Phenology and Monitoring of the Lesser Chestnut Weevil (Curculio sayi)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
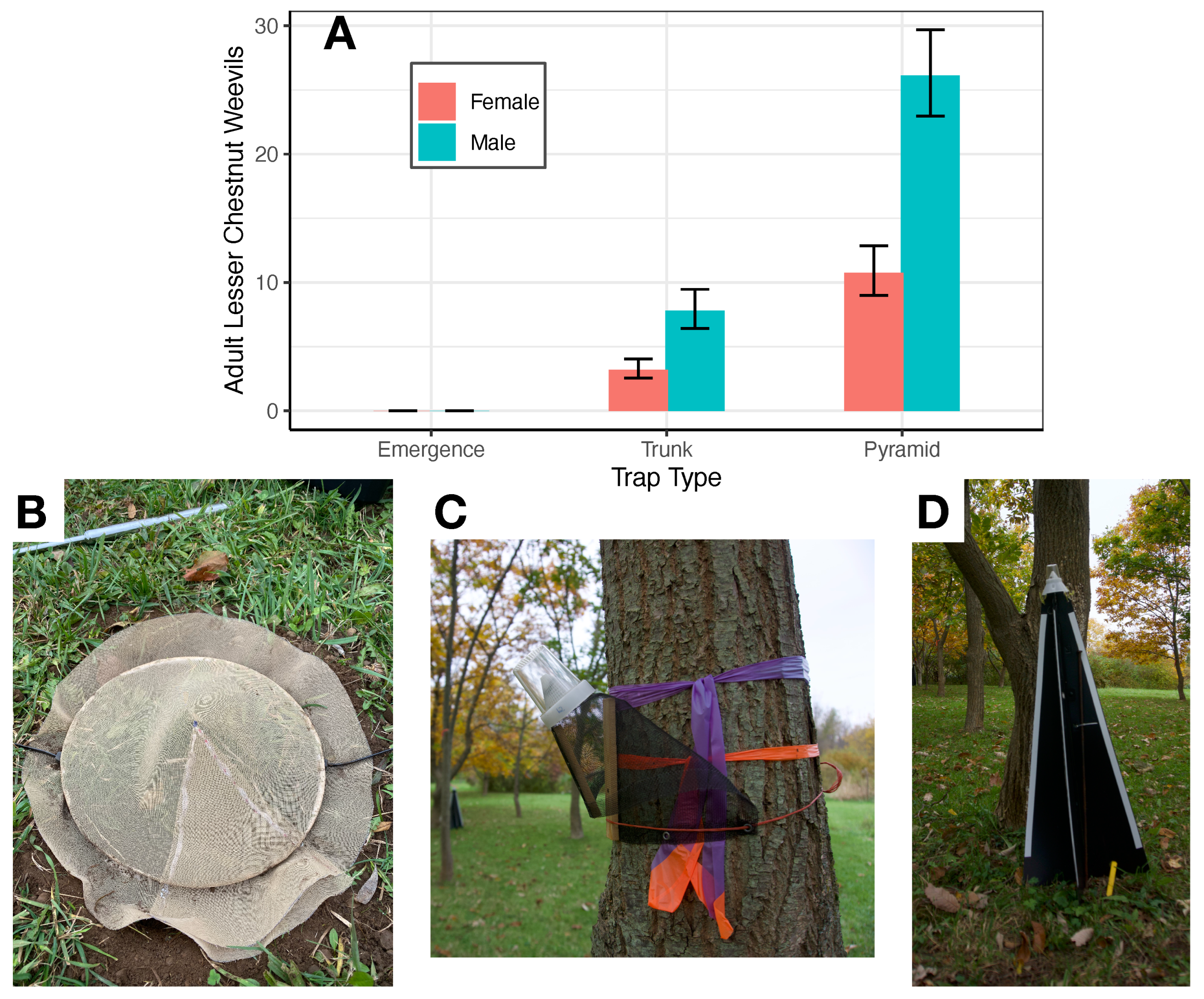
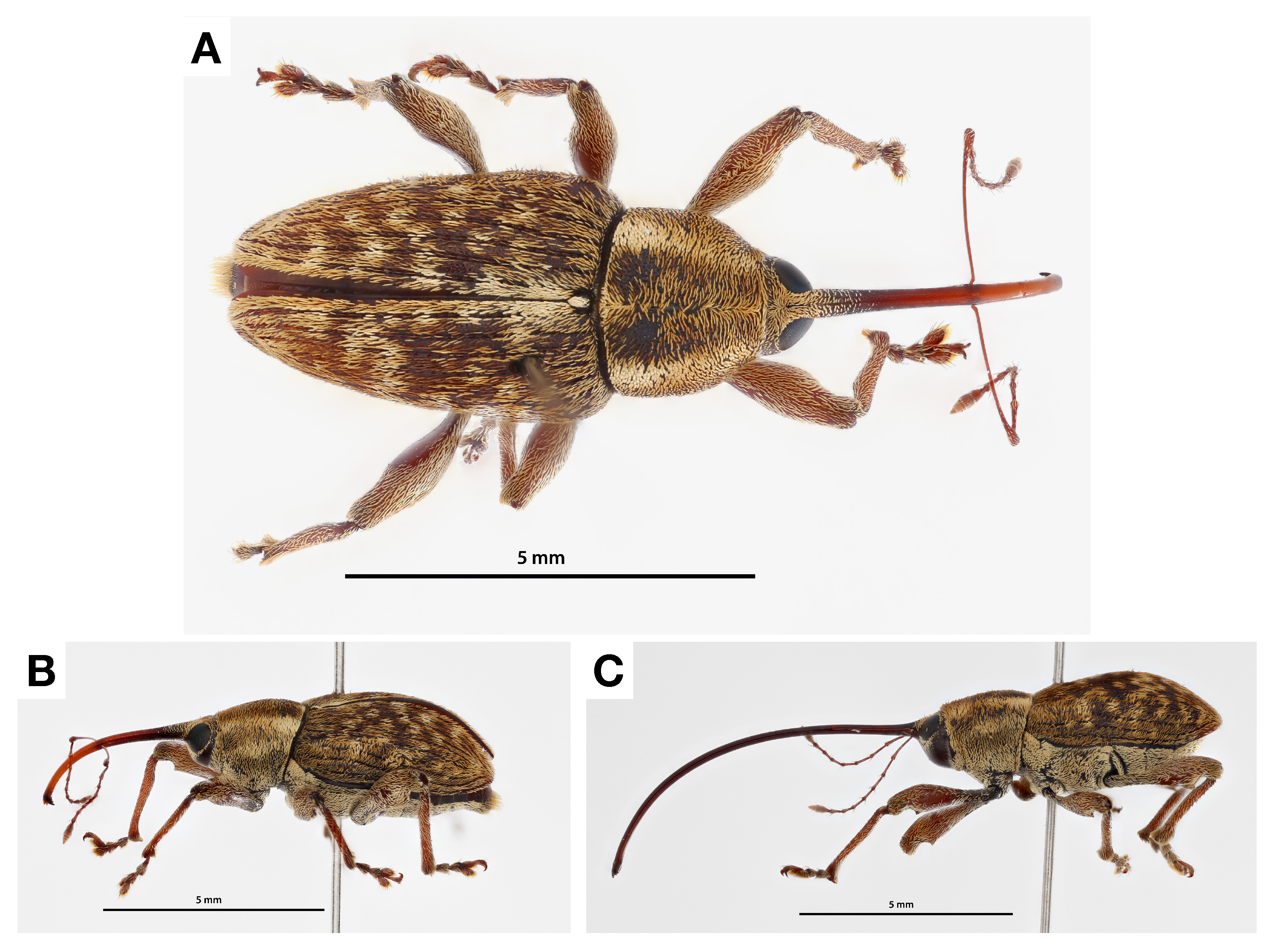
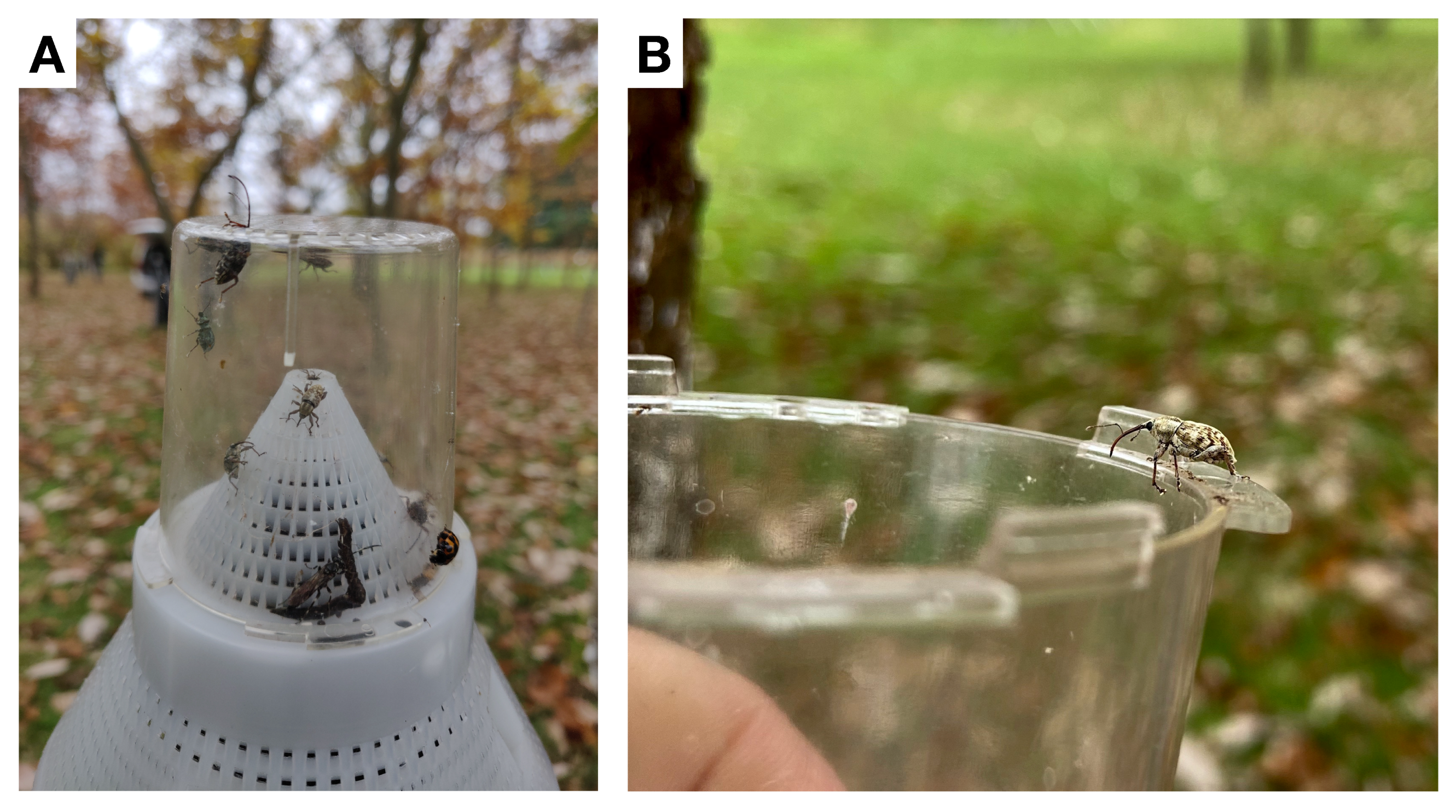
2.1. Phenology and Monitoring
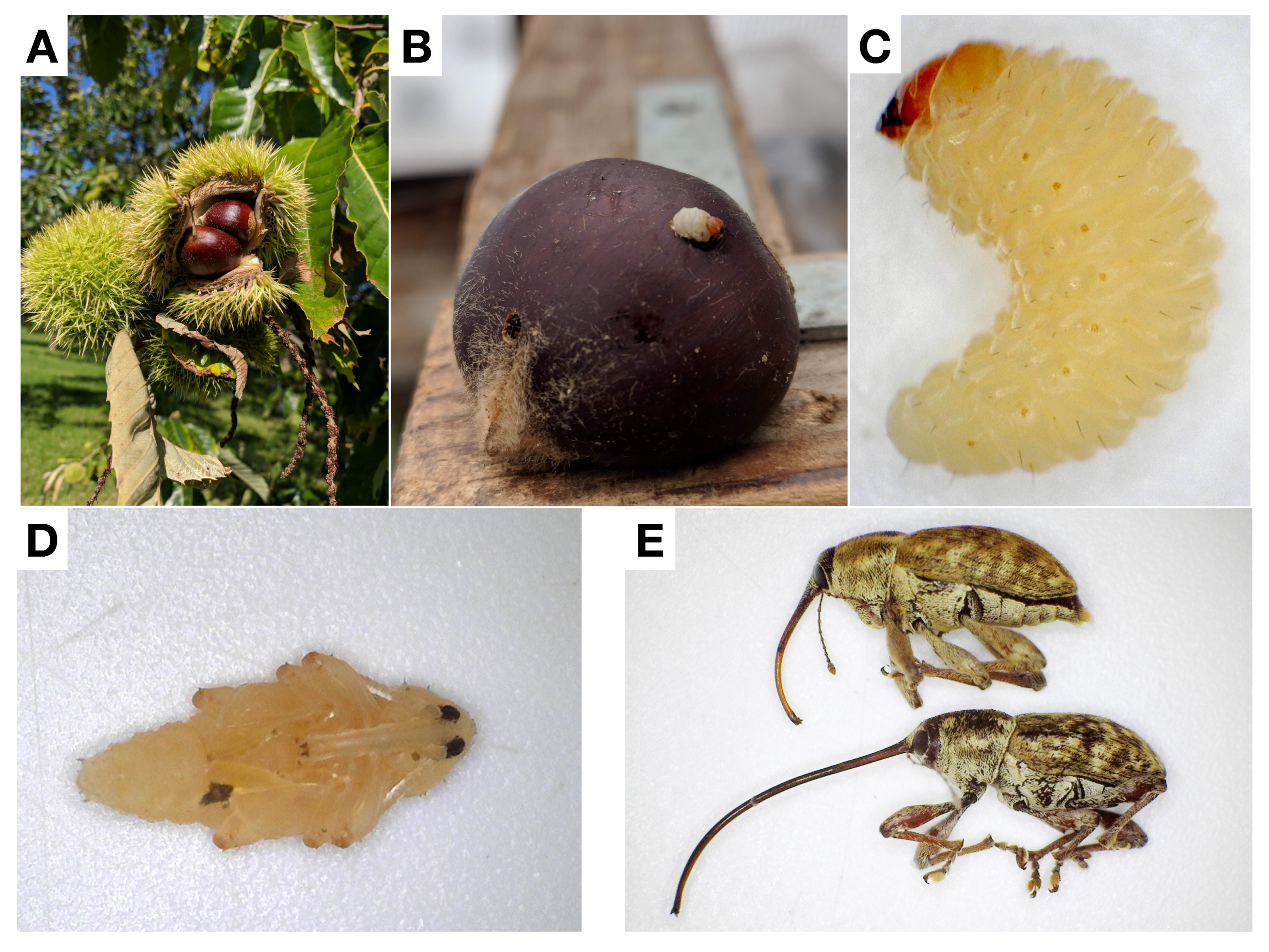
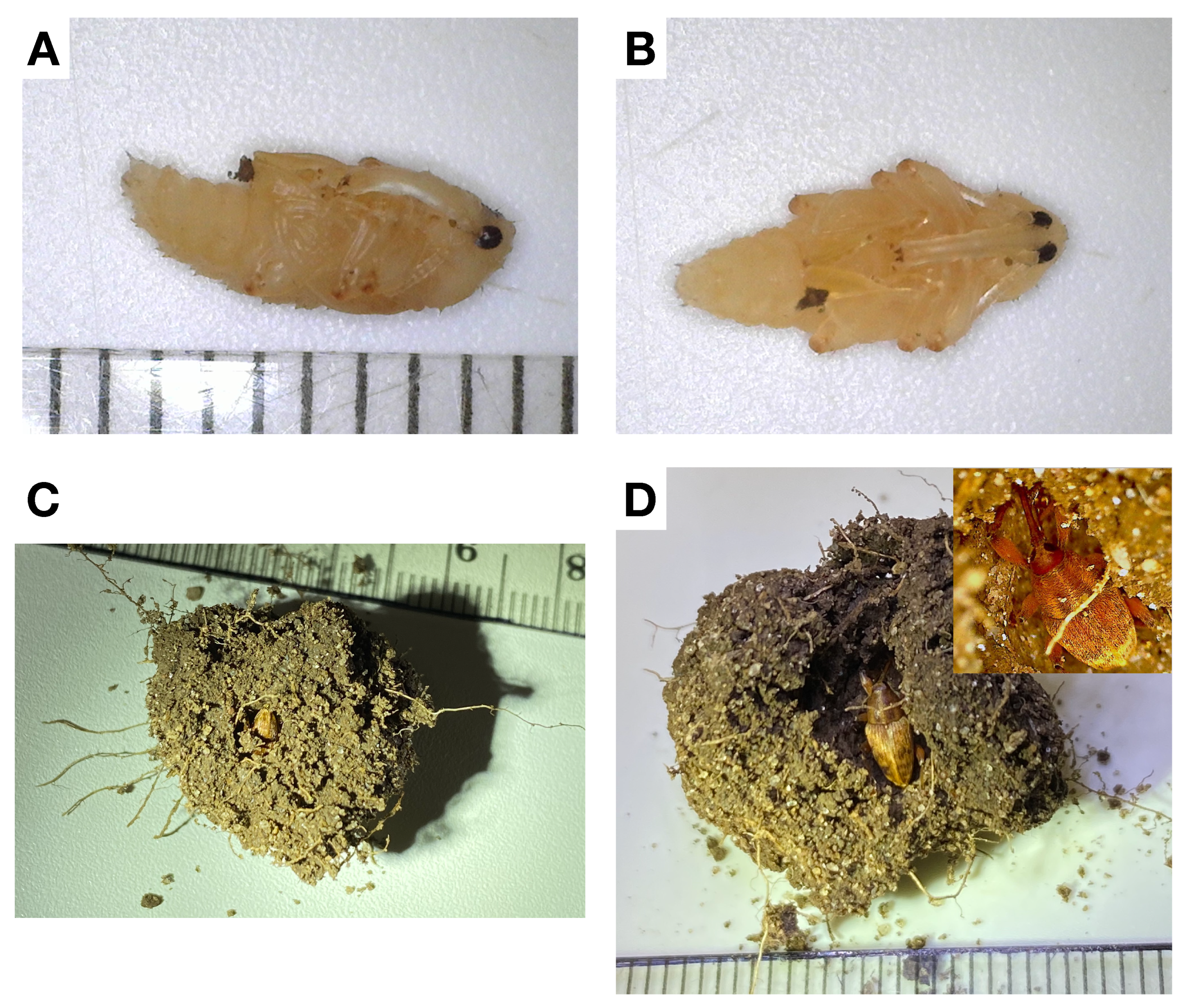
2.2. Lifecycle
2.3. Analysis
3. Results
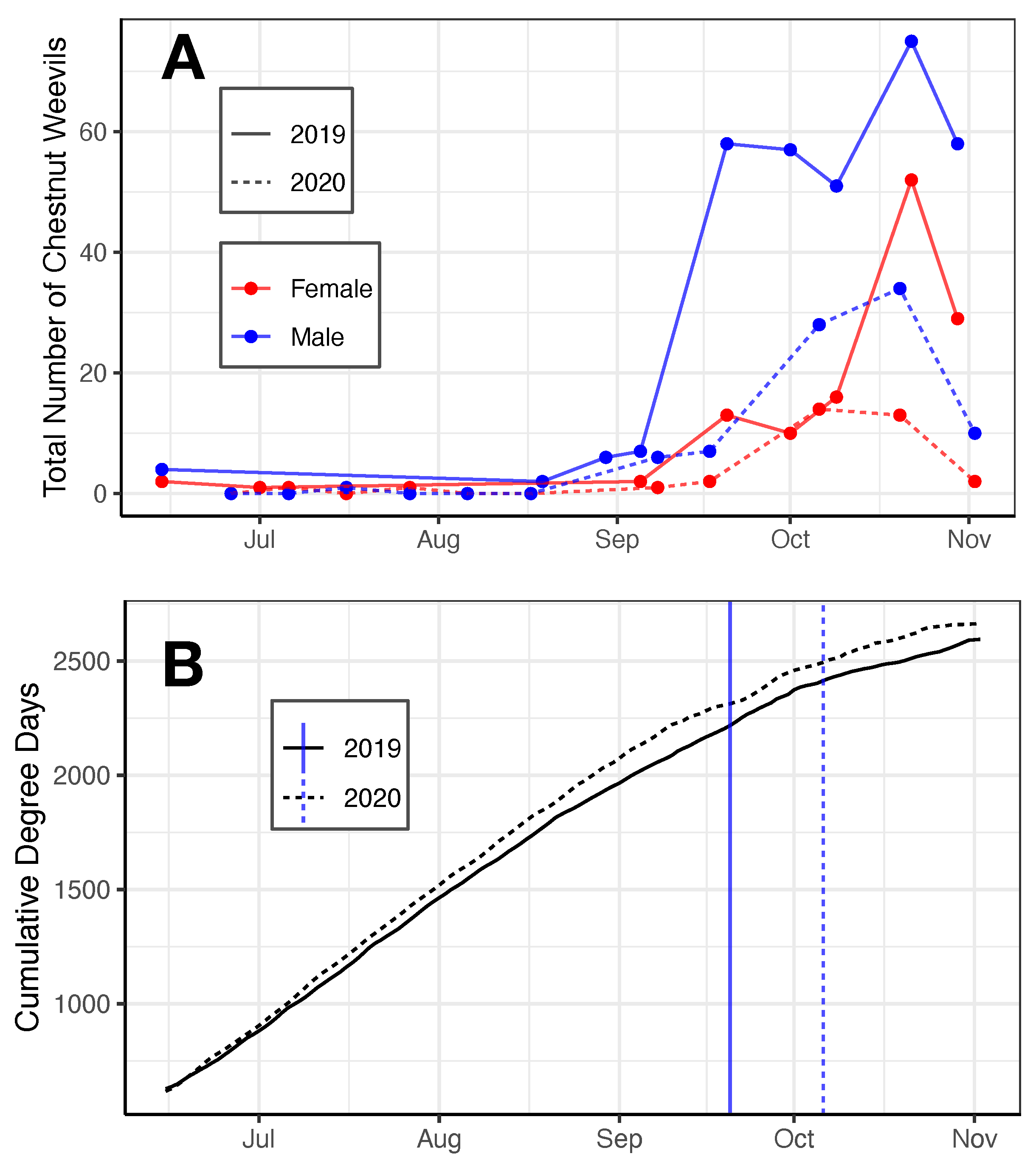
3.1. Phenology
3.2. Monitoring
3.3. Life Cycle
