Simple Summary
Cyclin-dependent kinases (CDKs), as biological macromolecules, play key regulatory roles in the cell cycle process. Cyclin-dependent kinase-5 (CDK5) is part of the CDK family; however, its effect on cell cycle progression is controversial and the mechanism remains unclear. In this article, we identified a homologous gene of the Cyclin-dependent kinase family, BmCDK5, in the silkworm, Bombyx mori. We proved that the BmCDK5 gene can regulate the cell cycle and promote cell proliferation in BmNS cells. Furthermore, we demonstrated that BmCDK5 can interact with BmCNN, and that they both affect cytoskeleton morphology but do not induce changes in microtubule protein expression; they can also promote cell proliferation.
Abstract
The ordered cell cycle is important to the proliferation and differentiation of living organisms. Cyclin-dependent kinases (CDKs) perform regulatory functions in different phases of the cell cycle process to ensure order. We identified a homologous gene of the Cyclin-dependent kinase family, BmCDK5, in Bombyx mori. BmCDK5 contains the STKc_CDK5 domain. The BmCDK5 gene was highly expressed in S phase. Overexpression of the BmCDK5 gene accelerates the process of the cell cycle’s mitotic period (M) and promotes cell proliferation; knocking out the BmCDK5 gene inhibited cell proliferation. Furthermore, we identified a protein, BmCNN, which can interact with BmCDK5 and represents the same express patterns as the BmCDK5 gene in the cell cycle phase and the spatial-temporal expression of B. mori. This study revealed that BmCDK5 and BmCNN play roles in promoting cell proliferation and regulating cytoskeleton morphology, but do not induce expression changes in microtubule protein. Therefore, our findings provide a new insight; the BmCDK5 gene has a regulatory effect on the cell cycle and proliferation of B. mori, which is presumably due to the interaction between BmCDK5 and BmCNN regulating changes in the cytoskeleton.
1. Introduction
Cyclin-dependent kinases (CDKs), known as serine/threonine kinases, play key roles in cell cycle regulation [1]. In general, CDK contains a regulatory cyclin subunit and a catalytic serine/threonine kinase subunit, so that it can be modulated by association with cyclins and CDKs inhibitors [2,3]. In mammals, 21 genes encode CDK according to sequence homology. The CDK family is divided into three cell cycle related subfamilies (CDK1, CDK4, and CDK5) and five transcription subfamilies (CDK7, CDK8, CDK9, CDK11, and CDK20) [1]. Cyclin-dependent kinase-5 (CDK5) is part of the CDK family and has high homology with other CDKs; however, it has unique activation modes and cellular functions compared to them [4]. Although CDK5 regulates a series of important cellular processes in cells [5,6], its effect on cell cycle progression is controversial and the mechanism remains unclear [7,8,9,10].
CDK5 was first isolated from bovine brain tissue extracts through biochemical methods [11]. CDK5 substrates include microtubule-associated protein 1B, which contains a Ksp-like motif, nerve filament protein containing KspxK τ Au and Kspxx, actin binding protein, and vesicle protein [12,13]. Studies show that CDK5 plays an important role in neuron migration, axon guidance, and synaptic formation by regulating the migration of the developing central nervous system and the cytoskeletal structure and organization of nerve cells [13,14]. Furthermore, CDK5 is related to the pathogenesis of neurodegenerative diseases such as Alzheimer’s disease (AD), amyotrophic lateral sclerosis (ALS), and Niemann’s Pick type-C disease (NPD); its hyperactivation results in the deep remodeling of the nerve cytoskeleton, and the loss of synapses eventually leads to neurodegenerative changes [15,16,17]. The current study demonstrated that CDK5 is also involved in inflammation-mediated neurodegeneration by mediating microtubule disassembly [18]. In addition, in Drosophila melanogaster, CDK5 regulates the timing and rate of mushroom body remodeling by dissolving the neuronal tubulin cytoskeleton; stress-induced CDK5 activity enhances cytoprotective basal autophagy [19,20]. However, the underlying molecular mechanism of CDK5 in cytoskeleton regulation function is unclear.
