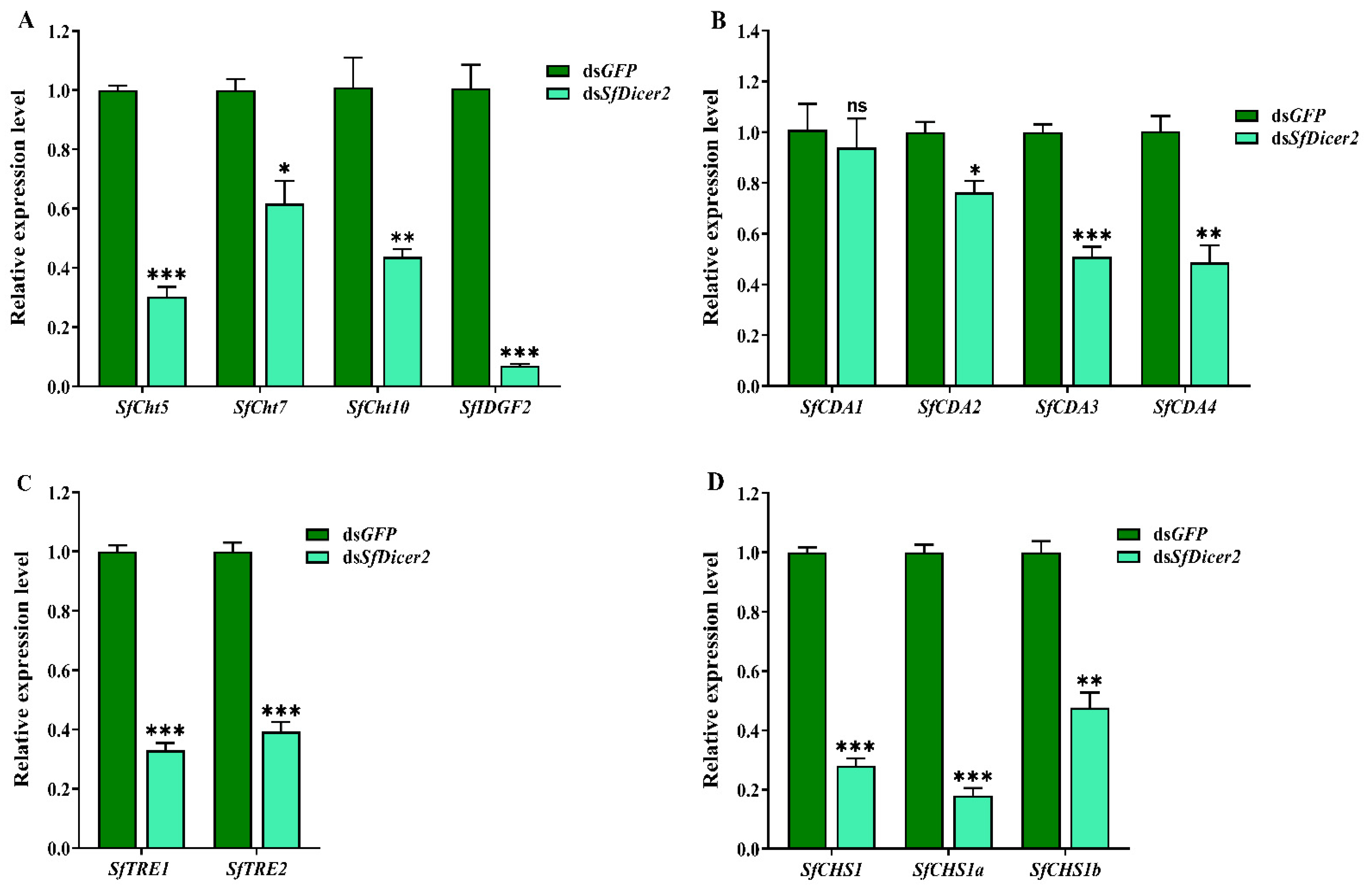
The samples were collected after silencing the SfDicer2 via RNAi for 48 h. To clarify the mechanism underlying the effect of silencing SfDicer2 on chitin synthesis and degradation in S. furcifera, we evaluated the expression of the genes associated with chitinase (SfCht5, SfCht7, SfCht10, and SfIDGF2), chitinase deacetylase (SfCDA1, SfCDA2, SfCDA3, and SfCDA4), trehalase (SfTRE1 and SfTRE2), and chitin synthase 1 (SfCHS1, SfCHS1a, and SfCHS1b) in S. furcifera (Figure 4, Table S4). The results indicated that after SfDicer2 was silenced for 48 h, the expression of SfCht5, SfCht7, SfCht10, and SfIDGF2 decreased significantly compared with that of the control (dsGFP) group (p < 0.05; Figure 4A). Similarly, the relative transcript levels of SfCDA2, SfCDA3, and SfCDA4 were significantly lower than those in the control (dsGFP) group; however, the relative expression of SfCDA1 did not vary significantly between the groups (Figure 4B). In addition, the relative expression of both trehalase (SfTRE1 and SfTRE2 (Figure 4C)) and three chitin synthase 1 genes (SfCHS1, SfCHS1a, and SfCHS1b (Figure 4D)) decreased significantly after SfDicer2 silencing. These findings suggest that SfDicer2 significantly affects the expression of chitinase, chitin deacetylase, trehalase, and chitin synthase 1 genes.
