First Interaction Network of Sarcosaprophagous Fauna (Acari and Insecta) Associated with Animal Remains in a Mediterranean Region (Northern Spain)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
Interaction Network
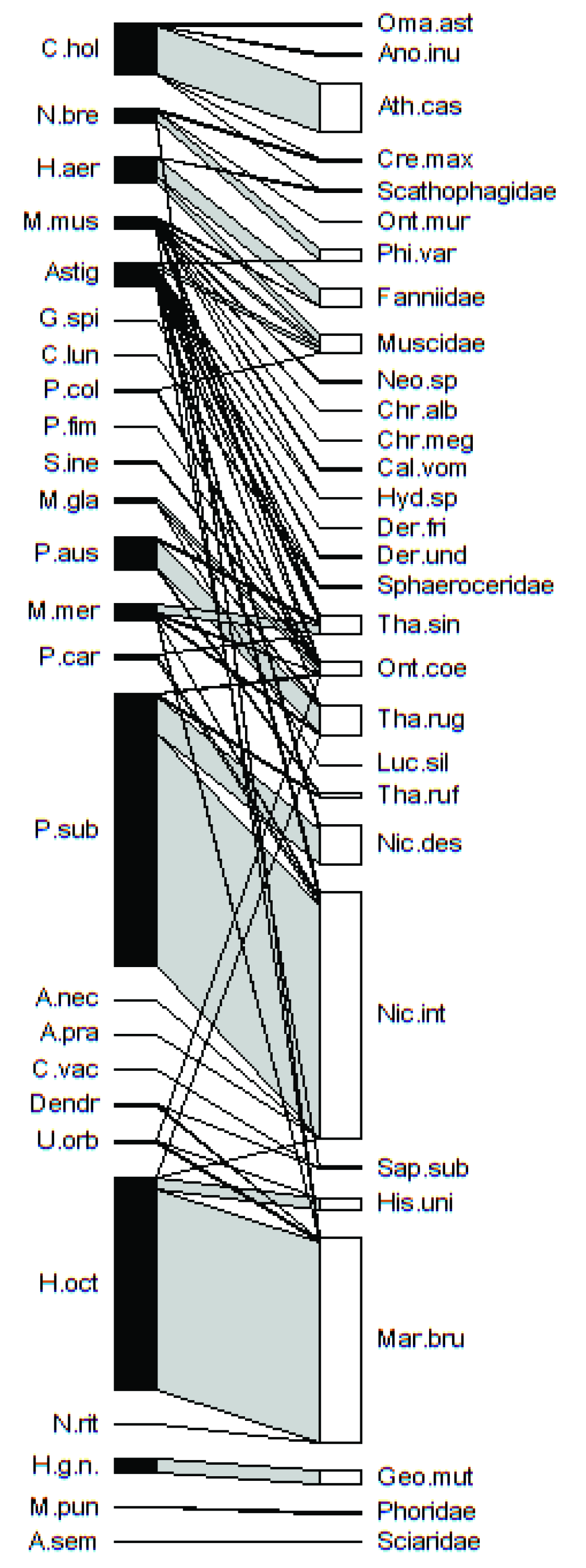
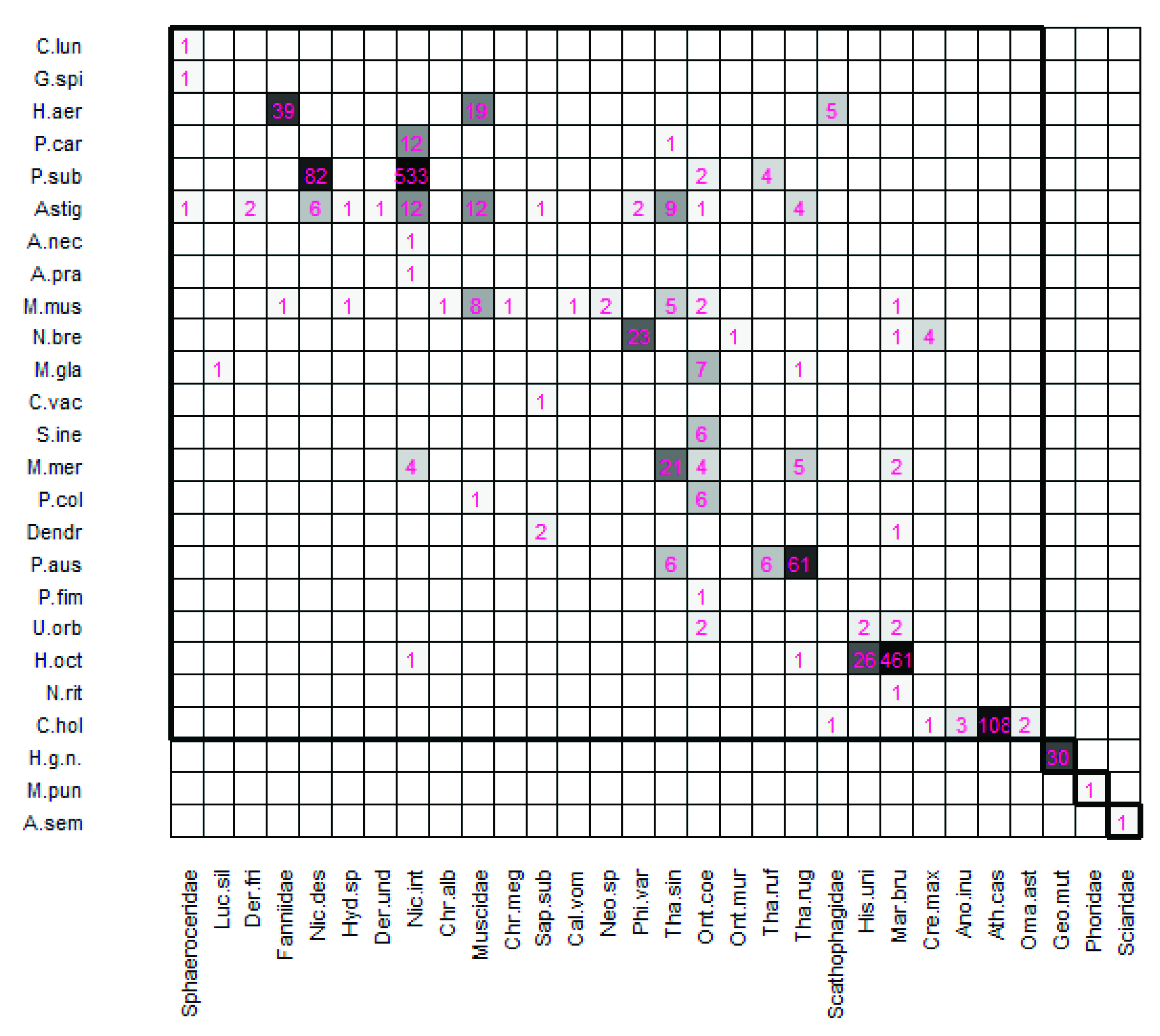
- It is composed of 1 shared compartment and 3 individual compartments. Each compartment is a subset formed by species of mites and insects that interact with each other, sharing species reciprocally, and that do not occur with the rest of the subsets. The first compartment is composed of 21 species of Mesostigmata, plus the order Astigmata, and 27 species of insects hosting more than one species. The second, third, and fourth compartments are individual compartments formed by a single mite species and a single host insect: Halolaelapidae sp. with Geotrupes (G.) mutator, Macrocheles punctoscutatus with Phoridae, and A. semiscissus with family Sciaridae (Figure 1).
- The asymmetry of the interaction network is 0.09, rescaled to [–1,1] according to Blüthgen et al. (2007) [61]. The asymmetry value is positive, close to 0, which means that there is a balance between the number of mite species (25) and the number of host species (30), although there is a very slight trend in favor of the hosts. This result indicates that in this network each species of mite corresponds to a host species, although in a few cases there is more than one.