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jacobs, D.F. Toward development of silvical strategies for forest restoration of American chestnut (Castanea dentata) using blight-resistant hybrids. Biol. Conserv. 2007, 137, 497–506. [Google Scholar] [CrossRef]
- Anagnostakis, S.L. Chestnut blight: The classical problem of an introduced pathogen. Mycologia 1987, 79, 23–37. [Google Scholar] [CrossRef]
- Kuhlman, E. The devastation of American chestnut by blight. In Proceedings of the American Chestnut Symposium, Morgantown, WV, USA, 4–5 January 1978; West Virginia University Press: Morgantown, WV, USA, 1978. [Google Scholar]
- Hepting, G.H. Death of the American chestnut. J. For. Hist. 1974, 18, 60–67. [Google Scholar] [CrossRef]
- Chittenden, F.H. The Nut Weevils; Number 99; US Department of Agriculture, Bureau of Entomology: Washington, DC, USA, 1908.
- Brooks, F.E.; Cotton, R.T. The Chestnut Curculios; Number 130; US Department of Agriculture: Washington, DC, USA, 1929.
- Johnson, W.T. On the Biology and Control of the North American Chestnut Weevils. Ph.D. Thesis, University of Chicago, Chicago, IL, USA, 1956. [Google Scholar]
- Clark, S.L.; Schlarbaum, S.E.; Saxton, A.M.; Baird, R. Eight-year blight (Cryphonectria parasitica) resistance of backcross-generation American chestnuts (Castanea dentata) planted in the southeastern United States. For. Ecol. Manag. 2019, 433, 153–161. [Google Scholar] [CrossRef]
- Metaxas, A.M. Chestnut (Castanea spp.) Cultivar evaluation for Commercial Chestnut Production in Hamilton County, Tennessee. Ph.D. Thesis, The University of Tennessee at Chattanooga, Chattanooga, TN, USA, 2013. [Google Scholar]