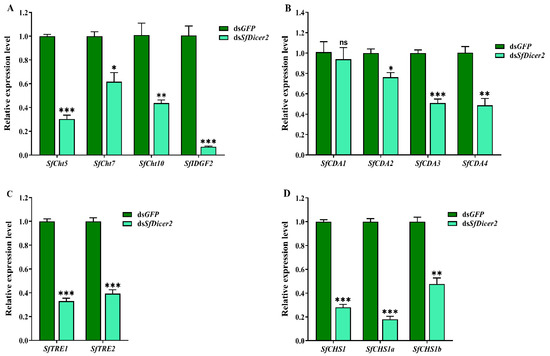
Figure 4.
Effects of silencing SfDicer2 on the expression of chitinase, chitin deacetylase, trehalase, and chitin synthase 1 genes. (A) The expression levels of chitinase genes: dsSfCht5, dsSfCht7, dsSfCht10, and dsSfIDGF2. (B) The expression levels of four chitin deacetylase genes: SfCDA1, SfCDA2, SfCDA3, and SfCDA4. (C) The expression levels of trehalase: SfTRE1 and SfTRE2. (D) The expression levels of chitin synthase 1 genes: SfCHS1, SfCHS1a, and SfCHS1b. Data in the figure are presented as the means ± SEs of three replicates. The significance of the differences between the treatment (dsSfDicer2) and control (dsGFP) groups was determined using a Student’s t-test for independent samples. The asterisks above the bars indicate significant differences (*, p < 0.05; **, p < 0.01; ***, p < 0.001; ns, no significance difference).
3.5. Analysis of the Effects of Silencing SfDicer2 on Wing Expansion-Related Genes
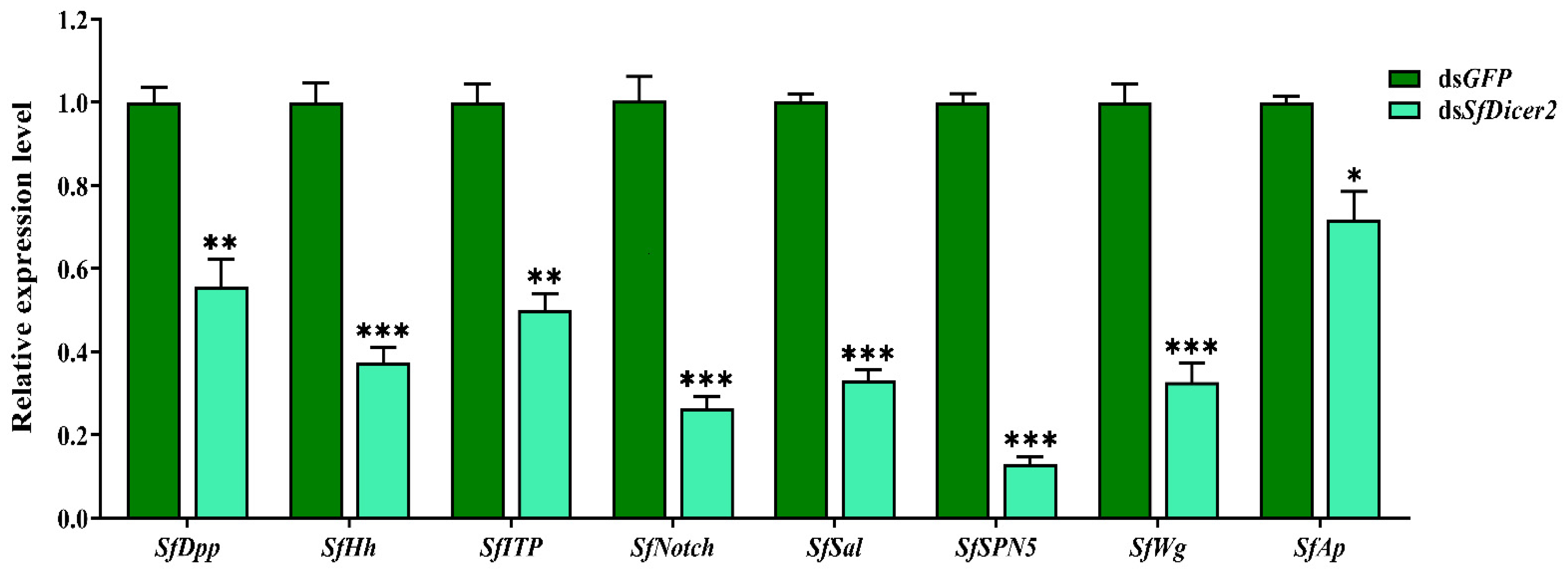
SfDPP, SfHh, SfITP, SfNotch, SfSal, SfSPN5, SfWg, and SfAp reportedly regulate wing expansion in insects. We used RT-qPCR to study the effect of silencing SfDicer2 on wing expansion-related genes in S. furcifera. Compared with the findings in the control (dsGFP) group, the expression of SfDPP, SfHh, SfITP, SfNotch, SfSal, SfSPN5, SfWg, and SfAp decreased significantly 48 h after dsSfDicer2 injection (p < 0.05 (Figure 5, Table S4). The specific silencing of the target gene could affect the transcript levels of some wing expansion-related genes, indicating that the silencing of SfDicer2 inhibits wing expansion.
