Simple Summary
Short- and long-term storage potential is an important feature of a biocontrol agent. We estimated survival during storage in the absence of natural food (aphids) and the post-storage fecundity of females of the predatory ladybird Cheilomenes propinqua in relation to temperature (from 7 to 27 °C), air humidity (from 50 to 80–90%), and feeding regime (starved or fed). The experiments showed that under the optimal conditions (temperatures of 15–17 °C and feeding on the grain moth eggs) C. propinqua females can be stored for up to 80 days for future use in mass rearing or the biological control of pests. Short-term storage or transportation (shipment) can occur at a much wider range of temperatures, i.e., from 12 to 27 °C. We conclude that C. propinqua can be successfully used for biological control in greenhouses by preventing colonization, although this would require the regular supplementation of food. Besides this, storage for more than 10 days at temperatures of 7 °C and lower results in 100% mortality; therefore, escaped individuals would not survive the winter even in the south of the temperate zone.
Abstract
The ability of the females of the predatory ladybird Cheilomenes propinqua to survive and to retain reproductive potential in the absence of natural food (aphids) was estimated under various hydrothermal (temperatures of 7, 12, 17, 22, and 27 °C; air humidities of 50% and 80–90%) and trophic (starved vs. fed on the frozen eggs of the grain moth Sitotroga cerealella) conditions. The post-storage reproductive potential was estimated using the mean number of eggs laid over 20 days. The experiments showed that fed females can be stored at temperatures of 15–17 °C for 20 days with a rather low (about 20%) and for up to 80 days with an acceptable (not more than 50%) loss of the reproductive potential. The successful short-term (up to 3 days) storage or transportation of fed and starved females can occur at temperatures from 12 to 27 °C. However, storage for more than 10 days at temperatures of 7 °C and lower results in the 100% mortality of both the starved and fed beetles. These data suggest that (1) in greenhouses, C. propinqua can be used for the biological control of aphids by preventing colonization, although this would require the regular supplementation of factitious food, and (2) escaped individuals would not survive the winter even in the south of the temperate zone.
Keywords:
biological control; storage; survival; fecundity; hatching; temperature; humidity; starvation; Cheilomenes propinqua 1. Introduction
The augmentative biological control of greenhouse insect pests, which is the most promising alternative to the extensive use of chemical pesticides, involves the mass rearing of a biocontrol agent [1,2,3,4,5]. The ability to survive in the absence of natural food is a substantial advantage of a mass-produced insect. This ability is important in two rather different processes: storage and transportation (shipment). The need for the storage of a biocontrol agent arises in a number of situations: a low-demand period, a need to accumulate insects for a large delivery, or a temporary lack of natural food, etc. [6]. In all of these cases, storage is usually a long-term process (up to several months) taking place in a mass-rearing facility under more- or less-controlled favorable conditions. Transportation (delivery to a consumer), in contrast, is a relatively short-term process, but the environmental conditions during this period can deviate considerably from the optimal values. Of course, maintaining optimal conditions during transportation is also possible, but this would make shipping more complicated and expensive. Thus, high storage and transportation potential is an essential prerequisite for a successful and cost-effective inundative biocontrol.
Generally speaking, the successful storage and transportation of an insect in the absence of its natural food depend on two main factors: species-specific starvation resistance (or the ability to survive on factitious food) and environmental conditions. In most aphidophagous ladybirds, both the starvation resistance and the ability to switch to an alternative food are relatively high because in nature aphids represent a very unstable source of food: an aphid colony can rapidly reach a high population density and then quickly disappear [7,8,9]. The most important abiotic environmental factors influencing the success of storage are temperature and humidity. A low temperature decreases their metabolism, thereby increasing resistance to starvation and other adverse factors. On the other hand, too low a temperature—approaching the tolerance limit—becomes an unfavorable factor. High air humidity helps an insect to cope with the desiccation which often accompanies starvation, although too high a humidity can promote the growth of mold or insect pathogens.
The object of our study, the predatory ladybird Cheilomenes propinqua vicina Mulsant (=Cydonia (Cheilomenes) vicina Mulsant) is widely distributed across Africa and the Middle East [10]. This polyphagous predator was found on various field crops, as well as in vineyards and orchards feeding on various aphids, soft scales, mealybugs, whiteflies, and psyllids [11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27]. In particular, C. propinqua was shown to be an important natural enemy of such serious pests as aphids Myzus persicae (Sulzer) [22,23], Aphis gossypii Glover [11,16,23,24,25,27], Aphis fabae Scopoli [21], Aphis craccivora Koch [24] and Lipaphis erysimi (Kalt) [23], soft scales of the genus Saissetia Deplanche [14], mealybugs Ferrisia virgata (Cockerell) [12] and Phenacoccus solenopsis Tinsley [26], whiteflies Bemisia tabaci (Gennadius) and Aleurodicus dispersus Russell [13], and citrus psylla Trioza erytreae (Del Guercio) [17]. However, we consider C. propinqua as a potential mass-reared agent for the biological control of greenhouse pests. In this context, it should be noted that—under the optimal conditions—this relatively small (body length 4–5 mm) and rapidly developing (10–11 days from egg to adult stage) ladybird shows rather high voracity (females consumed 40–80 aphids per day, depending on the prey species; when feeding on A. gossypii, larvae consumed a total average of about 500, and females consumed about 2000 individuals) and fecundity (15–30 eggs per day, with a total lifetime fecundity of about 1000 eggs per female) [21,28,29,30]. Such a combination of beneficial properties suggests that C. propinqua, as well as some other coccinellids [31], can be an effective agent for biological control. Indeed, greenhouse trials conducted in 2019–2020 in East Africa showed that C. propinqua can successfully control A. fabae on kalanchoe crops [21].
The use of an insect predator for the biological control of pests of protected crops, as was noted above, involves the development of optimal methods for its mass rearing, storage, packaging, distribution, and release, which in turn requires a basic knowledge of its ecophysiology. The influence of the temperature and prey species on the rate of development, fecundity, mortality, net reproductive rate, and some other biological parameters of C. propinqua has been investigated [11,20,28,30]. The storage potential, however, was not studied. The aims of the present study were to estimate the ability of C. propinqua females to survive and to retain reproductive potential in the absence of natural food under various hydrothermal and trophic conditions, and thereby to select the optimal methods for their storage and transportation.
2. Materials and Methods
The experiments were conducted with a laboratory population of C. propinqua originating from 42 adults collected on 10–14 September 2015 in Alexandria (31.200391° N, 29.9155046° E) and reared in the All-Russian Institute of Plant Protection on the wheat aphid (Schizaphis graminum Rond.) at a temperature of 24 °C and in a photoperiod of L:D = 16:8 (hereafter, light and dark periods are given in h). Before the study, the ladybirds were reared for several generations in the Laboratory of Experimental Entomology, Zoological Institute RAS, in glass cylinders covered with nylon tissue at a temperature of 25 °C and L:D = 16:8, feeding on nymphs and adults of the green peach aphid Myzus persicae (Sulz.), which were reared on Vicia faba L. seedlings.
To start the experiment, a cohort of the first instar larvae hatched over 24 h from eggs laid by 10–20 C. propinqua females was reared on the green peach aphid at a temperature of 25 °C and L:D = 10:14, i.e., under the short-day conditions that increase the survival of starving females [28]. The emerging adults were kept in groups of 20–50 individuals under the same conditions for 5 more days. Then, randomly selected females were distributed among the experimental treatments.
The study included two experiments. The aim of the first, ‘short-term’ experiment was to imitate short-term storage or transportation to a consumer, whereas the second, ‘long-term’ experiment aimed to estimate the long-term storage potential at the rearing facilities. In both experiments, females were stored in plastic Petri dishes (90 mm × 15 mm) lined with paper (9–10 individuals per dish). The dishes were placed in tightly (but not hermetically) closed plastic containers.
The experimental treatments differed in the following factors.
- Duration of storage: 3, 6, 9, and 12 days in the short-term experiment; 20, 40, 60, and 80 days in the long-term experiment; and 0 (no storage) in the controls.
- Temperature: 7, 12, 17, 22, and 27 °C in the short-term experiment; 12, 15, and 17 °C in the long-term experiment (in all of the treatments, the insects were stored in the dark). These ranges of temperatures were used because we presumed that the storage temperature at the rearing facility can be strictly controlled, whereas the transportation conditions can vary considerably. Regarding the long-term experiment, our (unpublished) pilot test demonstrated that at temperatures of 10 °C and lower C. propinqua adults are not able to feed, whereas the results of our previous study [28] suggest that feeding on some factitious food is necessary for their long-term storage. On the other hand, another earlier study [30] showed that at 20 °C (in contrast to 15 °C) C. propinqua females matured and laid eggs, suggesting that the temperature of 20 °C is too high for long-term storage.
- Humidity: in both experiments, two levels of air humidity were used, i.e., low (about 50%) and high (80–90%). The low humidity was maintained using a saturated solution of calcium nitrate, and the high humidity was maintained using saturated sucrose solution.
- Trophic regime: fed vs. starved. Frozen eggs of the Angoumois grain moth Sitotroga cerealella Oliv. glued to a piece of hard paper with 30% sugar solution were used as a factitious food. In the long-term experiment, this food was offered to all of the females, whereas in the short-term experiment, it was provided in only half of the treatments (excluding those with a temperature of 7 °C, which—as was noted above, —is too low for feeding). The food was always provided in excess, as needed (e.g., daily at 27 °C and weekly at 12 °C).