- The interaction uniformity value (H2’) (which measures the specialization of the network) is 0.86. This value indicates a general specialization in the associations established between certain species of mites and their host insects (network values close to one mean a specialized network).
- Astigmata mites interact with myriapods, crustaceans, and insects; we newly report the presence of hypopal stages on Dermestidae (D. frischii and D. undulatus), Geotrupidae (G. (G.) mutator), Histeridae (H. unicolor, M. brunneus and S. (S.) subnitescens), Scarabaeidae (O. (P.) coenobita), Silphidae (N. interruptus, N. vespilloides, Th. ruficornis, Th. rugosus and Th. sinuatus), Calliphoridae (C. vomitoria, Ch. albiceps, Ch. megacephala and L. silvarum), Fanniidae, Muscidae, and Scathophagidae.
- M. muscaedomesticae interacts with the largest number of insect species. Its newly reported host species are Ch. albiceps, M. brunneus, Neomyia sp., O. (P.) coenobita, and Th. sinuatus.
- C. holsaticus was detected for the first time in association with Scathophagidae.
- M. merdarius has M. brunneus, N. interruptus, O. (P.) coenobita, Th. rugosus, and Th. sinuatus as newly identified hosts.
- The host insects of N. breviunguiculata was hitherto unknown. This is the first record of this Uropodina being associated with C. maxillosus, P. (P.) varians, M. brunneus, and O. murinus.
- Deutonymphs of P. subterraneus now have O. (P.) coenobita and Th. ruficornis among their list of hosts.
- P. austroasiaticus has Th. sinuatus as a new host with high specificity.
- M. glaber has L. silvarum and Th. rugosus as new hosts.
- U. orbicularis—previously cited as a generalist phoretic mite on coprophilic and saprophylic insects—is for the first time cited as phoretic on M. brunneus.
- The genus Dendrolaelaps joins M. brunneus and S. (S.) subnitescens (Histeridae) among the previously reported hosts.
- P. coleoptratorum shows high specificity in this region and uses Muscidae flies and O. (P.) coenobita as hosts.
- A. necrophilus interacted uniquely and specifically with N. interruptus.
- A. pratensis was for the first time found on N. interruptus (Silphidae); it was also found (in the same trap) on its usual hosts (O. (P.) coenobita and Hister spp.), which could indicate that this species switches hosts in the trap to survive.
- The new record of A. semiscissus females on Sciaridae supports the specificity of the relationship.
- There are few records of C. vacua as a phoretic mite; it is newly reported to live on S. (S.) subnitescens.
- The interactions established by M. punctoscutatus with Phoridae flies, N. ritzemai with M. brunneus, and Halolaelapidae sp. with G. (G.) mutator, are new phoretic sites for these species.
- Deutonymphs of P. fimetorum are specialists for O. (P.) coenobita.
- S. inexpectatus had O. (P.) coenobita as a unique host.
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Catts, E.P.; Goff, M.L. Forensic Entomology in Criminal Investigations. Annu. Rev. Entomol. 1992, 37, 253–272. [Google Scholar] [CrossRef] [PubMed]
- Faran, N.K.; Khatoon, S.; Kumar, V.; Choudhary, S. Forensic Entomology: Insect Clock. In Latest Trends in Zoology and Entomology Sciences; Ganguly, S., Faran, N.K., Eds.; AkiNik Publications: Nueva Delhi, India, 2018; pp. 46–52. [Google Scholar]