- Zarnowski, J.; Zarnowski, D. The Hazelnut and Chestnut Handbook: All You Need to Know to Grow Hazelnuts and Chestnuts from 2 to 20,000 Trees; Z’s Nutty Ridge LLC: McGraw, NY, USA, 2020. [Google Scholar]
- Revord, R.S.; Nave, J.M.; Miller, G.; Meier, N.; Webber, J.B.; Gold, M.A.; Wahl, T. Descriptions of Chestnut Cultivars for Nut Production in the Eastern and Midwestern United States. HortScience 2021, 56, 1315–1324. [Google Scholar] [CrossRef]
- Beccaro, G.; Alma, A.; Bounous, G.; Gomes-Laranjo, J. The Chestnut Handbook: Crop & Forest Management; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Keesey, I.W.; Barrett, B.A. Seasonal occurrence and soil distribution of the lesser chestnut weevil, Curculio sayi (Coleoptera: Curculionidae) in mid-Missouri. J. Kans. Entomol. Soc. 2008, 81, 345–354. [Google Scholar] [CrossRef]
- Lizotte, E. Managing Chestnut Weevil in Michigan in 2020. Available online: https://www.canr.msu.edu/news/managing-chestnut-weevil-in-michigan#:~:text=Chestnut%20weevil%20with%20damaged%20kernel.&text=Large%20and%20lesser%20chestnut%20weevil%20both%20lay%20eggs%20on%20developing,making%20their%20way%20to%20consumers (accessed on 1 July 2022).