Microtubule, as a highly dynamic polymer, is a major component of the cytoskeleton, which is important for fundamental cellular events and developmental processes [21,22]. In addition, the centrosome is the major microtubule organizing center, which ensures orderly cell mitosis in most cell types [23]. The centrosome consists of a pair of centrioles surrounded by the pericentriolar material or matrix (PCM) [24]. Furthermore, centrosomin (CNN) is a key component of the centrosome and is abundantly distributed within the PCM [25,26]. In D. melanogaster, CNN plays an important role in the formation of functional centrosomes and is also essential for the organization of actin and microtubule cytoskeleton [27,28].
In this article, we identified a silkworm homologous gene of the Cyclin-dependent kinase family, BmCDK5. We proved that the BmCDK5 gene can regulate the cell cycle and promote cell proliferation. Additionally, we identified that BmCNN can interact with BmCDK5. Furthermore, we demonstrated that BmCDK5 and BmCNN both affect cytoskeleton morphology, but do not induce expression changes in microtubule protein; they can also promote cell proliferation.
2. Materials and Methods
2.1. Gene Identification and Homology Analysis
All gene sequences were obtained from the National Center for Biotechnology Information (NCBI). Sequence alignments were constructed using GeneDoc 3.0 software. Protein domains were predicted by SMART software (http://smart.embl-heidelberg.de/; accessed on 19 May 2020). The phylogenetic tree was built using MEGA 6.0 software. Primer Premier 5.0 software was used to design the primers. The sgRNAs for the knock out cloning vector were designed by CRISPR direct (http://crispr.dbcls.jp/; accessed on 11 September 2019).
2.2. Gene Cloning
The PCR products of the target sequences and the expression vectors (Invitrogen, Waltham, MA, USA) were ligated with Solution I ligation Enzyme (Takara, Tokyo, Japan) after digestived with the same type of restriction enzymes (EcoR I and Xho I for PIZ-BmCNN-OE plasmid; BamHI and EcoR I for PIZ-BmCDK5-OE plasmid; Takara, Tokyo, Japan). The expression efficiencies or knock out efficiencies of the cloning vectors are shown in Figure S1. All primers used in this study are listed in Table S1.
2.3. Silkworm Strain
Larval cDNA was extracted from third-day fifth-instar Dazao larvae; the silkworm strain Dazao was maintained at the Gene Resource Library of Domesticated Silkworm of Southwest University, Chongqing, China.
2.4. Cell Cultures and Cell Transfections
The cell line used in this study is BmN-SWU1 (BmNS) from the ovary of Dazao strain B. mori, which were cultured in insect medium (TC-100, United States Biological, MA, USA) at 27 °C. In the transient transfections experiment, we used the TransIT®-LT1 transfection reagent (Mirus, Beijing, China) to transfect plasmids into cells using a ratio of 1 μL:2 μg when the cell density was 80%.
2.5. Flow Cytometry
Cells were incubated with cell cycle detection reagent (Beyotime, Shanghai, China) at 37 °C for 30 min after fixing in 70% ethanol at 4 °C overnight after 48 h of transfection. Cell cycle phase changes were analyzed immediately using flow cytometry (CYTOFLEX, Beckman Coulter, Brea, CA, USA).
2.6. 5-Ethynyl-2′-deoxyuridine (EdU) Assay
EdU is a thymidine kinase analogue that inserts the thymidine kinase (T) into duplicated DNA during cell proliferation; it can be visually detected after reacting with Apollo fluorescent dyes for EdU. In this study, cell proliferation activity was detected via EdU lablling (Beyotime, Shanghai, China) after 48 h of transfection; green fluorescence represented EdU positive cells or EdU+ cells [29].
2.7. Cell Counting Kit-8 Assay (CCK-8)
The transfected cells were counted at 0 h, 48 h, and 72 h after transfection to determine cell proliferation ability using CCK-8 assay. Cells were divided into 96-well plates with 100 μL per well, with three replicates. Next, the CCK-8 solution (5 mg/mL; Biogroud, Chongqing, China) was added to each well. The well plate was placed in a 37 °C incubator for 3 h. Finally, the OD value of each sample was measured under a 540 nm wavelength to calculate the cellular viabilities.