- Gómez-Gómez, A.; Martín-Vega, D.; Botías-Talamantes, C.; Baz-Ramos, A.; Díaz-Aranda, L.M. La Entomología Forense En España: Pasado, Presente y Perspectivas de Futuro. Forensic Entomology in Spain: Past, Present and Future Perspectives. Cuad. Med. Forense 2007, 13, 21–32. [Google Scholar] [CrossRef]
- Hall, R.D. Medicocriminal Entomology. In Entomology and Death: A Procedural Guide; Catts, E.P., Haskell, N.H., Eds.; Joyce Print Shop: Clemson, SC, USA, 1990; pp. 1–8. [Google Scholar]
- Hall, R.D.; Huntington, T.E. Introduction: Perceptions and Status of Forensic Entomology. In Forensic Entomology: The Utility of Arthropods in Legal Investigations; Byrd, J.H., Castner, J.L., Eds.; CRC Press: Boca Raton, FL, USA, 2009; pp. 1–16. ISBN 9780849392153. [Google Scholar]
- Keh, B. Scope and Applications of Forensic Entomology. Annu. Rev. Entomol. 1985, 30, 137–154. [Google Scholar] [CrossRef] [PubMed]
- Amendt, J.; Campobasso, C.P.; Gaudry, E.; Reiter, C.; LeBlanc, H.N.; Hall, M.J.R. Best Practice in Forensic Entomology-Standards and Guidelines. Int. J. Legal Med. 2007, 121, 90–104. [Google Scholar] [CrossRef]
- Erzinclioĝlu, Y.Z. The Application of Entomology to Forensic Medicine. Med. Sci. Law 1983, 23, 57–63. [Google Scholar] [CrossRef]
- Nuorteva, P. Sarcosaprophagous Insects as Forensic Indicators. In Forensic Medicine: A Study in Trauma and Environmental Hazards; Tedeschi, C.G., Eckert, W.G., Tedeschi, L.G., Eds.; W. B. Saunders: Philadelphia, PA, USA, 1977; pp. 1072–1095. [Google Scholar]
- Smeeton, W.M.I.; Koelmeyer, T.D.; Holloway, B.A.; Singh, P. Insects Associated with Exposed Human Corpses in Auckland, New Zealand. Med. Sci. Law 1984, 24, 167–174. [Google Scholar] [CrossRef]
- Smith, K.G.V. A Manual of Forensic Entomology; British Museum/Comstock Publishing Associates: New York, NY, USA, 1986. [Google Scholar]
- Perotti, M.A.; Braig, H.R.; Goff, M.L. Phoretic Mites and Carcasses: Acari Transported by Organisms Associated with Animal and Human Decomposition. In Current Concepts in Forensic Entomology; Amendt, J., Campobosso, C.P., Grassberger, M., Goff, M.L., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 69–91. ISBN 9781402096846. [Google Scholar]
- Anderson, G.S.; VanLaerhoven, S.L. Initial Studies on Insect Succession on Carrion in Southwestern British Columbia. J. Forensic Sci. 1996, 41, 617–625. [Google Scholar] [CrossRef]
- Kashyap, V.K.; Pillay, V.V. Efficacy of Entomological Method in Estimation of Postmortem Interval: A Comparative Analysis. Forensic Sci. Int. 1989, 40, 245–250. [Google Scholar] [CrossRef]
- Goff, M.L. Gamasid Mites as Potential Indicators of Postmortem Interval. In Progress in Acarology; Channabasavanna, G., Viraktamatch, C., Eds.; Oxford and IBH Publishing: New Delhi, India, 1989; Volume 1, pp. 443–450. [Google Scholar]
- Rasmy, A.H. The Humans Lie but the Spiders Do Not Lie: An Overview on Forensic Acarology. Egypt. J. Forensic Sci. 2011, 1, 109–110. [Google Scholar] [CrossRef] [Green Version]