After the end of the storage period, all of the surviving females were used for the estimation of their reproductive potential. To this aim, they were individually placed in the same size (90 mm × 15 mm) Petri dishes and kept for 20 days under the optimal conditions (25 °C and L:D = 16:8), feeding on the green peach aphid. The eggs laid during this period were counted daily. In addition, a batch of eggs laid during this period was incubated under the same condition, and the hatching percentage was calculated. At the end of the estimation period, the hind femur length of each female was measured using a stereo microscope with an accuracy of 0.025 mm as an indicator of its size, and then the experiment was finished (females that died during the storage or estimation period were also measured). Thus, the percentage of females that survived to the end of the storage period was calculated for each treatment of each experiment, and for each female the number of eggs laid over 20 days, the percentage of hatched eggs, and the hind femur length were recorded. Then, the final reproductive potential was calculated for each female as the product of fecundity and the percentage of hatched eggs (the reproductive potential of females that did not survive the storage period was taken to be zero).
The short-term experiment included 72 treatments (combinations of 4 storage periods, 5 temperatures, 2 levels of humidity, and 2 trophic regimes for 4 of the 5 temperatures). The long-term experiment included 24 treatments (combinations of 4 storage periods, 3 temperatures, and 2 levels of humidity). In addition, control treatments were carried out, in which the females were not stored. The experiments were performed with 27–30 females per experimental treatment, and 58 control females (a total of 2665 females were used in the present study).
For the statistical analysis of the binary parameter (survival), probit analysis was used; the treatments were compared by the chi-square test. The fecundity and the percentage of hatched eggs were analyzed by GLM and ANOVA. For the final reproductive potential (which was often bimodally distributed), a non-parametric Kruskal–Wallis test was used. All of the calculations were conducted using SYSTAT 10.2 (http://systat.software.informer.com/10.2 (accessed on 1 July 2022)).
3. Results
3.1. Survival
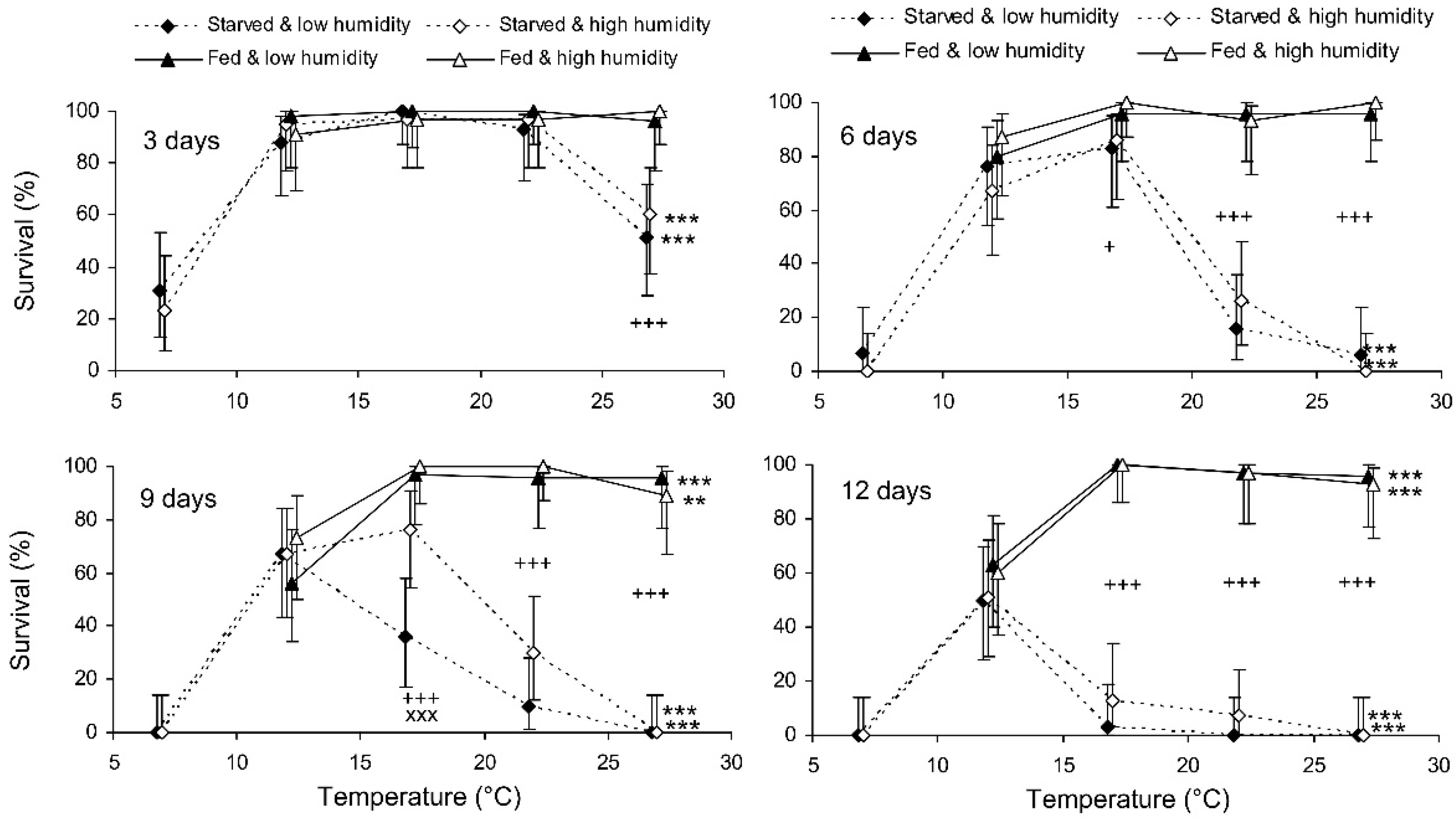
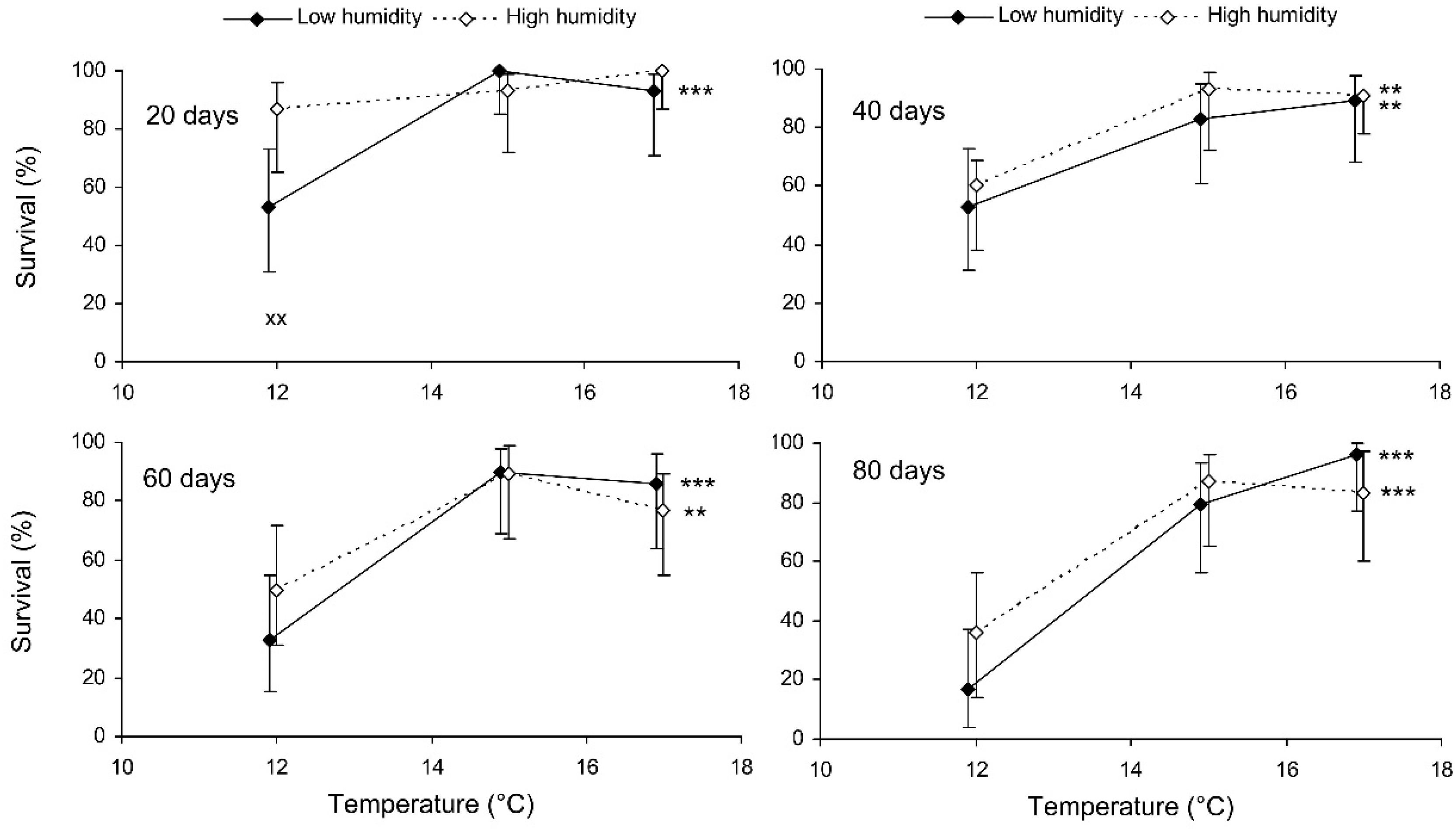
The binary probit analysis of the results of the short-term experiment showed that, as expected, the survival of C. propinqua females decreased with the storage period, and fed individuals survived better than starved ones (Table 1). In addition, survival decreased with the temperature and increased with the humidity (although the last effect was marginally not significant). In Figure 1, it can be seen that the survival percentage of starved females decreased at extreme temperatures of 7 and 27 °C. Fed females did not show any decrease in survival at 27 °C, but at 12 °C their survival decreased after 9 days of storage (reminder: the storage potential of fed females was not tested at 7 °C because feeding is not possible at this temperature). In total, the highest survival of starved females was observed at 12 °C, whereas fed females showed equally high survival at a wide range of temperatures from 17 to 27 °C. The impact of the trophic factor increased with the temperature: at 27 °C, the difference in survival between starved and fed females was already significant after 3 days of storage, whereas at 12 °C it was not significant even after 12 days. The survival of starved females after 6 and more days of storage tended to increase with the humidity, but in most cases this effect was not statistically significant. The long-term experiment gave similar results (Table 1, Figure 2). The survival of fed females at 15–17 °C was independent of the humidity, and was markedly higher than that at 12 °C. Under the optimal conditions, about 80% of the females survived for at least 80 days.