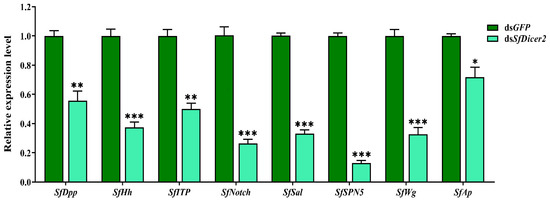
Figure 5.
Effects of silencing SfDicer2 on the expression of wing expansion-related genes. The data in the figure are presented as the means ± SEs of three replicates. The significance of the differences between the treatment (dsSfDicer2) and control (dsGFP) groups was determined using a Student’s t-test for independent samples. The asterisks above the bars indicate significant differences (*, p < 0.05, **, p < 0.01; ***, p < 0.001).
4. Discussion
Dicer2 is a core element in the RNAi pathway and serves as a cleavage enzyme in the siRNA pathway. An amino acid sequence characterization and phylogenetic tree analysis in S. furcifera revealed that the Dicer2 protein of S. furcifera was most closely related to that of N. lugens and L. striatellus. Previous studies have elucidated that Dicers of various species have similar domains, most of which include a HELICASE_ATP_BIND_1 or N-terminal DEAD domain, a HELICASE_CTER domain, a DICER_DSRBF domain, a PAZ domain, two RNase III domains, and a dsRBD domain [25,26]. The activity of helicases is crucial for processing dsRNA in the RNAi pathway [14]. The PAZ domain and tandem RNase III domains are responsible for excising siRNAs, preferentially from the ends of dsRNA molecules [27]. dsRBD is a typical domain of Dicer found in D. melanogaster [28]. Our results demonstrated that S. furcifera Dicer2 contains seven conserved domains similar to those in N. lugens and L. striatellus. This indicated that the protein is highly conserved in planthoppers.
Dicer2 is an endoribonuclease responsible for fragmenting exogenous dsRNA into siRNA, and it plays an essential role in the RNAi antiviral immune mechanism [26,29]. In this study, the expression of SfDicer2 in various developmental stages and tissues of S. furcifera was detected using RT-qPCR. The results showed that the target gene can play an important role in the growth and development of S. furcifera. However, the observed expression patterns were inconsistent with those in other insects. For instance, a study on D. melanogaster revealed that Dicer2 was expressed in all developmental stages and that its transcript levels were higher in pupae and adults in the late developmental stage than those in the early developmental stage. However, the expression of Dicer2 was lower in the nymphal stage than in the adult stage, which may be attributable to the different expression patterns of Dicer2 in different insects [16]. The evidence suggests that the third instar is a sensitive period of wing morph determination in N. lugens, and the wing bud development of the third-instar nymph determines the length of the fifth-instar wing bud [30]. The period between 24 and 36 h of molting in the fifth instar is a sensitive period for wing morph determination in N. lugens males and females, respectively [31]. This is consistent with the results of our study. Along with its high expression in 1-day-old first-instar nymphs and 2- and 3-day-old third-instar nymphs, SfDicer2 exhibited high transcript levels in 1- and 3-day-old fifth-instar nymphs, suggesting that SfDicer2 can play a vital role in wing differentiation. Furthermore, SfDicer2 participates in the growth, development, and reproduction of insects. The relative transcript level of SfDicer2 was high in the fat body, head, and integument, and the gene was distributed in the other tissues of S. furcifera. The fat body participates in the growth, development, and reproduction of insects as a crucial energy store and as a metabolic organ [31]. Therefore, this gene may participate in the growth and development of S. furcifera.