- Mégnin, P. La Faune Des Cadavres: Application de L’entomologie À La Médecine Légale; Masson, G., Ed.; Gauthier-Villars et Fils: Paris, France, 1894. [Google Scholar]
- Perotti, M.A.; Goff, M.L.; Baker, A.S.; Turner, B.D.; Braig, H.R. Forensic Acarology: An Introduction. Exp. Appl. Acarol. 2009, 49, 3–13. [Google Scholar] [CrossRef] [Green Version]
- Kreitlow, K.L. Insect Succession in a Natural Environment. In Forensic Entomology: The Utility of Arthropods in Legal Investigations; Byrd, J.H., Castner, J.L., Eds.; CRC Press: Boca Raton, FL, USA, 2009; pp. 251–270. ISBN 9780849392153. [Google Scholar]
- Turner, B. Forensic Entomology: A Template for Forensic Acarology? Exp. Appl. Acarol. 2009, 49, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Braig, H.R.; Perotti, M.A. Carcases and Mites. Exp. Appl. Acarol. 2009, 49, 45–84. [Google Scholar] [CrossRef] [PubMed]
- Açikgöz, H.N.; Kilinçarslan, L.E.; Açikgöz, A. Role of Acari in Forensic Medicine: Review. Türkiye Klin. J. Forensic Med. 2012, 9, 42–45. [Google Scholar]
- OConnor, B.M. Astigmatid Mites (Acari: Sarcoptiformes) of Forensic Interest. Exp. Appl. Acarol. 2009, 49, 125–133. [Google Scholar] [CrossRef]
- Athias-Binche, F. Dispersal in Varying Environments: The Case of Phoretic Uropodid Mites. Can. J. Zool. 1993, 71, 1793–1798. [Google Scholar] [CrossRef]
- Hunter, P.E.; Rosario, R.M.T. Associations of Mesostigmata with Other Arthropods. Annu. Rev. Entomol. 1988, 33, 393–417. [Google Scholar] [CrossRef]
- White, P.S.; Morran, L.; de Roode, J. Phoresy. Curr. Biol. 2017, 27, R578–R580. [Google Scholar] [CrossRef]
- Farish, D.J.; Axtell, R.C. Phoresy Redefined and Examined in Macrocheles muscaedomesticae (Acarina: Marcochelidae). Acarologia 1971, 13, 16–29. [Google Scholar]
- Perotti, M.A.; Braig, H.R. Phoretic Mites Associated with Animal and Human Decomposition. Exp. Appl. Acarol. 2009, 49, 85–124. [Google Scholar] [CrossRef]
- Halffter, G.; Matthews, E.G. The Natural History of Dung Beetles. A Supplement on Associated Biota. Rev. Latinoam. Microbiol. 1971, 13, 147–164. [Google Scholar]
- Krantz, G.W.; Walter, D.E. A Manual of Acarology, 3rd ed.; Texas Tech University Press: Lubbock, TX, USA, 2009; ISBN 9780896726208. [Google Scholar]
- Athias-Binche, F. Phenotypic Plasticity, Polymorphisms in Variable Environments and Some Evolutionary Consequences in Phoretic Mites (Acarina): A Review. Ecologie 1995, 26, 225–241. [Google Scholar]
- Binns, E.S. Phoresy as Migration-Some Functional Aspects of Phoresy in Mites. Biol. Rev. 1982, 57, 571–620. [Google Scholar] [CrossRef]
- Moraza, M.L.; Pérez-Martínez, S. The Genus Uroseius Berlese (Acari: Mesostigmata: Uropodina: Trachytidae) in the Iberian Peninsula with Description of a New Species Associated with Animal Remains. Syst. Appl. Acarol. 2019, 24, 929–944. [Google Scholar] [CrossRef]
- Pérez-Martínez, S.; Moraza, M.L.; Saloña-Bordas, M.I. Gamasina Mites (Acari: Mesostigmata) Associated with Animal Remains in the Mediterranean Region of Navarra (Northern Spain). Insects 2019, 10, 5. [Google Scholar] [CrossRef] [Green Version]
- Rasmy, A.H. Forensic Acarology: A New Area for Forensic Investigation. Acarines J. Egypt. Soc. Acarol. 2007, 1, 5–6. [Google Scholar] [CrossRef] [Green Version]