2.8. Co-Immunoprecipitation (Co-IP) Assay and Western Blotting
The interacting plasmids were co-transferred into BmNS cells which were collected and lysed after transfection for 72 h. The 50 µL protein A + G Dynabeads (Life, Rockville, MD, USA) were incubated with anti-Flag/HA-tags antibody (Sigma, Rockville, MD, USA) or mouse control IgG (Beyotime, Shanghai, China) in 200 µL PBS (Phosphate Buffer Saline) for 30 min at 4 °C. Next, the lysed protein samples were added to the mixed systems for 1 h at 4 °C, after washing the Dynabeads two times with PBS. Finally, a 5 × sample loading buffer was added to the mixed systems, which were then boiled for 10 min to prepare the samples for the SDS-PAGE experiment. Following this, the protein bands were transferred onto a hydrophilic polyvinylidene fluoride (PVDF) membrane (Yeasen, Shanghai, China). The PVDF was incubated with primary antibodies and secondary antibodies (Beyotime, Shanghai, China), and visualized using a Western ECL Substrate (Bio-Rad, Hercules, CA, USA).
2.9. Immunofluorescence
Cells were incubated with 1% Triton X-100 (Beyotime, Shanghai, China) at 48 h after transfection for 15 min at room temperature (RT) (after fixing with 4% paraformaldehyde (Beyotime, Shanghai, China) for 15 min at RT) and then incubated with blocking buffer (Beyotime, Shanghai, China) for 1 h at RT. The primary antibodies, anti-Flag/anti-HA (1:200; Beyotime, Shanghai, China) were incubated with the cells for 1 h at 4 °C. After washing four times for 6 min using PBS, the cells were incubated with secondary antibodies (1:500; Life, MD, USA) and 4-diamidino-2-phenylindole, DAPI (1:500; Beyotime, Shanghai, China) to stain the cell nuclei. After washing four times for 6 min, the cells were observed using a confocal microscope (FV3000, Olympus, Tokyo, Japan) [30].
2.10. Total RNA Extraction and Quantitative Real-Time PCR