Table 1.
Influence of the trophic regime, temperature, humidity, and duration of storage on the survival of Cheilomenes propinqua females (the results of the binary probit analysis: the regression coefficient ± SE, t-ratio, and significance of influence).
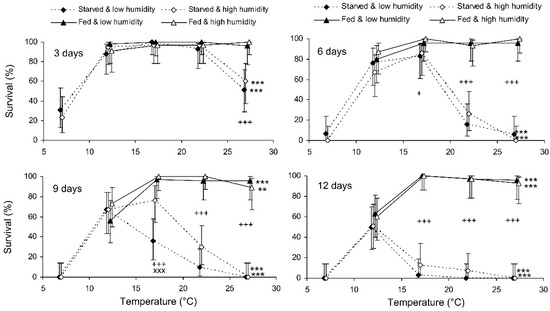
Figure 1.
Survival of Cheilomenes propinqua females in relation to the temperature, humidity, trophic regime, and duration of storage (the results of the short-term experiment). Percentages with 95% confidence intervals are shown. The duration of storage is indicated in the graphs. Asterisks at the right end of the line indicate the significant influence of temperature: **—p < 0.01 or ***—p < 0.001 by the chi-square test. Plus symbols indicate a significant influence of the trophic regime at the corresponding temperature: +—p < 0.05, +++—p < 0.001, according to the Mantel–Haenszel chi-square test (humidity was used as a strata variable). X-crosses indicate the significant influence of humidity at the corresponding temperature, ×××—p < 0.001, according to the Mantel–Haenszel chi-square test (the trophic regime was used as a strata variable).
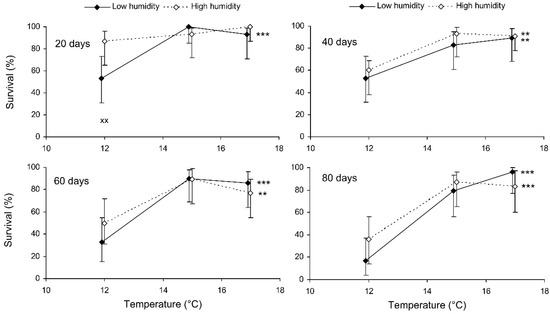
Figure 2.
Survival of fed Cheilomenes propinqua females in relation to the temperature, humidity, and duration of storage (the results of the long-term experiment). Percentages with 95% confidence intervals are shown. The duration of storage is indicated in the graphs. Asterisks at the right end of the line indicate a significant influence of temperature—**, p < 0.01, ***, p < 0.001—according to the chi-square test. X-crosses indicate the significant influence of humidity at the corresponding temperature, ××, p < 0.01, according to the Mantel–Haenszel chi-square test (the trophic regime was used as a strata variable).
3.2. Fecundity
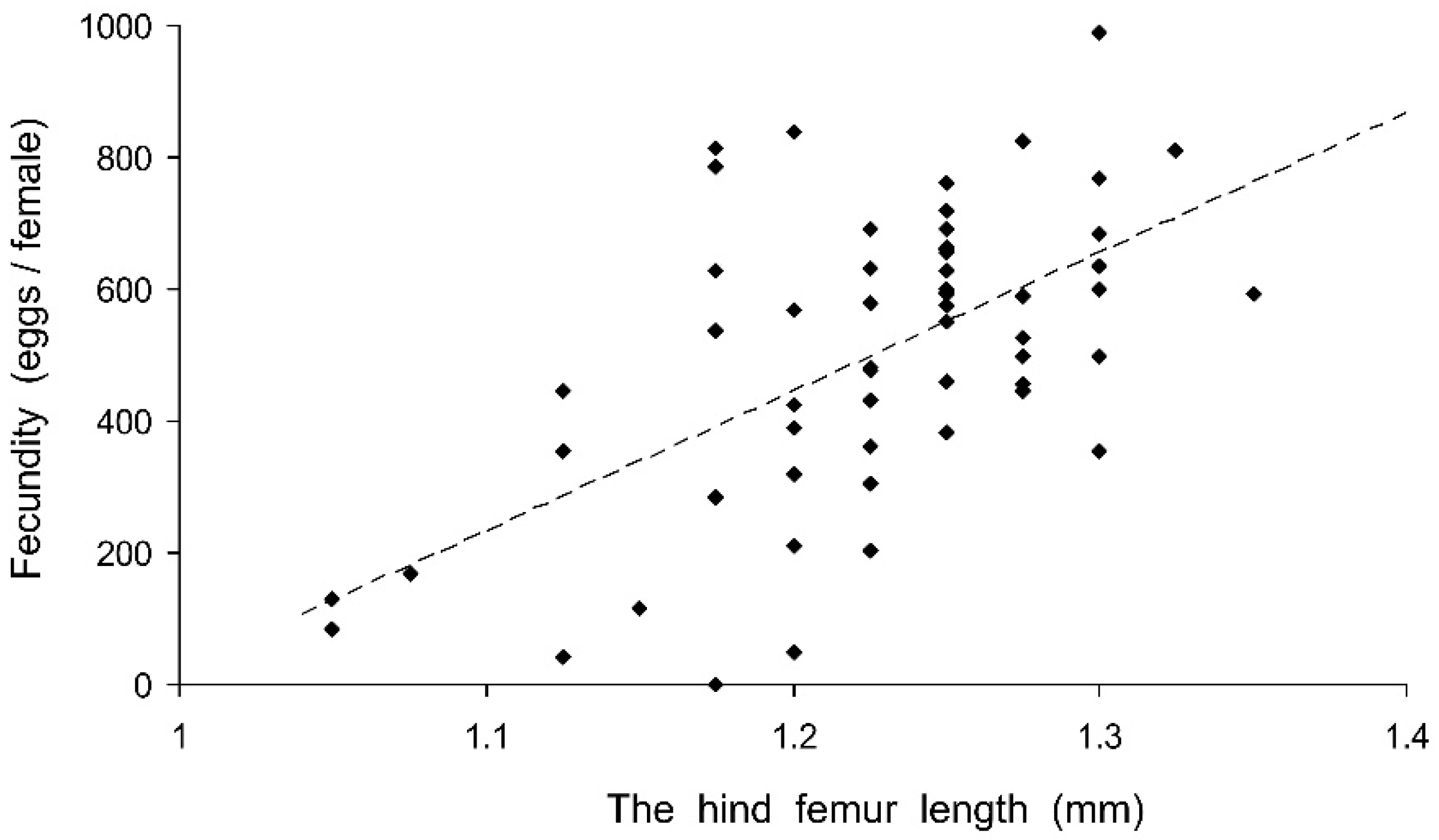
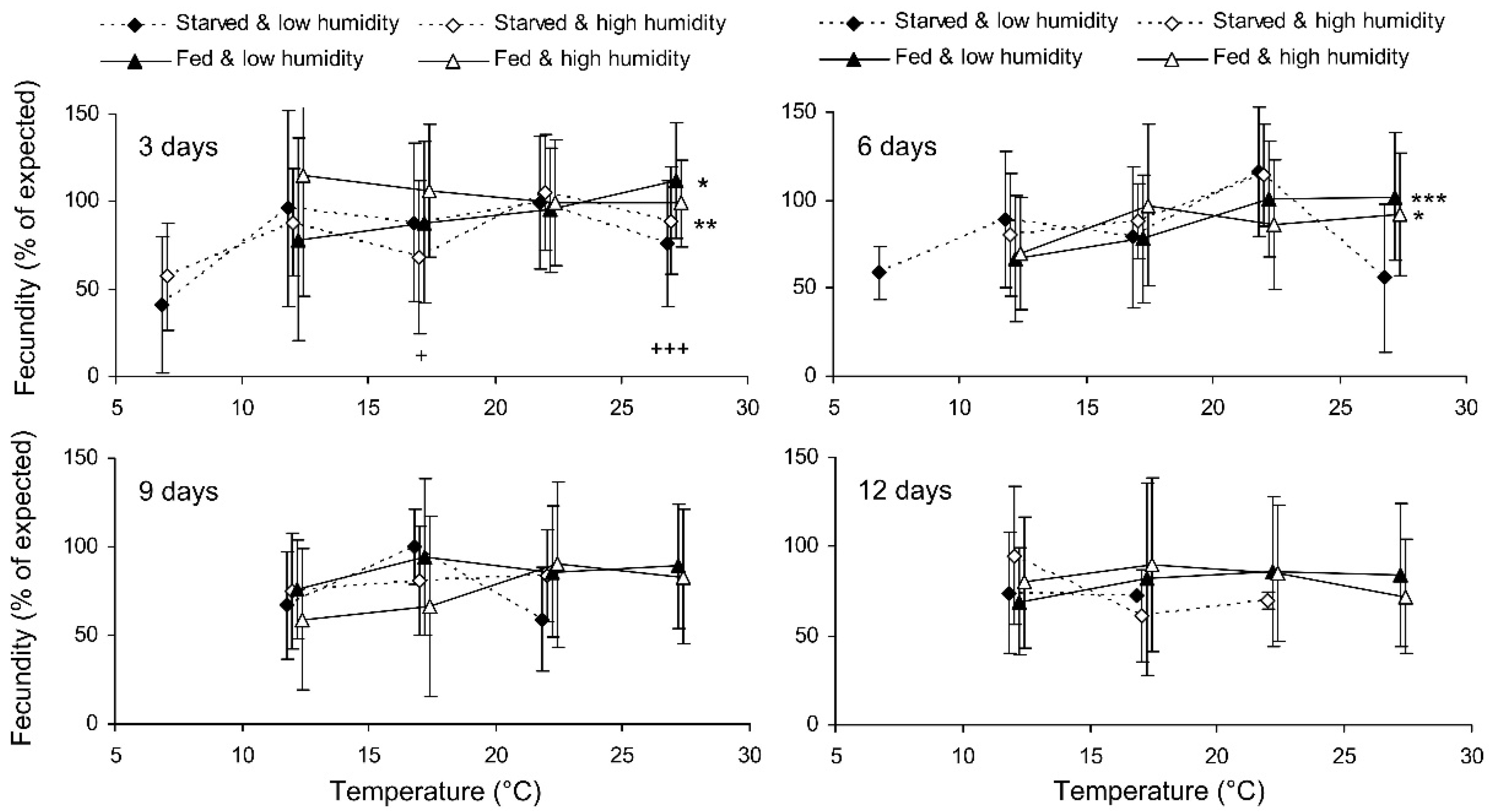
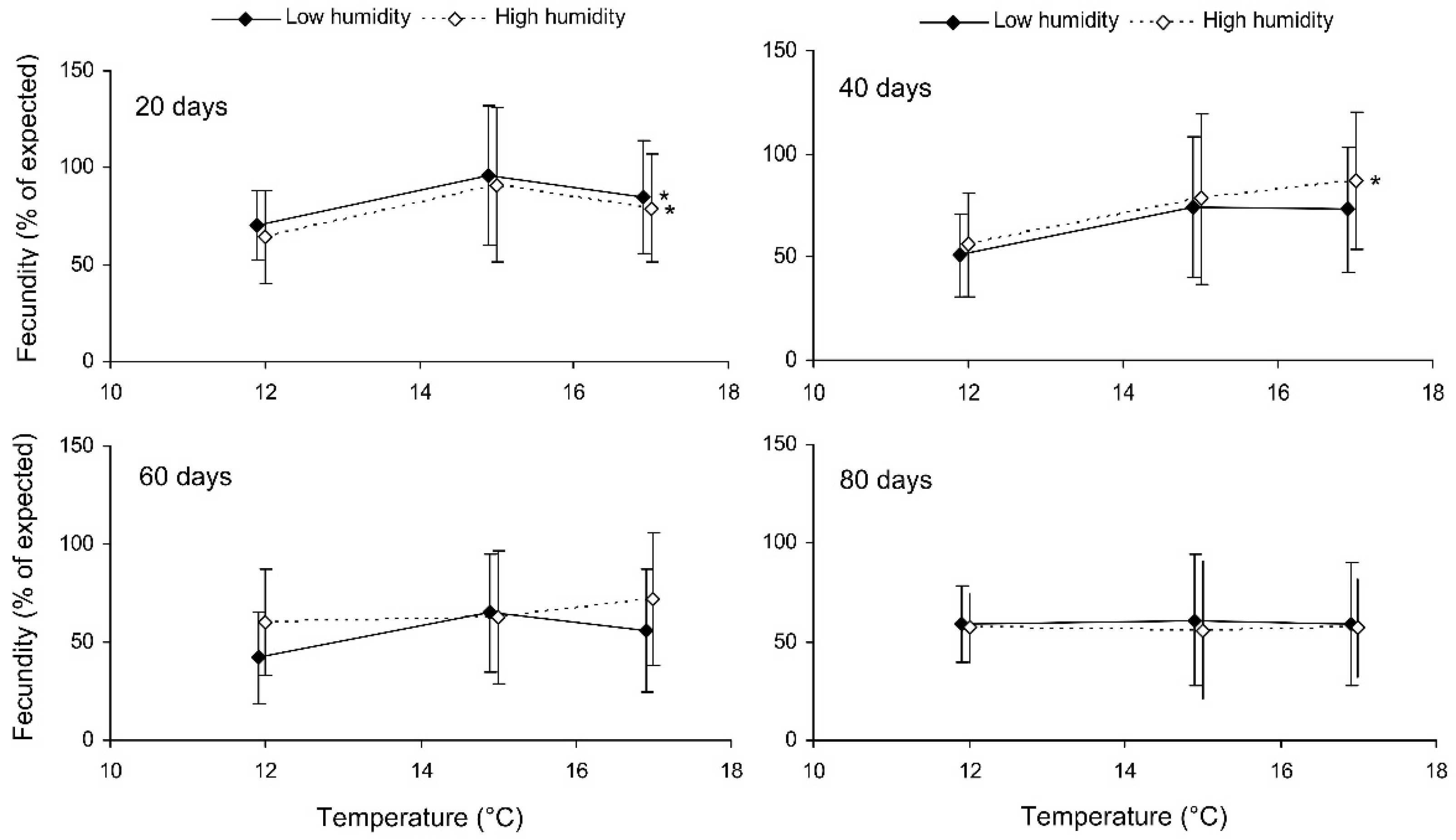