- Perotti, M.A.; Braig, H.R. Acarology in Criminolegal Investigations. The Human Acarofauna during Life and Death. In Forensic Entomology: The Utility of Arthropods in Legal Investigations; Byrd, J.H., Castner, J.L., Eds.; CRC Press: Boca Raton, FL, USA, 2009; pp. 637–649. ISBN 9780849392153. [Google Scholar]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World Map of the Köppen-Geiger Climate Classification Updated. Meteorol. Zeitschrift 2006, 15, 259–263. [Google Scholar] [CrossRef]
- Goff, M.L. Estimating of Postmortem Interval Using Arthropods Development and Succesional Patterns. Forensic Sci. Rev. 1993, 5, 81–93. [Google Scholar]
- Schoenly, K.G.; Haskell, N.H.; Mills, D.K.; Bieme-Ndi, C.; Larsen, K.; Lee, Y. RECREATING DEATH’S ACRE IN THE SCHOOL YARD: Using Pig Carcasses as Model Corpses To Teach Concepts of Forensic Entomology & Ecological Succession. Am. Biol. Teach. 2006, 68, 402–410. [Google Scholar] [CrossRef]
- Athias-Henriot, C. Mésostigmates (Uro. Excl.) Édaphiques Méditerráneens (Acaromorpha, Anactinotrichida). Acarologia 1961, 3, 381–509. [Google Scholar]
- Bregetova, N.G.; Vainshtein, B.A.; Kadite, B.A.; Koroleva, E.V.; Petrovs, A.D.; Tikhomirov, S.I.; Shcherbat, G.I. A Key to the Soil-Inhabiting Mites of the Mesostigmata; Gilyarov, M.S., Ed.; Nauka: Leningrad, Rusia, 1977. [Google Scholar]
- Evans, G.O. Adults of the Genus Crassicheles Karg (Acari: Mesostigmata). Int. J. Acarol. 1980, 6, 33–38. [Google Scholar] [CrossRef]
- Evans, G.O.; Browning, E. British Mites of the Subfamily Macrochelinae Trägardh (Gamasina-Macrochelidae). Bull. Br. Mus. Nat. Hist. 1956, 4, 1–56. [Google Scholar]
- Ferragut Pérez, F.; Pérez Moreno, I.; Iraola Calvo, V.M.; Escudero Colomar, L.A. Ácaros Depredarores En Las Plantas Cultivadas. Familia Phytoseiidae; Ediciones Agrotécnicas: Madrid, Spain, 2010; ISBN 978-84-87480-53-9. [Google Scholar]
- Hyatt, K.H. Mites of the Subfamily Parasitinae (Mesostigmata: Parasitidae) in the British Isles. Bull. Br. Mus. Nat. Hist. 1980, 38, 237–378. [Google Scholar] [CrossRef]
- Hyatt, K.H.; Emberson, R.M. A Review of the Macrochelidae (Acari: Mesostigmata) of the British Isles. Bull. Br. Mus. Nat. Hist. 1988, 54, 63–125. [Google Scholar]
- Karg, W. The Family Acari (Acarina), Milben, Unterordnung Parasitiformes (Anactinochaeta), Uropodina Kramer, Schildkrötenmilben; VEB Gustav Fischer Verlag Jena: Raubmilben, Germany, 1989; ISBN 3334003086. [Google Scholar]
- Karg, W. Acari (Acarina), Milben Parasitiformes (Anactinochaeta), Cohors Gamasina Leach; Gustav Fischer Verlag Jena: Raubmilben, Germany, 1993; ISBN 3334604454. [Google Scholar]
- Krantz, G.W. A Manual of Acarology; Texas Tech Press: Lubbock, TX, USA, 1978; ISBN 0882460641. [Google Scholar]
- Krantz, G.W.; Ainscough, B.D. Acarina: Mesostigmata (Gamasida). In Soil Biology Guide; Dindal, D.L., Ed.; Wiley & Sons: New York, NY, USA, 1990; pp. 583–665. [Google Scholar]
- Mašán, P. Mites (Acarina) Associated with Burying and Carrion Beetles (Coleoptera, Silphidae) and Description of Poecilochirus mrciaki Sp. n. (Mesostigmata, Gamasina). Biol. Bratislava 1999, 54, 515–524. [Google Scholar]