- Wells, J.M.; Payne, J. Mycoflora and market quality of chestnuts treated with hot water to control the chestnut weevil. Plant Dis. 1980, 64, 999–1001. [Google Scholar] [CrossRef]
- Wells, J.M.; Cole, R.J.; Kirksey, J.W. Emodin, a toxic metabolite of Aspergillus wentii isolated from weevil-damaged chestnuts. Appl. Microbiol. 1975, 30, 26–28. [Google Scholar] [CrossRef] [PubMed]
- USDA Soil Survey. Natural Resources Conservation Service, United States Department of Agriculture. Available online: http://websoilsurvey.sc.egov.usda.gov/ (accessed on 1 July 2022).
- Whitehead, D.R.; Chamorro, M.L.; Anderson, R.S. An illustrated key to the species of Curculio Linnaeus (Coleoptera: Curculionidae) of North America east of the Mississippi River. Proc. Entomol. Soc. Wash. 2018, 120, 616–641. [Google Scholar] [CrossRef]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019. [Google Scholar]
- Lenth, R.V. Emmeans: Estimated Marginal Means, Aka Least-Squares Means; 2022; R package version 1.7.4-1. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 1 July 2022).
- Zeileis, A.; Hothorn, T. Diagnostic Checking in Regression Relationships. R News 2002, 2, 7–10. [Google Scholar]
- Barrett, B.A.; Keesey, I.W. The seasonal occurrence, soil distribution and flight characteristics of Curculio sayi (Coleoptera: Curculionidae) in mid-Missouri. Ph.D. Thesis, University of Missouri–Columbia, Columbia, MO, USA, 2007. [Google Scholar]
- Keesey, I.W.; Barrett, B.A.; Lin, C.H.; Lerch, R.N. Electroantennographic responses of the small chestnut weevil Curculio sayi (Coleoptera: Curculionidae) to volatile organic compounds identified from chestnut reproductive plant tissue. Environ. Entomol. 2012, 41, 933–940. [Google Scholar] [CrossRef]
- Keesey, I.W.; Barrett, B.A. Behavioral and electroantennographic responses of the lesser chestnut weevil, Curculio sayi (Coleoptera: Curculionidae), to odors emanating from different chestnut plant tissues. J. Kans. Entomol. Soc. 2012, 85, 145–154. [Google Scholar] [CrossRef]
- Menu, F.; Debouzie, D. Coin-flipping plasticity and prolonged diapause in insects: Example of the chestnut weevil Curculio elephas (Coleoptera: Curculionidae). Oecologia 1993, 93, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Menu, F. Strategies of emergence in the chestnut weevil Curculio elephas (Coleoptera: Curculionidae). Oecologia 1993, 96, 383–390. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filgueiras, C.C.; Willett, D.S. Phenology and Monitoring of the Lesser Chestnut Weevil (Curculio sayi). Insects 2022, 13, 713. https://doi.org/10.3390/insects13080713
Filgueiras CC, Willett DS. Phenology and Monitoring of the Lesser Chestnut Weevil (Curculio sayi). Insects. 2022; 13(8):713. https://doi.org/10.3390/insects13080713
Chicago/Turabian StyleFilgueiras, Camila C., and Denis S. Willett. 2022. "Phenology and Monitoring of the Lesser Chestnut Weevil (Curculio sayi)" Insects 13, no. 8: 713. https://doi.org/10.3390/insects13080713
APA StyleFilgueiras, C. C., & Willett, D. S. (2022). Phenology and Monitoring of the Lesser Chestnut Weevil (Curculio sayi). Insects, 13(8), 713. https://doi.org/10.3390/insects13080713