Preliminary analysis of the data for the controls showed that their fecundity positively correlated with size, which was highly variable both in the control and experimental females. Therefore, the data were transformed to exclude the ‘size factor’. First, the expected fecundity was calculated for each female based on the regression equation for the controls (Figure 3). Second, the observed fecundity was expressed as a percentage of the expected fecundity with the formula Ft = 100 (Fo/Fe), where Ft is the transformed fecundity, Fo is the observed fecundity, and Fe is the expected fecundity. Then, this percentage was used for further statistical treatment. The GLM analysis of the total results of the short-term experiment showed that the fecundity of the surviving females increased with the temperature and decreased with the duration of storage, whereas the impacts of the humidity and trophic regime were not significant (Table 2). However, the analysis of some particular datasets revealed the significant difference between fed and starved individuals (Figure 4). The long-term experiment yielded very similar results (Table 2, Figure 5). In general, the influence of environmental factors on the fecundity of C. propinqua females was weaker than that on survival (compare Figure 1 and Figure 4, Figure 2 and Figure 5).
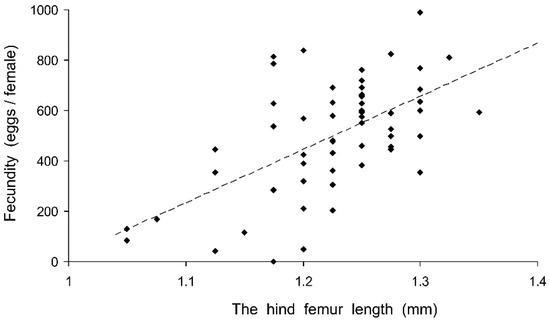
Figure 3.
Fecundity of Cheilomenes propinqua females in relation to their size, as estimated by the hind femur length. Each symbol corresponds to one control female. The dashed line shows the linear regression Y = 2079 X − 2046 (r = 0.584, n = 58, p < 0.001).

Table 2.
Influence of the trophic regime, temperature, humidity, and duration of storage on the fecundity of Cheilomenes propinqua females (the results of the GLM analysis: the regression coefficient ± SE, t-ratio, and significance of influence).
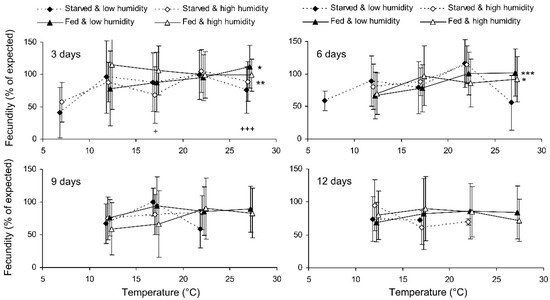
Figure 4.
Fecundity of Cheilomenes propinqua females in relation to the temperature, humidity, trophic regime, and duration of storage (the results of the short-term experiment). The means and SD of the percentage of the expected fecundity calculated based on the female size are shown. The duration of storage is indicated in the graphs. Asterisks at the right end of the line indicate a significant influence of temperature—*—p < 0.05, **—p < 0.01, ***—p < 0.001—according to a one-way ANOVA test. Plus symbols indicate the significant influence of the trophic regime at the corresponding temperature, +—p < 0.05, +++—p < 0.001, according to a two-way ANOVA test (humidity was the second factor).