- Mašán, P.; Halliday, B. Review of the European Genera of Eviphididae (Acari: Mesostigmata) and the Species Occurring in Slovakia. Zootaxa 2010, 2585, 1–122. [Google Scholar] [CrossRef]
- Shcherbak, G.I. New Species of Gamasid from the Genus Hypoaspis Canestrini (Acarina: Gamasoidea). Vestn. Zool. 1971, 5, 76–79. [Google Scholar]
- Vitzthum, G.H. Acarologische Beobachtungen. Zweite Reihe. Zool. Jahrbüchern 1918, 6, 1–40. [Google Scholar]
- Team, R.C. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Dormann, C.; Gruber, B.; Fründ, J. Introducing the Bipartite Package: Analysing Ecological Networks. Interaction 2008, 8, 8–11. [Google Scholar]
- Dormann, C.F.; Fründ, J.; Blüthgen, N.; Gruber, B. Indices, Graphs and Null Models: Analyzing Bipartite Ecological Networks. Open Ecol. J. 2009, 2, 7–24. [Google Scholar] [CrossRef]
- Dormann, C.F. How to Be a Specialist? Quantifying Specialisation in Pollination Networks. Netw. Biol. 2011, 1, 1–20. [Google Scholar]
- Borgatti, S.P.; Everett, M.G. Network Analysis of 2-Mode Data. Soc. Netw. 1997, 19, 243–269. [Google Scholar] [CrossRef]
- Newman, M.; Barabási, A.L.; Watts, D.J. The Structure and Dynamics of Networks; Princeton University Press: Princeton, NJ, USA, 2006; ISBN 9780691113579. [Google Scholar]
- Blüthgen, N.; Menzel, F.; Hovestadt, T.; Fiala, B.; Blüthgen, N. Specialization, Constraints, and Conflicting Interests in Mutualistic Networks. Curr. Biol. 2007, 17, 341–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okabe, K. Ecological Characteristics of Insects That Affect Symbiotic Relationships with Mites. Entomol. Sci. 2013, 16, 363–378. [Google Scholar] [CrossRef]
- Walter, D.E.; Proctor, H.C. Mites-Ecology, Evolution and Behaviour; CABI: Wallingford, UK, 1999; ISBN 0851993753. [Google Scholar]
- Bahrami, F.; Arbabi, M.; Shoushtari, R.V.; Kazemi, S. Mesostigmatic Mites Associated with Coleoptera and Biodiversity Calculation of These Mites Phoretic on Dung Beetles in Golestan Province (North of Iran). Middle-East J. Sci. Res. 2011, 9, 345–366. [Google Scholar]
- Beninger, C.W. A Study of the Ecology and Reproductive Biology of the Carrion Beetle Assemblage in the Mer Bleue Bog Area with Specific Reference to the Habitat Associations of Nicrophorus vespilloides Herbst and N. Defodiens Mannerheim (Coleoptera: Silphidae). Ph.D. Thesis, Carleton University, Ottawa, ON, Canada, 1989. [Google Scholar]
- Brown, J.M.; Wilson, D.S. Poecilochirus carabi: Behavioral and Life-History Adaptations to Different Hosts and the Consequences of Geographical Shifts in Host Communities. In Mites; Houck, M.A., Ed.; Springer Science+Business Media: Dordrecht, The Netherland, 1994; pp. 1–22. [Google Scholar]
- Schwarz, H.H.; Müller, J.K. The Dispersal Behaviour of the Phoretic Mite Poecilochirus carabi (Mesostigmata, Parasitidae): Adaptation to the Breeding Biology of Its Carrier Necrophorus vespilloides (Coleoptera, Silphidae). Oecologia 1992, 89, 487–493. [Google Scholar] [CrossRef] [PubMed]