dsRNA is fragmented by Dicer2 to generate a 12–23-bp siRNA [32,33]. The resulting siRNA combines with AGO2 to form RISC, thus prompting the silencing of the target gene [8,9]. Therefore, Dicer2 plays a two-pronged role in the RNAi process. First, it functions upstream of RNAi to convert dsRNA to siRNA. Second, it functions downstream of RNAi for the formation of stable protein complexes (including Dicer2 and R2D2) after siRNA production, followed by mRNA shear [12,34]. The impact of dsRNA on the physiology of insects has also been studied in several species. It was found that dsRNA triggers the upregulation of RNAi and other immune-related genes in insects. Upon the delivery of (exogenous) dsRNA, the gene expression of Dicer2 is particularly upregulated to 395 times in Manduca sexta [35], 5 times in Blattella germanica [36], and 2.3 times in Acyrthosiphon pisum [37]. Therefore, dsRNA treatment can affect Dicer2 expression. To demonstrate whether dsRNA affects Dicer2 expression, we set up dsGFP and water as controls. Obviously, the relative mRNA expression level of SfDicer2 in both the dsGFP treatment group and the nuclease-free water treatment group were basically consistent with no significant indigenous change, indicating that treatment with dsRNA had no effect on the expression of Dicer2.
As a key effector of the RNAi-silencing mechanism, Dicer2 has considerable significance in the life activities of insects [15,16]. Previous studies have detected Ago2 and Dicer2 transcripts in the eggs, first-/second-instar larvae, third-/fourth-instar larvae, pupae, and male and female adults of Aedes albopictus, suggesting that siRNA-mediated RNAi plays a regulatory role in its growth and development [38]. In this study, SfDicer2 RNAi was associated with significant mortality and deformity, contradicting the previous findings that Dicer2 had no specific effect on the growth and development of N. lugens [17]. In a study on N. lugens, RT-qPCR results revealed that the transcript level of NlDicer2 was lowest (55%) on the first day after feeding the third-instar nymphs for 4 days. Only 7% of the insects died after being fed dsDicer2 for 4 days, and an 80% survival rate was achieved by N. lugens after 8 days without any notable deformity. These results could be explained by the high interference efficiency achieved with the injection method in this study. At 24 and 48 h after the injection of dsPxDicer2 into Plutella xylostella, the expression of the target genes decreased by 73.1% and 73.6%, respectively, compared with the expression at 0 h. Moreover, the mortality rate reached 77%. However, no obvious phenotypic changes were observed [39]. Some studies also revealed that silencing Drosha, Dicer2, Pasha, and Ago1 is not conducive to the transformation of Diabrotica virgifera virgifera larvae to adults [40]. The expression of these four transcripts increased from the third-instar stage to the pupal stage in this insect. In most cases, treatment with dsRNA before this stage reduced the protein’s abundance to below the level required to complete metamorphosis [41]. According to the binding efficiency of Dicer2 to dsRNA or the cleavage efficiency of mRNA in different insects, the degradation efficiency of mRNA varies [42], resulting in different outcomes.
Chitin is an important component of an insect’s exoskeleton, and its synthesis and degradation are crucial for insects to successfully molt. Studies revealed that silencing chitin synthase 1 is fatal to Locusta migratoria, which is attributable to molting difficulties [43]. Tang et al. studied the function of the trehalase gene in Tribolium castaneum and silenced it using RNAi technology, which resulted in molting deformity and death [44]. GpTre1 and GpTre2 play significant roles in the growth of Glyphodes pyloalis by influencing chitin metabolism [45]. Similarly, silencing the trehalase genes (TRE1 and TRE2) inhibits the growth of N. lugens [46]. Deacetylases play a vital role in insect molting, and interfering with their expression may lead to molting disorders and death [47]. For instance, SfCDA1, SfCDA2, and SfCDA4 play crucial roles in the molting of S. furcifera [48]. SfCht5, SfCht7, SfCht10, and SfIDGF2 also play essential roles in the molting of S. furcifera. Silencing the SfCht5, SfCht7, SfCht10, and SfIDGF2 genes resulted in the downregulation of chitin synthesis and decreased expression of chitin deacetylase genes, which subsequently affected the normal growth and development of insects [49]. These data are in agreement with the observed mortality rate of S. furcifera after the silencing of SfDicer2 in this study, including the high number of deaths in which the epidermis did not split or the insect became slender and molting failed. The transcript levels of four chitinase, two chitin deacetylase, two trehalase, and three chitin synthase 1 genes in S. furcifera decreased significantly after SfDicer2 was silenced. Therefore, we speculated that SfDicer2 affects the molting of S. furcifera by regulating the expression of chitinase, chitin deacetylase, trehalase, and chitin synthase 1 genes.