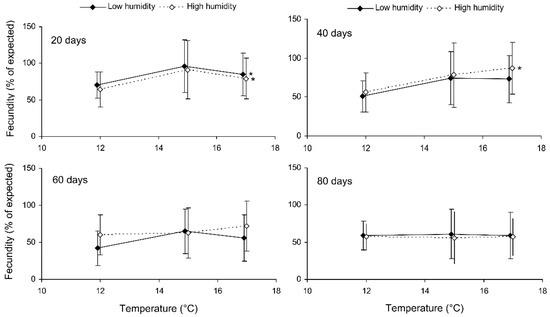
Figure 5.
Fecundity of fed Cheilomenes propinqua females in relation to the temperature, humidity, and duration of storage (the results of the long-term experiment). The means and SD of the percentage of the expected fecundity calculated based on the females’ size are shown. The duration of storage is indicated in the graphs. Asterisks at the right end of the line indicate a significant influence of temperature, *—p < 0.05, according to a one-way ANOVA test.
3.3. Egg Hatching
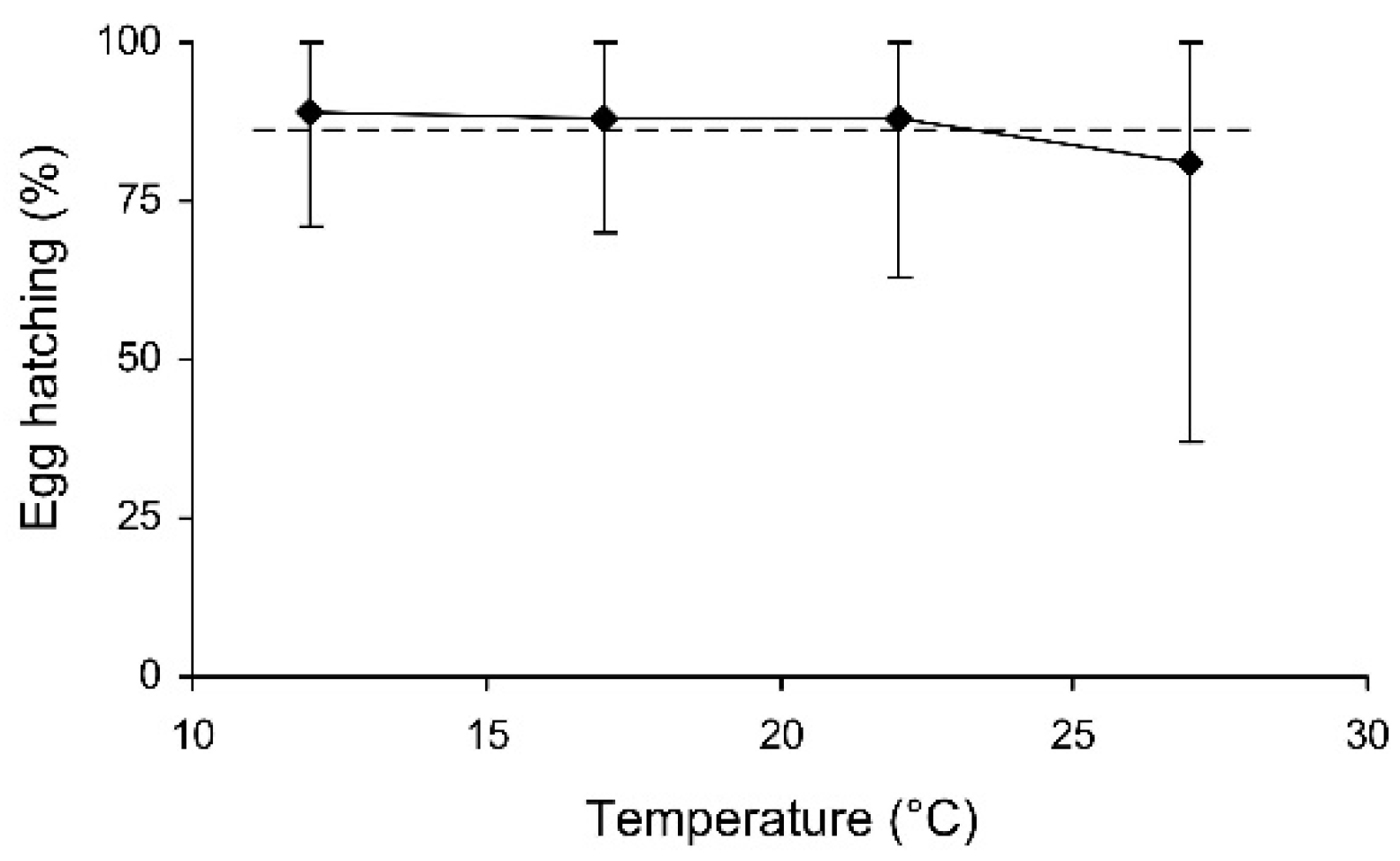
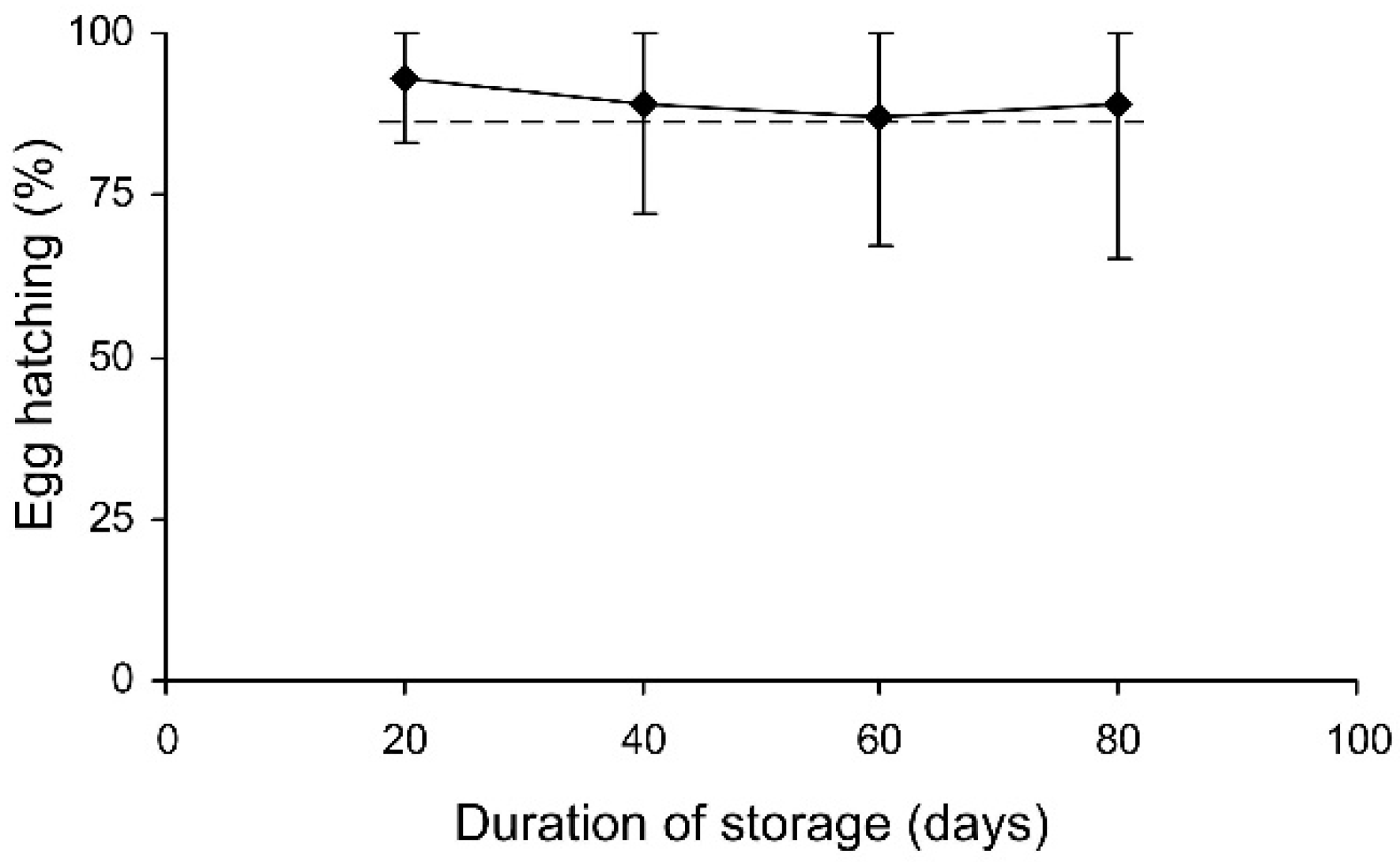
In the short-term experiment, the percentage of hatched eggs was somewhat lower in females which were stored at 27 °C (Table 3, Figure 6). In the long-term experiment, the thermal effect was not significant (evidently because of the limited range of temperatures used), but egg hatching slightly decreased with the duration of storage (Table 3, Figure 7). However, in both experiments the percentage of hatching of the eggs laid by the experimental females was very close to that of the controls (Figure 6 and Figure 7).

Table 3.
Influence of the trophic regime, temperature, humidity, and duration of storage on the percentage of hatched eggs in Cheilomenes propinqua (the results of the GLM analysis: the regression coefficient ± SE, t-ratio, and significance of influence).
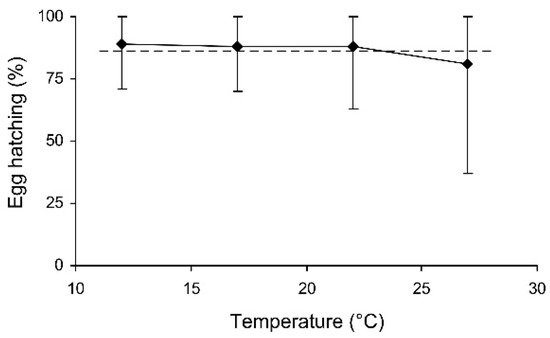
Figure 6.