- De Gasperin, O.; Kilner, R.M. Interspecific Interactions and the Scope for Parent-Offspring Conflict: High Mite Density Temporarily Changes the Trade-Off between Offspring Size and Number in the Burying Beetle, Nicrophorus vespilloides. PLoS ONE 2016, 11, eD150969. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, H.H.; Starrach, M.; Koulianos, S. Host Specificity and Permanence of Associations between Mesostigmatic Mites (Acari: Anactinotrichida) and Burying Beetles (Coleoptera: Silphidae: Nicrophorus). J. Nat. Hist. 1998, 32, 159–172. [Google Scholar] [CrossRef]
- Nannelli, R.; Sabbatini Peverieti, R. Segnalazioni Faunistiche Italiane. Boll. Della Soc. Entomol. Ital. 2006, 138, 73–80. [Google Scholar]
- Desch, C.E. Human Hair Follicle Mites and Forensic Acarology. Exp. Appl. Acarol. 2009, 49, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Morand, S. Agrégation et Régulation Dans Les Systèmes Hôtes-Parasites: Exemple Des Nématodes Parasites de l’escargot Helix Aspersa. Bull. Société Zool. Fr. 1988, 114, 14–19. [Google Scholar]
- Bajerlein, D.; Przewoźny, M. When a Beetle Is Too Small to Carry Phoretic Mites? A Case of Hydrophilid Beetles (Coleoptera: Hydrophilidae) and Uropoda orbicularis (Acari: Mesostigmata). Can. J. Zool. 2012, 90, 368–375. [Google Scholar] [CrossRef]
- Mitchell, R. An Analysis of Dispersal in Mites. Am. Nat. 1970, 104, 425–431. [Google Scholar] [CrossRef]
- Kinn, D.N.; Witcosky, J.J. Variation in Southern Pine Beetle Attack Height Associated with Phoretic Uropodid Mites. Can. Entomol. 1978, 110, 249–251. [Google Scholar] [CrossRef]
| Mite sp. | Astig | N.bre | N.rit | U.orb | C.lun | G.spi | P.col | P.fim | P.aus | P.car | P.sub | Dendr |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | 52 * | 29 | 1 | 6 | 1 | 1 | 7 | 1 | 73 | 13 | 621 | 3 |
| S | 12 | 4 | 1 | 3 | 1 | 1 | 2 | 1 | 3 | 2 | 4 | 2 |
| BI | 0.85 | 0.36 | 0 | 0.8 | 0 | 0 | 0.29 | 0 | 0.29 | 0.15 | 0.25 | 0.67 |
| SI | 0.36 | 0.8 | 1 | 0.56 | 1 | 1 | 0.86 | 1 | 0.83 | 0.92 | 0.86 | 0.74 |
| H.aer | H.oct | H.g.n. | A.nec | A.pra | C.hol | S.ine | M.gla | M.mer | M.mus | M.pun | A.sem | A.vac |
| 63 | 489 | 30 | 1 | 1 | 115 | 6 | 9 | 36 | 23 | 1 | 1 | 1 |
| 3 | 4 | 1 | 1 | 1 | 5 | 1 | 3 | 5 | 10 | 1 | 1 | 1 |
| 0.53 | 0.11 | 0 | 0 | 0 | 0.1 | 0 | 0.42 | 0.57 | 0.84 | 0 | 0 | 0 |
| 0.68 | 0.94 | 1 | 1 | 1 | 0.94 | 1 | 0.79 | 0.61 | 0.41 | 1 | 1 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Martínez, S.; Moraza, M.L. First Interaction Network of Sarcosaprophagous Fauna (Acari and Insecta) Associated with Animal Remains in a Mediterranean Region (Northern Spain). Insects 2022, 13, 610. https://doi.org/10.3390/insects13070610
Pérez-Martínez S, Moraza ML. First Interaction Network of Sarcosaprophagous Fauna (Acari and Insecta) Associated with Animal Remains in a Mediterranean Region (Northern Spain). Insects. 2022; 13(7):610. https://doi.org/10.3390/insects13070610
Chicago/Turabian StylePérez-Martínez, Sandra, and María Lourdes Moraza. 2022. "First Interaction Network of Sarcosaprophagous Fauna (Acari and Insecta) Associated with Animal Remains in a Mediterranean Region (Northern Spain)" Insects 13, no. 7: 610. https://doi.org/10.3390/insects13070610
APA StylePérez-Martínez, S., & Moraza, M. L. (2022). First Interaction Network of Sarcosaprophagous Fauna (Acari and Insecta) Associated with Animal Remains in a Mediterranean Region (Northern Spain). Insects, 13(7), 610. https://doi.org/10.3390/insects13070610