Insect wing expansion is also a crucial biological process regulated by multiple genes and signaling pathways. The development and differentiation of D. melanogaster wing primordium are mainly regulated by the Hedgehog (Hh), Decapentaplegic (Dpp), and Wingless (Wg) genes [50,51]. Morgan et al. first identified the gene Notch in D. melanogaster. Wing defects were observed when the partial function of Notch was missing [52]. After dsNotch injection, 10% of L. migratoria specimens displayed wing disorders after molting [53]. The elongation of insect wings is regulated by other genes, such as ion transport protein (ITP) and serpin-5 (SPN5) [54,55]. For example, ITP regulates wing expansion in N. lugens [56]. After Dpp expression, the wings of N. lugens exhibited different degrees of wing deformity [57]. In a study on S. furcifera, the silencing of Dpp resulted in abnormal phenotypes, such as curling or folding [58]. Similarly, Wg may affect wing elongation in S. furcifera. After the administration of dsWg in S. furcifera, 65.63% of the wings curled or failed to stretch normally [59]. Similar observations were observed in our study, which suggests that SfDicer2 plays an important role in regulating wing expansion in S. furcifera. Seven wing expansion-related genes (SfDPP, SfHh, SfITP, SfNotch, SfSal, SfSPN5, SfWg, and SfAp) were significantly downregulated after SfDicer2 was silenced. Therefore, SfDicer2 may affect wing expansion by regulating the expression of wing expansion-related genes.
These results provide a theoretical basis for the use of Dicer2 as a target gene for pest control in future studies.
5. Conclusions
We identified SfDicer2 using the genomic and transcriptomic data of S. furcifera. An RT-qPCR revealed that SfDicer2 is expressed in different developmental stages and tissues of S. furcifera. Moreover, this gene may be associated with the growth and development of the insect. The silencing of SfDicer2 resulted in significant inhibition of molting and wing expansion in S. furcifera and the downregulation of chitinase, chitin deacetylase, trehalase, and chitin synthase 1 and wing expansion-related genes. The results of our research may help clarify the spatiotemporal expression characteristics and biological functions of SfDicer2 to further understand its internal molecular mechanism in the regulation of insect growth and development. They can also provide a theoretical basis for future studies on the use of Dicer2 as a target gene for pest control.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/insects13080677/s1, Table S1: Relative expression levels of SfDicer1 in the various development stages and tissues of S. furcifera. Table S2: Effects of SfDicer2 silencing at the transcriptional level of SfDicer2. Table S3: Statistics after 8 days of interference. Table S4: Effects of silencing SfDicer2 on the expression of molting and wing expansion-related genes.
Author Contributions
H.Y. and D.-C.J. conceived and designed the study; and Z.-Y.J. conducted experiments for the detection of gene expression and interference efficiency; X.-B.Y. and completed the sample preparation; Q.-H.Z. and G.-Y.L. performed RNAi, analyzed the data, and drafted the manuscript; Q.-H.Z., H.Y., G.-Y.L., Z.-Y.J., D.-C.J. and X.-B.Y. reviewed and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (3216170196 and 31960537) and the program of Excellent Innovation Talents in Guizhou Province (20206003). It was also supported by the Natural Science Foundation Project of Chongqing (cstc2021jcyj-msxmX0235), the Chongqing Normal University Fund Project (grant no. 21XLB010), and the Science and Technology Foundation of Qiandongnan (no. J2019108).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data used/generated in this study have been included in the text and supplementary materials of this manuscript.
Acknowledgments
We thank Mingfu Gong Cao Zhou and Zhao Wang for his assistance with the manuscript revision.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, Y.; Zhang, H.; Li, H.; Miao, X. Second-generation sequencing supply an effective way to screen RNAi targets in large scale for potential application in pest insect control. PLoS ONE 2011, 6, e18644. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Peng, Y.; Zhang, H.; Wang, K.; Zhao, C.; Zhu, G.; Reddy Palli, S.; Han, Z. Off-target effects of RNAi correlate with the mismatch rate between dsRNA and non-target mRNA. RNA Biol. 2021, 18, 1747–1759. [Google Scholar] [CrossRef]