The percentage of hatched eggs of Cheilomenes propinqua females in relation to the temperature (the results of the short-term experiment). Medians and quartiles are shown. The dashed line shows hatching in the controls.
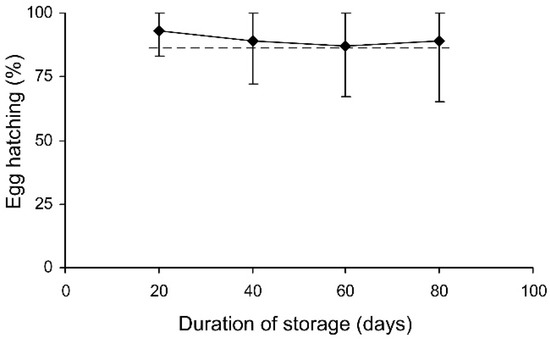
Figure 7.
The percentage of hatched eggs of Cheilomenes propinqua females in relation to the duration of storage (the results of the long-term experiment). Medians and quartiles are shown. The dashed line shows hatching in the controls.
3.4. Final Reproductive Potential
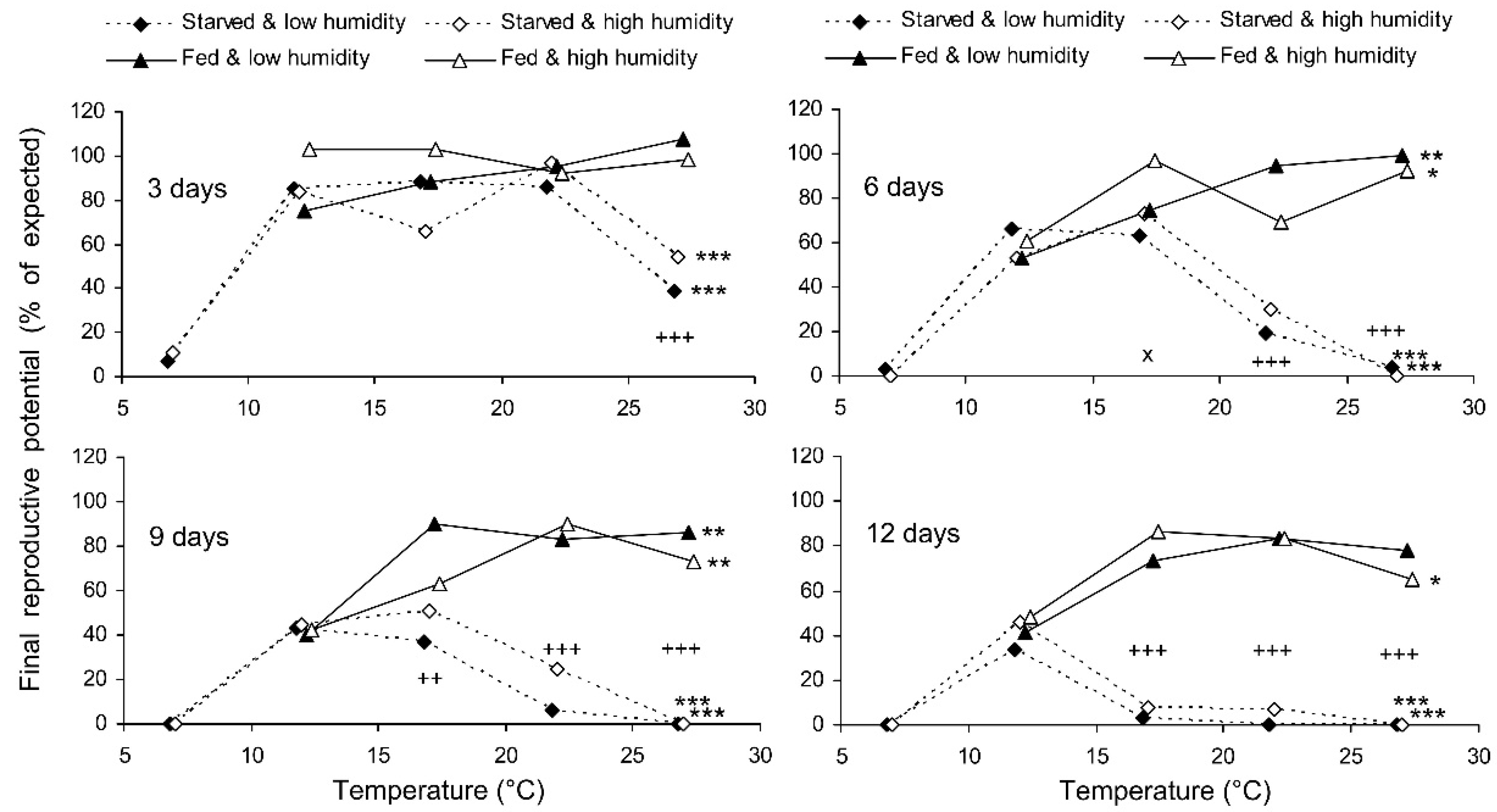
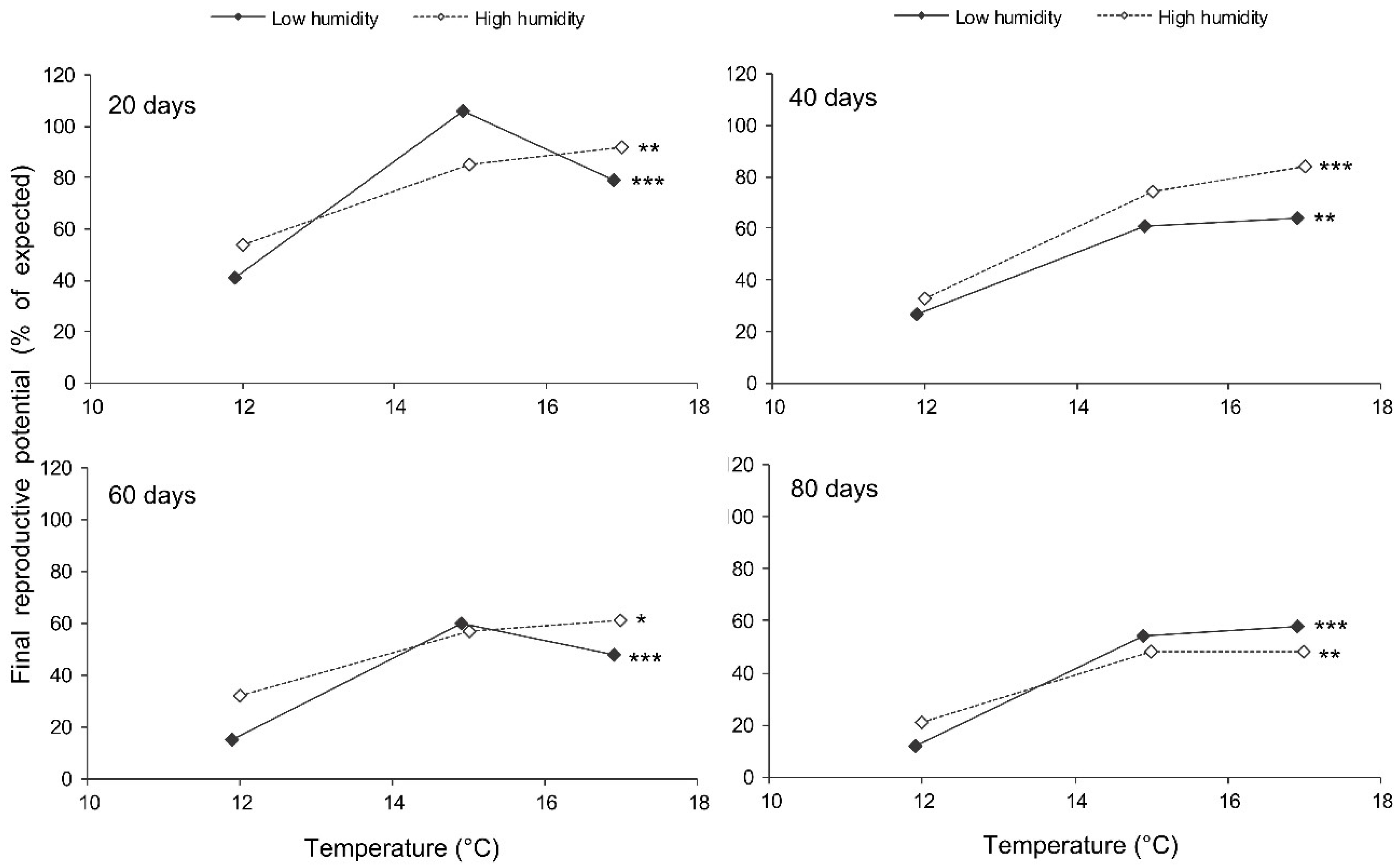
The final reproductive potential, an estimation of the mean number of progeny per female (including both individuals that survived the storage and those that did not) was expressed as the percentage of the expected value calculated with the same formula that was used for fecundity. This indicator represents a product of the three above parameters (survival, fecundity, and hatching); therefore, it shows the same dependencies on the same factors. In the short-term experiment, the fed females retained high reproductive potential when stored at a wide range of temperatures from 17 to 27 °C, both at low (about 50%) and at high (80–90%) air humidities. Under these conditions, their reproductive potential even after 12 days of storage constituted about 80% of that in controls (Figure 8). The starved females retained about 80% of the initial reproductive potential for only 3 days of storage at 12–22 °C. Then, their reproductive potential gradually decreased, being the highest in females stored at 12 °C (about 40% of that in controls after 12 days of storage). In the long-term experiment, the best results were obtained at 15 and 17 °C: the reproductive potential was about 80% and 50% of that in controls after 20 and 80 days of storage, respectively (Figure 9).