- Xie, Z.; Johansen, L.K.; Gustafson, A.M.; Kasschau, K.D.; Lellis, A.D.; Zilberman, D.; Jacobsen, S.E.; Carrington, J.C. Genetic and functional diversification of small RNA pathways in plants. PLoS Biol. 2004, 2, E104. [Google Scholar] [CrossRef] [Green Version]
- Hatfield, S.D.; Shcherbata, H.R.; Fischer, K.A.; Nakahara, K.; Carthew, R.W.; Ruohola-Baker, H. Stem cell division is regulated by the microRNA pathway. Nature 2005, 435, 974–978. [Google Scholar] [CrossRef]
- Ghildiyal, M.; Zamore, P.D. Small silencing RNAs: An expanding universe. Nat. Rev. Genet. 2009, 10, 94–108. [Google Scholar] [CrossRef] [Green Version]
- Karlikow, M.; Goic, B.; Saleh, M.C. RNAi and antiviral defense in Drosophila: Setting up a systemic immune response. Dev. Comp. Immunol. 2014, 42, 85–92. [Google Scholar] [CrossRef] [Green Version]
- Tijsterman, M.; Plasterk, R.H. Dicers at RISC; the mechanism of RNAi. Cell 2004, 117, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Carthew, R.W.; Sontheimer, E.J. Origins and mechanisms of miRNAs and siRNAs. Cell 2009, 136, 642–655. [Google Scholar] [CrossRef] [Green Version]
- Zhu, K.Y.; Palli, S.R. Mechanisms, applications, and challenges of insect RNA interference. Annu. Rev. Entomol. 2020, 65, 293–311. [Google Scholar] [CrossRef] [Green Version]
- Golden, D.E.; Gerbasi, V.R.; Sontheimer, E.J. An inside job for siRNAs. Mol. Cell 2008, 31, 309–312. [Google Scholar] [CrossRef]
- Kawamura, Y.; Saito, K.; Kin, T.; Ono, Y.; Asai, K.; Sunohara, T.; Okada, T.N.; Siomi, M.C.; Siomi, H. Drosophila endogenous small RNAs bind to Argonaute 2 in somatic cells. Nature 2008, 453, 793–797. [Google Scholar] [CrossRef]
- Liu, Q.; Rand, T.A.; Kalidas, S.; Du, F.; Kim, H.E.; Smith, D.P.; Wang, X. R2D2, a bridge betweenthe initiation and effector steps of the Drosophila RNAi pathway. Science 2003, 301, 1921–1925. [Google Scholar] [CrossRef]
- Kalidas, S.; Sanders, C.; Ye, X.; Strauss, T.; Kuhn, M.; Liu, Q.; Smith, D.P. Drosophila R2D2 mediates follicle formation in somatic tissues through interactions with Dicer-1. Mech. Dev. 2008, 125, 475–485. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.S.; Nakahara, K.; Pham, J.W.; Kim, K.; He, Z.; Sontheimer, E.J.; Carthew, R.W. Distinct roles for Drosophila Dicer-1 and Dicer-2 in the siRNA/miRNA silencing pathways. Cell 2004, 117, 69–81. [Google Scholar] [CrossRef] [Green Version]
- Lim, D.H.; Kim, J.; Kim, S.; Carthew, R.W.; Lee, Y.S. Functional analysis of dicer-2 missense mutations in the siRNA pathway of Drosophila. Biochem. Biophys. Res. Commun. 2008, 371, 525–530. [Google Scholar] [CrossRef] [Green Version]
- Lim, D.H.; Lee, L.; Oh, C.T.; Kim, N.H.; Hwang, S.; Han, S.J.; Lee, Y.S. Microarray analysis of Drosophila dicer-2mutants reveals potential regulation of mitochondrial metabolism by endogenous siRNAs. J. Cell. Biochem. 2013, 114, 418–427. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Lu, K.; Zhou, J.L.; Zhou, Q. Molecular characterization and gene functional analysis of Dicer-2 gene from Nilaparvata lugens (Hemiptera: Geometroidea). Insect Sci. 2013, 20, 61–68. [Google Scholar] [CrossRef]
- Zhou, G.H.; Wen, J.J.; Cai, D.J.; Li, P.; Xu, D.; Zhang, S. Southern rice black-streaked dwarf virus: A new proposed Fijivirus species in the family Reoviridae. Chin. Sci. Bull. 2008, 53, 3677–3685. [Google Scholar] [CrossRef]
- Zhu, Z.R.; Cheng, J.A.; Liu, Y.G. A comparison of population biology of macropterous and brachypterous adults of Sogatella furcifera (Horvath). Chin. J. Rice Sci. 2001, 03, 70–73. (In Chinese) [Google Scholar]
- Zhou, C.; Yang, H.; Wang, Z.; Long, G.Y.; Jin, D.C. Comparative transcriptome analysis of Sogatella furcifera (Horváth) exposed to different insecticides. Sci. Rep. 2018, 8, 8773. [Google Scholar] [CrossRef]
- Wang, L.; Tang, N.; Gao, X.L.; Chang, Z.; Zhang, L.; Zhou, G.; Guo, D.; Zeng, Z.; Li, W.; Akinyemi, I.A.; et al. Genome sequence of a rice pest, the white-backed planthopper (Sogatella furcifera). GigaScience 2017, 6, giw004. [Google Scholar]
- Wang, Y.Y.; Yu, J.M.; Dai, L.L.; Cheng, H. Cloning and expression of two mevalonate pathway genes of Dendroctonus armandi. J. Northwest Univ. 2020, 35, 140–149. [Google Scholar]