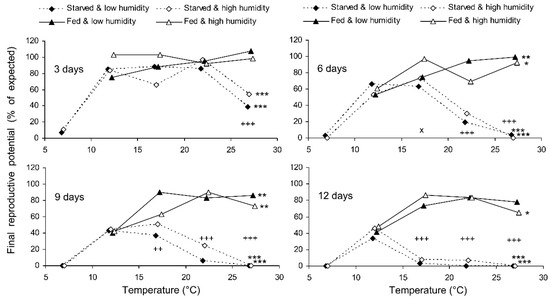
Figure 8.
Final reproductive potential of Cheilomenes propinqua females in relation to the temperature, humidity, trophic regime, and duration of storage (the results of the short-term experiment). Asterisks at the right end of the line indicate a significant influence of temperature: *—p < 0.05, **—p < 0.01, ***—p < 0.001, according to the Kruskal–Wallis test. Plus symbols indicate a significant influence of the trophic regime at the corresponding temperature: ++—p < 0.01, +++—p < 0.001, according to the Kruskal–Wallis test. X-crosses indicate the significant influence of humidity at the corresponding temperature: ×—p < 0.05 according to the Kruskal–Wallis test.
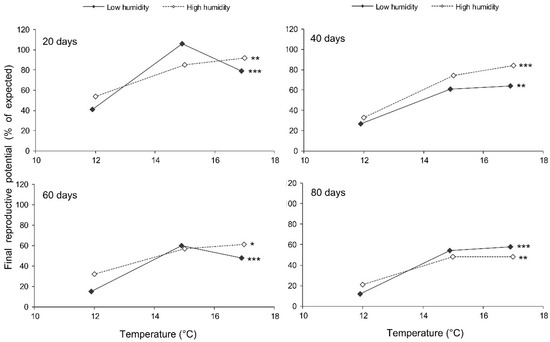
Figure 9.
Final reproductive potential of fed Cheilomenes propinqua females in relation to the temperature, humidity, and duration of storage (the results of the long-term experiment). Asterisks at the right end of the line indicate the significant influence of temperature: *—p < 0.05, **—p < 0.01, ***—p < 0.001, according to the Kruskal–Wallis test.
4. Discussion
The storage potential of predatory ladybirds used for the biological control of insect pests varies greatly among species. For example, Semyanov [32] reported the mortality after 3 months’ storage under the standard conditions (12 °C, feeding on sucrose solution) of Cheilomenes (Menochilus) sexmaculata was 80%, that of Leis biplagiata was 50%, that of Harmonia sedecimnotata was 30%, and that of Leis dimidiata was 4% (in the last species, the mortality was 13–14% after 6 months of storage), and suggested that the storage potential is largely determined by the adult size. Other authors using other methods of storage also reported very different species-specific storage potentials [6]. The longest shelf life, as expected, was observed in Coccinellidae species of the temperate climate entering the long winter diapause in nature. For example, several authors demonstrated the high survival and relatively high fecundity of Harmonia axyridis after very long (up to eight months) storage [33,34,35,36,37].
The optimal storage conditions also correlate with the natural habitats of a ladybird. The above-mentioned H. axyridis (its geographic range includes Southern Siberia), as well as some other species of the temperate zone, show maximal storage potential at near-zero temperatures [33,34,37,38,39], whereas the subtropical and tropical ladybirds Scymnus coccivora and C. propinqua should be stored at 15–20 °C [40].
In practice, based on the results of the present study, we conclude that:
- In rearing facilities, C. propinqua females can be successfully stored for future use in mass rearing or in the biological control of pests. Under the optimal conditions (temperatures of 15–17 °C, air humidities of 50–90%, feeding on the frozen eggs of the grain moth) females can be stored for 20 days with a rather low (about 20%) and for up to 80 days with an acceptable (not more than 50%) loss of the reproductive potential. Moreover, about 80% of beetles not only survive 80 days storage but retain relatively high viability for another 20 days. Although the fecundity of these females significantly decreases, it is still enough to eliminate an initial aphid colony which usually represents a progeny of a single foundress.
- Short-term (up to 3 days) storage or transportation (shipment) can occur at a much wider range of temperatures: from 12 to 27 °C and from 12 to 22 °C for fed and starved females, respectively. Moreover, the mortality of fed females stored for up to 12 days at temperatures of 17–27 °C was still not higher than 10%, although in the absence of food they could be stored at 12–17 °C for not more than 6 days.
- In greenhouses, C. propinqua can be successfully used for the biological control of aphids by preventing colonization (a “standing army” approach [4,5,41,42,43]). Indeed, considering the 20-day viability of adults after long storage, their biweekly releases would be enough to protect the plants without even relying on the predators’ progeny. It should, however, be noted that the application of this method would require the regular supplementation of factitious food, as is also recommended by many biocontrol practitioners [4,5,41,42,43,44,45].
- Storage for more than 10 days at temperatures of 7 °C and lower results in the 100% mortality of both starved and fed females of C. propinqua. Hence, beetles of the studied population which escape from greenhouses or rearing facilities would not survive even the mild winter of the south temperate climate zone.
Author Contributions
Conceptualization, S.Y.R. and N.A.B.; methodology, S.Y.R., N.A.B. and A.N.O.; experimentation, A.N.O., O.S.B.-M., K.G.S. and S.Y.R.; formal analysis, S.Y.R.; writing and visualization, S.Y.R. and N.A.B.; project administration and funding acquisition, N.A.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Russian Science Foundation, grant number 20-66-47010.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data supporting the reported results can be obtained upon request from the corresponding author (S.Y.R.).
Acknowledgments
We are grateful to L.S. Ramenskaya and M.Y. Dolgovskaya (Zoological Institute RAS) for their assistance in conducting the experiments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- van Lenteren, J.C. IOBC Internet Book of Biological Control, Version 6. 2012. Available online: https://www.iobc-global.org/publications_iobc_internet_book_of_biological_control.html (accessed on 12 June 2022).
- van Lenteren, J.C.; Alomar, O.; Ravensberg, W.J.; Urbaneja, A. Biological control agents for control of pests in greenhouses. In Integrated Pest and Disease Management in Greenhouse Crops; Gullino, M.L., Albajes, R., Nicot, P.C., Eds.; Springer: Cham, Switzerland, 2020; pp. 409–439. [Google Scholar]
- van Lenteren, J.C.; Bueno, V.H.; Klapwijk, J.N. Augmentative biological control. In Biological Control: Global Impacts, Challenges and Future Directions of Pest Management; Mason, P.G., Ed.; Csiro Publishing: Clayton, Australia, 2021; pp. 90–109. [Google Scholar]
- Messelink, G.J.; Bennison, J.; Alomar, O.; Ingegno, B.L.; Tavella, L.; Shipp, L.; Palevsky, E.; Wäckers, F.L. Approaches to conserving natural enemy populations in greenhouse crops: Current methods and future prospects. BioControl 2014, 59, 377–393. [Google Scholar] [CrossRef]
- Parrella, M.P.; Lewis, E. Biological control in greenhouse and nursery production: Present status and future directions. Am. Entomol. 2017, 63, 237–250. [Google Scholar] [CrossRef]
- Rathee, M.; Ram, P. Impact of cold storage on the performance of entomophagous insects: An overview. Phytoparasitica 2018, 46, 421–449. [Google Scholar] [CrossRef]
- Hodek, I. Diapause/Dormancy. In Ecology and Behaviour of the Ladybird Beetles (Coccinellidae); Hodek, I., van Emden, H.F., Honěk, A., Eds.; Wiley-Blackwell: Chichester, UK, 2012; pp. 275–342. [Google Scholar]
- Hodek, I.; Evans, E.W. Food relationships. In Ecology and Behaviour of the Ladybird Beetles (Coccinellidae); Hodek, I., van Emden, H.F., Honěk, A., Eds.; Wiley-Blackwell: Chichester, UK, 2012; pp. 141–274. [Google Scholar]
- Nedved, O.; Honěk, A. Life history and development. In Ecology and Behaviour of the Ladybird Beetles (Coccinellidae); Hodek, I., van Emden, H.F., Honěk, A., Eds.; Wiley-Blackwell: Chichester, UK, 2012; pp. 54–109. [Google Scholar]
- Kovář, I. Family Coccinellidae Latreille, 1807. In Catalogue of Palaearctic Coleoptera; Löbl, I., Smetana, A., Eds.; Apollo Books: Stenstrup, Denmark, 2007; Volume 4, pp. 568–631. [Google Scholar]
- Abdel-Salam, A.H.; El-Serafi, H.A.; Bayoumy, M.H.; Abdel-Hady, A.A. Effect of temperature and aphid-host plant variety on performance and thermal requirements of Coccinella undecimpunctata L. and Cheilomenes propinqua isis (Mulsant). J. Plant Prot. Pathol. 2018, 9, 375–380. [Google Scholar]
- Adly, D.; Fadl, H.A.; Mousa, S.F.M. Survey and seasonal abundance of mealybug species, their parasitoids and associated predators on guava trees in Egypt. Egypt. J. Biol. Pest Control 2016, 26, 657–664. [Google Scholar]
- Atuncha, H.; Ateka, E.; Amata, R.; Mwirichia, R.; Kasina, M.; Mbevi, B.; Wakoli, E. Evaluation of predation potential of coccinellids on cassava whiteflies. J. Entomol. Nematol. 2013, 5, 84–87. [Google Scholar] [CrossRef]
- Badary, H. Ecological aspects of Sassetia spp. (Coccidae: Coccoidae: Hemiptera) and their natural enemies in Egypt. Egypt. Acad. J. Biol. Sci. A Entomol. 2011, 4, 163–174. [Google Scholar] [CrossRef]
- Bayoumy, M.H.; Michaud, J.P. Cannibalism in two subtropical lady beetles (Coleoptera: Coccinellidae) as a function of density, life stage, and food supply. J. Insect Behav. 2015, 28, 387–402. [Google Scholar] [CrossRef]
- Borkakati, R.N.; Venkatesh, M.R.; Saikia, D.K. Insect pests of Brinjal and their natural enemies. J. Entomol. Zool. Stud. 2019, 7, 932–937. [Google Scholar]
- Catling, H.D. The bionomics of the South African citrus psylla Trioza erytreae (Del Guercio) (Homoptera: Psyllidae) 4. The influence of predators. J. Entomol. Soc. S. Afr. 1970, 33, 341–348. Available online: https://hdl.handle.net/10520/AJA00128789_3140 (accessed on 1 July 2022).