- An, X.K.; Hou, M.L.; Liu, Y.D. Reference gene selection and evaluation for gene expression studies using qRT-PCR in the white-backed planthopper, Sogatella furcifera (Hemiptera: Delphacidae). J. Econ. Entomol. 2016, 109, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Lv, X.; Wang, W.; Han, Z.; Liu, S.; Yang, W.; Li, M.; Wang, L.; Song, L. The Dicer from oyster Crassostrea gigas functions as an intracellular recognition molecule and effector in anti-viral immunity. Fish Shellfish Immunol. 2019, 95, 584–594. [Google Scholar] [CrossRef]
- Ji, X.H. The mechanism of RNase III action: How dicer dices. Curr. Top. Microbiol. Immunol. 2008, 320, 99–116. [Google Scholar] [PubMed]
- Carmell, M.A.; Hannon, G.J. RNase III enzymes and the initiation of gene silencing. Nat. Struct. Mol. Biol. 2004, 11, 214–218. [Google Scholar] [CrossRef]
- Bronkhorst, A.W.; Leef, K.W.R.; Vodovar, N.; Ince, I.A.; Blanc, H.; Vlak, J.M.; Saleh, M.C.; van Rij, R.P. The DNA virus Invertebrate iridescent virus 6 is a target of the Drosophila RNAi machinery. Proc. Natl. Acad. Sci. USA 2012, 109, E3604–E3613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamada, S. Dual wing-form determination mechanism in the brown planthopper, Nilaparvata lugens STL(Homoptera:Delphacidae). Appl. Entomol. Zool. 1991, 26, 590–592. [Google Scholar] [CrossRef]
- Jiang, Y.Q. Sensitive Period of Wing-Morph Determination in the Brown Planthopper. Master’s Thesis, Zhejiang University, Hangzhou, China, August 2016. (In Chinese). [Google Scholar]
- Arrese, E.L.; Soulages, J.L. Insect fat body: Energy, metabolism, and regulation. Annu. Rev. Entomol. 2010, 55, 207–225. [Google Scholar] [CrossRef] [Green Version]
- Kandasamy, S.K.; Zhu, L.; Fukunaga, R. The C-terminal dsRNA-binding domain of Drosophila Dicer-2 is crucial for efficient and high-fidelity production of siRNA and loading of siRNA to Argonaute2. RNA 2017, 23, 1139–1153. [Google Scholar] [CrossRef] [Green Version]
- Cagliari, D.; Dias, N.P.; Dos Santos, E.Á.; Rickes, L.N.; Kremer, F.S.; Farias, J.R.; Lenz, G.; Galdeano, D.M.; Garcia, F.R.M.; Smagghe, G.; et al. First transcriptome of the Neotropical pest Euschistus heros (Hemiptera: Pentatomidae) with dissection of its siRNA machinery. Sci. Rep. 2020, 10, 4856. [Google Scholar] [CrossRef]
- Gao, L.; Wang, Y.; Abbas, M.; Zhang, T.; Ma, E.; Xing, S.; Zhu, K.Y.; Zhang, J. Molecular characterizations and functional analyses of LmR2D2 in the Locusta migratoria siRNA pathway. Insects 2021, 12, 812. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.L.; Zheng, X.L. Cloning of AGO2 and Dcr-2 gene fragments and analysis of their transcription level in different developmental stages of Aedes albopictus. Chin. J. Parasitol. Parasit. Dis. 2012, 30, 214–217. (In Chinese) [Google Scholar]
- Garbutt, J.S.; Reynolds, S.E. Induction of RNA interference genes by double-stranded RNA; implications for susceptibility to RNA interference. Insect Biochem. Mol. Biol. 2012, 42, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Lozano, J.; Gomez-Orte, E.; Lee, H.J.; Belles, X. Super-induction of Dicer-2 expression by alien double-stranded RNAs: An evolutionary ancient response to viral infection? Dev. Genes Evol. 2012, 222, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; An, X.; Jiang, Y.D.; Ding, B.Y.; Shang, F.; Christiaens, O.; Taning, C.N.T.; Smagghe, G.; Niu, J.; Wang, J.J. Induction of RNAi core machinery’s gene expression by exogenous dsRNA and the effects of pre-exposure to dsRNA on the gene silencing efficiency in the pea aphid (Acyrthosiphon pisum). Front. Physiol. 2019, 10, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Shen, X.J. Genome-Wide Characterization and Expression Profiling of the RNAi Pathway Genes in Plutella xylostella (L.) and Functional Analysis of PxDcr-2; Fujian Agriculture and Forestry University: Fuzhou, China, 2016. (In Chinese) [Google Scholar]
- Davis-Vogel, C.; Ortiz, A.; Procyk, L.; Robeson, J.; Kassa, A.; Wang, Y.; Huang, E.; Walker, C.; Sethi, A.; Nelson, M.E.; et al. Knockdown of RNA interference pathway genes impacts the fitness of western corn rootworm. Sci. Rep. 2018, 8, 7858. [Google Scholar] [CrossRef] [PubMed]