- Ghanim, A.A.; Abou-El-naga, A.A.; El-Serafi, H.A.; Jabbar, A.S. Studies on the population densities of certain insect pests attacking grapevine trees and their associated predators in Mansoura district. J. Plant Prot. Pathol. 2015, 6, 1089–1104. [Google Scholar] [CrossRef][Green Version]
- Magina, F.L.; Kilambo, D.L.; Maerere, A.P.; Teri, J.M. Innovative strategies for control of coffee insect pests in Tanzania: A review. Huria J. Open Univ. Tanzan 2016, 22, 63–72. [Google Scholar]
- Mahmoud, A.K.; Yousif, N.E.; Satti, A.A. Effect of three aphid species on the life tables data of the coccinellid predator, Cheilomenes propinqua vicina (Mulsant). Environ. Nat. Resour. Int. J. 2016, 1, 49–55. [Google Scholar]
- Nordey, T.; Boni, S.B.; Agbodzavu, M.K.; Mwashimaha, R.; Mlowe, N.; Ramasamy, S.; Deletre, E. Comparison of biological methods to control Aphis fabae Scopoli (Hemiptera: Aphididae) on kalanchoe crops in East Africa. Crop Prot. 2021, 142, 105520. [Google Scholar] [CrossRef]
- Ramadan, M.M.; Hassan, M.A.; Afifi, M. Ecological studies on the Peach green aphid, Myzus persicae and its natural enemies. J. Plant Prot. Pathol. 2022, 13, 29–35. [Google Scholar] [CrossRef]
- Sæthre, M.G.; Godonou, I.; Hofsvang, T.; Tepa-Yotto, G.T.; James, B. Aphids and their natural enemies in vegetable agroecosystems in Benin. Int. J. Trop. Insect Sci. 2011, 31, 103–117. [Google Scholar] [CrossRef]
- Sæthre, M.G.; Godonou, I.; Leclercq, S.; Yoto, G.T.; James, B. Assessment of aphid ecology in vegetable systems and potential for biological control agents. Acta Hortic. 2011, 911, 227–230. [Google Scholar]
- Samways, M.J. Interrelationship between an entomogenous fungus and two ant-homopteran (Hymenoptera: Formicidae-Hemiptera: Pseudococcidae & Aphididae) mutualisms on guava trees. Bull. Entomol. Res. 1983, 73, 321–331. [Google Scholar] [CrossRef]
- Spodek, M.; Ben-Dov, Y.; Mondaca, L.; Protasov, A.; Erel, E.; Mendel, Z. The cotton mealybug, Phenacoccus solenopsis Tinsley (Hemiptera: Pseudococcidae) in Israel: Pest status, host plants and natural enemies. Phytoparasitica 2018, 46, 45–55. [Google Scholar] [CrossRef]
- Youssif, M.A.I. Coccinellid species and their insect parasitoids in pear orchards at El-Khattara district, Sharkia Governorate, Egypt. J. Entomol. Zool. Stud. 2019, 7, 780–790. [Google Scholar]
- Reznik, S.; Ovchinnikov, A.; Ovchinnikova, A.; Bezman-Moseyko, O.; Belyakova, N. Photoperiodic, thermal and trophic responses of a predatory ladybird Cheilomenes propinqua. J. Appl. Entomol. 2021, 145, 134–144. [Google Scholar] [CrossRef]
- El-Heneidy, A.H.; El-Serafy, H.A.; Mohamed, N.E.; Elbanna, E.H.E. Biological characteristics of two coccinellid predators when reared on Aphis gossypii Glover and an artificial diet under laboratory condition. J. Plant Prot. Path. 2021, 12, 575–578. [Google Scholar] [CrossRef]
- Yarpuzlu, F.; Uygun, N. Effects of different temperatures on development and fecundity of a predatory beetle, Cheilomenes propinqua (Mulsant) (Coleoptera: Coccinellidae). Türkiye Biyolojik Mücadele Dergisi 2010, 1, 97–107. [Google Scholar]
- Michaud, J.P. Coccinellids in biological control. In Ecology and Behaviour of the Ladybird Beetles (Coccinellidae); Hodek, I., van Emden, H.F., Honěk, A., Eds.; John Wiley & Sons: Chichester, UK, 2012; pp. 488–519. [Google Scholar]
- Semyanov, V.P. Rearing, Long-term Storage, and Use of Tropical Coccinellidae for Aphid Control in Greenhouses; KMK: Moscow, Russia, 2006. (In Russian) [Google Scholar]
- Awad, M.; Kalushkov, P.; Nedvědová, T.; Nedvěd, O. Fecundity and fertility of ladybird beetle Harmonia axyridis after prolonged cold storage. BioControl 2013, 58, 657–666. [Google Scholar] [CrossRef]
- Ruan, C.C.; Du, W.M.; Wang, X.M.; Zhang, J.J.; Zang, L.S. Effect of long-term cold storage on the fitness of pre-wintering Harmonia axyridis (Pallas). BioControl 2012, 57, 95–102. [Google Scholar] [CrossRef]
- Seo, M.J.; Youn, Y.N. Effective preservation methods of the Asian ladybird, Harmonia axyridis (Coleoptera: Coccinellidae), as an application strategy for the biological control of aphids. J. Asia-Pac. Entomol. 2002, 5, 209–214. [Google Scholar] [CrossRef]
- Sun, G.; Zang, L.; Wang, X.; Ruan, C.; Liu, X. Effect of long-term cold storage on fecundity of pre-wintering Harmonia axyridis (Pallas) and the fitness of its progeny. J. Environ. Entomol. 2016, 38, 286–292. [Google Scholar]
- Sun, Y.X.; Hao, Y.N.; Zhou, J.J.; Liu, C.Z.; Wang, S.S. Effects of long-term cold storage on maternal and progeny fitness of laboratory-reared Harmonia axyridis adults. BioControl 2022, 67. [Google Scholar] [CrossRef]
- Khan, A.; ul Ain, Q.; Ghafoor, A.; Riaz, A.; Suleman, N. Effect of low temperature on the storage of adult and larvae of Coccinella septempunctata (Coleoptera; Coccinellidae). Int. J. Agric. Environ. Res. 2019, 5, 274–283. [Google Scholar]
- Sakaki, S.; Jalali, M.A.; Kamali, H.; Nedvěd, O. Effect of low-temperature storage on the life history parameters and voracity of Hippodamia variegata (Coleoptera: Coccinellidae). Eur. J. Entomol. 2019, 116, 10–15. [Google Scholar] [CrossRef]
- Baskaran, R.K.M.; Suresh, K. Optimum thermal regimes for adult storage of Scymnus coccivora Ayyar (Coleoptera: Coccinellidae). J. Biol. Control 2006, 20, 135–139. [Google Scholar] [CrossRef]
- Kozlova, E.G.; Krasavina, L.P.; Orlova, G.S. Experience of biocontrol use in cucumber protection from the complex of greenhouse pests. Zashchita i Karantin Rastenii 2019, 11, 39–42. (In Russian) [Google Scholar]
- Dader, B.; Colomer, I.; Adan, A.; Medina, P.; Vinuela, E. Compatibility of early natural enemy introductions in commercial pepper and tomato greenhouses with repeated pesticide applications. Insect Sci. 2020, 27, 1111–1124. [Google Scholar] [CrossRef] [PubMed]
- Benson, C.M.; Labbe, R.M. Exploring the role of supplemental foods for improved greenhouse biological control. Ann. Entomol. Soc. Am. 2021, 114, 302–321. [Google Scholar] [CrossRef]
- Brenard, N.; Sluydts, V.; De Bruyn, L.; Leirs, H.; Moerkens, R. Food supplementation to optimize inoculative release of the predatory bug Macrolophus pygmaeus in sweet pepper. Entomol. Exp. Appl. 2018, 166, 574–582. [Google Scholar] [CrossRef]
- Brenard, N.; Sluydts, V.; Christianen, E.; Bosmans, L.; De Bruyn, L.; Moerkens, R.; Leirs, H. Biweekly supplementation with Artemia spp. cysts allows efficient population establishment by Macrolophus pygmaeus in sweet pepper. Entomol. Exp. Appl. 2019, 167, 406–414. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).