- Davis-Vogel, C.; Van Allen, B.; Van Hemert, J.L.; Sethi, A.; Nelson, M.E.; Sashital, D.G. Identification and comparison of key RNA interference machinery from western corn rootworm, fall armyworm, and southern green stink bug. PLoS ONE 2018, 13, e0203160. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Li, H.C.; Miao, X.X. Feasibility, limitation and possible solutions of RNAi-based technology for insect pest control. Insect Sci. 2013, 20, 15–30. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Zhang, J.; Li, D.; Sun, Y.; Guo, Y.; Ma, E.; Zhu, K.Y. Silencing of two alternative splicing-derived mRNA variants of chitin synthase 1 gene by RNAi is lethal to the oriental migratory locust, Locusta migratoria manilensis (Meyen). Insect Biochem. Mol. Biol. 2010, 40, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Wei, P.; Zhao, L.; Shi, Z.; Shen, Q.; Yang, M.; Xie, G.; Wang, S. Knockdown of five trehalase genes using RNA interference regulates the gene expression of the chitin biosynthesis pathway in Tribolium castaneum. BMC Biotechnol. 2016, 16, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, Z.M.; Ding, J.H.; Jiang, D.L.; Liu, Z.X.; Li, Y.J.; Wang, J.; Wang, J.; Sheng, S.; Wu, F.A. Characterization and Functional Analysis of trehalase Related to Chitin Metabolism in Glyphodes pyloalis Walker (Lepidoptera: Pyralidae). Insects 2021, 12, 370. [Google Scholar] [CrossRef]
- Zhao, L.; Yang, M.; Shen, Q.; Liu, X.; Shi, Z.; Wang, S.; Tang, B. Functional characterization of three trehalase genes regulating the chitin metabolism pathway in rice brown planthopper using RNA interference. Sci. Rep. 2016, 6, 27841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quan, G.; Ladd, T.; Duan, J.; Wen, F.; Doucet, D.; Cusson, M.; Krell, P.J. Characterization of a spruce budworm chitin deacetylase gene: Stage- and tissue-specific expression, and inhibition using RNA interference. Insect Biochem. Mol. Biol. 2013, 43, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.B.; Zhou, C.; Gong, M.F.; Yang, H.; Long, G.Y.; Jin, D.C. Identification and RNAi-based functional analysis of four chitin deacetylase genes in Sogatella furcifera (Hemiptera: Delphacidae). J. Insect Sci. 2021, 21, 9. [Google Scholar] [CrossRef]
- Yang, X.B.; Zhou, C.; Long, G.Y.; Yang, H.; Chen, C.; Jin, D. Characterization and functional analysis of chitinase family genes involved in nymph-adult transition of Sogatella furcifera. Insect Sci. 2021, 28, 901–916. [Google Scholar] [CrossRef]
- Strigini, M.; Cohen, S.M. Formation of morphogen gradients in the Drosophila wing. Semin. Cell Dev. Biol. 1999, 10, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Erickson, J.L. Formation and maintenance of morphogen gradients: An essential role for the endomembrane system in Drosophila melanogaster wing expanse. Fly 2011, 5, 266–271. [Google Scholar] [CrossRef]
- Morgan, T.H. The theory of the gene. Am. Nat. 1917, 51, 513–544. [Google Scholar] [CrossRef]
- Xu, Z.L.; Zhang, F.B.; Wu, Z.C.; Zhang, J.; Zhang, J.Z.; Zhao, X.M. Function analysis of LmNotch in the wing development of Locusta migratoria. Chin. J. Biol. Control. 2021, 18, 1–12. (In Chinese) [Google Scholar]
- Dircksen, H.; Tesfai, L.K.; Albus, C.; Nässel, D.R. Ion transport peptide splice forms in central and peripheral neurons throughout postembryogenesis of Drosophila melanogaster. J. Comp. Neurol. 2008, 509, 23–41. [Google Scholar] [CrossRef]
- Charron, Y.; Madani, R.; Combepine, C.; Gajdosik, V.; Hwu, Y.; Margaritondo, G.; Vassalli, J.D. The serpin Spn5 is essential for wing expansion in Drosophila melanogaster. Int. J. Dev. Biol. 2008, 52, 933–942. [Google Scholar] [CrossRef]
- Yu, B.; Li, D.T.; Wang, S.L.; Xu, H.J.; Bao, Y.Y.; Zhang, C.X. Ion transport peptide (ITP) regulates wing expansion and cuticle melanism in the brown planthopper, Nilaparvata lugens. Insect Mol. Biol. 2016, 25, 778–787. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, F.; Wu, C.; Zhao, J.; Cai, W.; Hua, H. Decapentaplegic function in wing vein development and wing morph transformation in brown planthopper, Nilaparvata lugens. Dev. Biol. 2019, 449, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Long, G.Y.; Yang, J.P.; Jin, D.C.; Yang, H.; Zhou, C.; Wang, Z.; Yang, X.B. Silencing of decapentaplegic (Dpp) gene inhibited the wing expansion in the white-backed planthopper, Sogatella furcifera (Horváth) (Hemiptera: Delphacidae). Arch. Insect Biochem. Physiol. 2022, 110, E21879. [Google Scholar] [CrossRef]
- Yu, J.L.; An, Z.F.; Liu, X.D. Wingless gene cloning and its role in manipulating the wing dimorphism in the white-backed planthopper, Sogatella furcifera. BMC Mol. Biol. 2014, 15, 20. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).