The sample was lysed by TRIzol reagent (Invitrogen, Waltham, MA, USA), and then RNA was extracted (using the RNA extraction Kit; Omega, Norcross, GA, USA), which underwent reverse transcription into cDNA using retrotranscriptional reagent (Takara, Kusatsu, Japan). After mixing the cDNA with HieffTM qPCR SYBR® Green Master Mix (Yeasen, Shanghai, China), quantitative real-time PCR (qRT-PCR) was performed on the samples using the real-time PCR system (Analytik Jena, Jena, Germany) with sw22934 as the internal control [31].
2.11. Statistical Analysis
All experiments were performed at least three times with biological replications; the data were stated as the mean ± SD of three independent experiments. The student’s t-test was used to determine all statistically significant differences between treatments. The notation “ns” indicates “not statistically significant”, or p ≥ 0.05; “*” indicates “statistically significant” or 0.01 ≤ p < 0.05; and “**” indicates “highly significant” or p < 0.01 [31].
3. Results
3.1. Cloning and Identification of BmCDK5 Gene
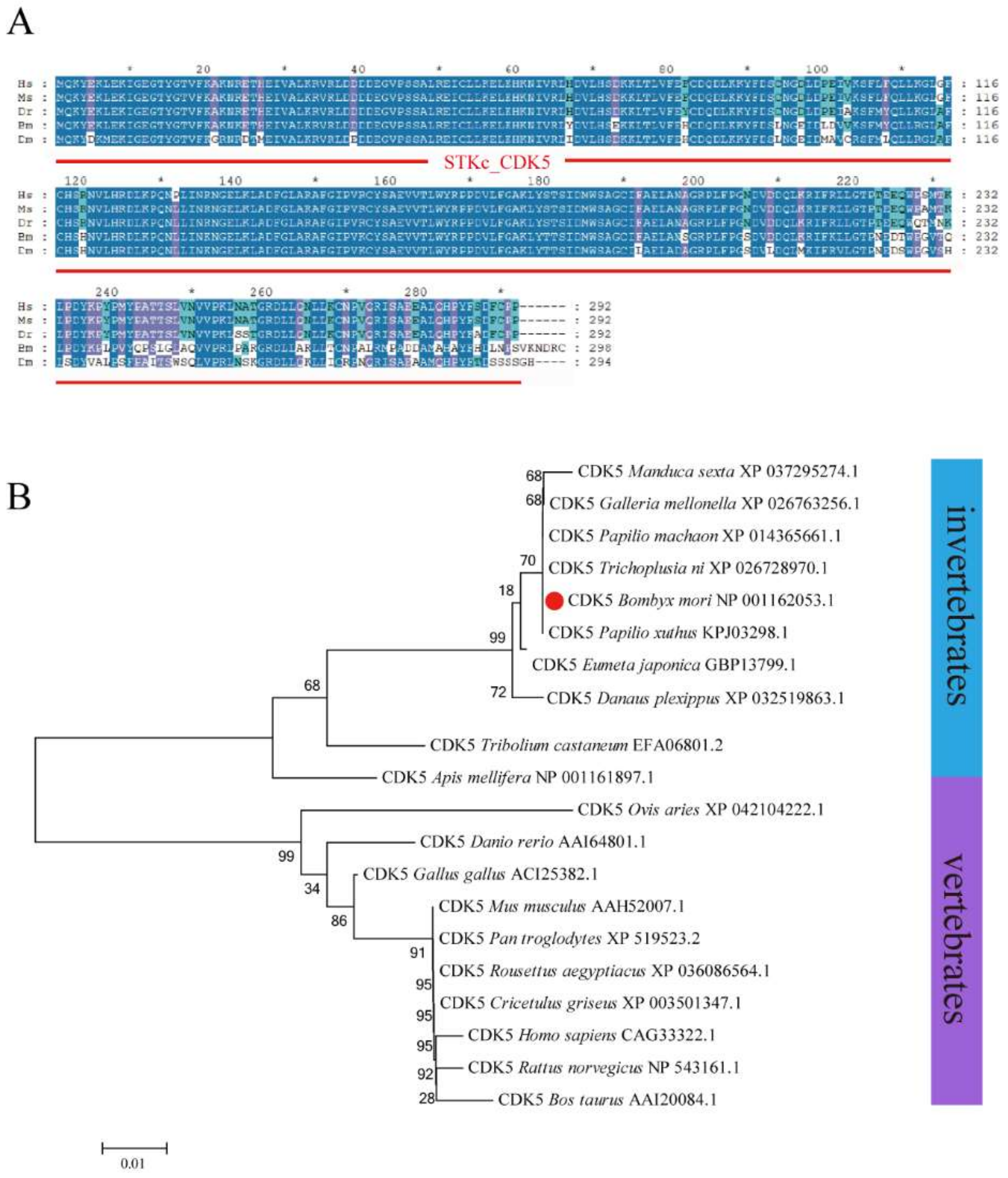
A full-length BmCDK5 gene was cloned from the larval cDNA of the Dazao strain, which encompassed a coding sequence (CDS) of 897 bp encoding a protein of 298 amino acids. Multiple sequence analysis demonstrated that BmCDK5 is highly conserved in general (Figure 1A). Phylogenetic analysis showed that BmCDK5 and Tribolium castaneum CDK5 could be clustered together (Figure 1B). These results suggest that BmCDK5 is a member of the CDK5 family.
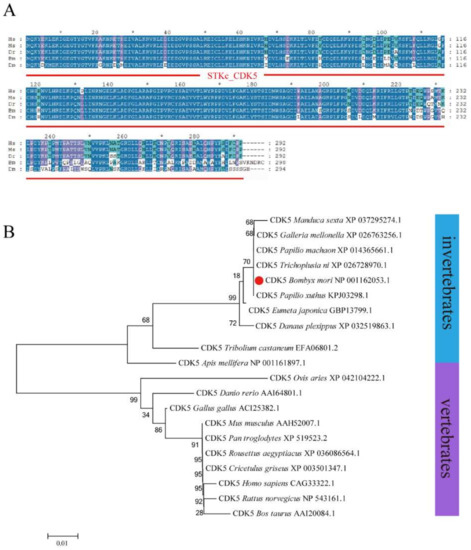
Figure 1.
Homology and evolution analysis of BmCDK5. (A) Multiple sequence alignment of CDK5 from Homo sapiens, Mus musculus, Danio rerio, Bombyx mori and Drosophila melanogaster, containing a conservative CDK5 domain (red line). (B) Phylogenetic tree of CDK5 from invertebrates and vertebrates; BmCDK5 is marked by red origin. * 0.01 ≤ p < 0.05.
3.2. Expression Pattern of BmCDK5 Gene
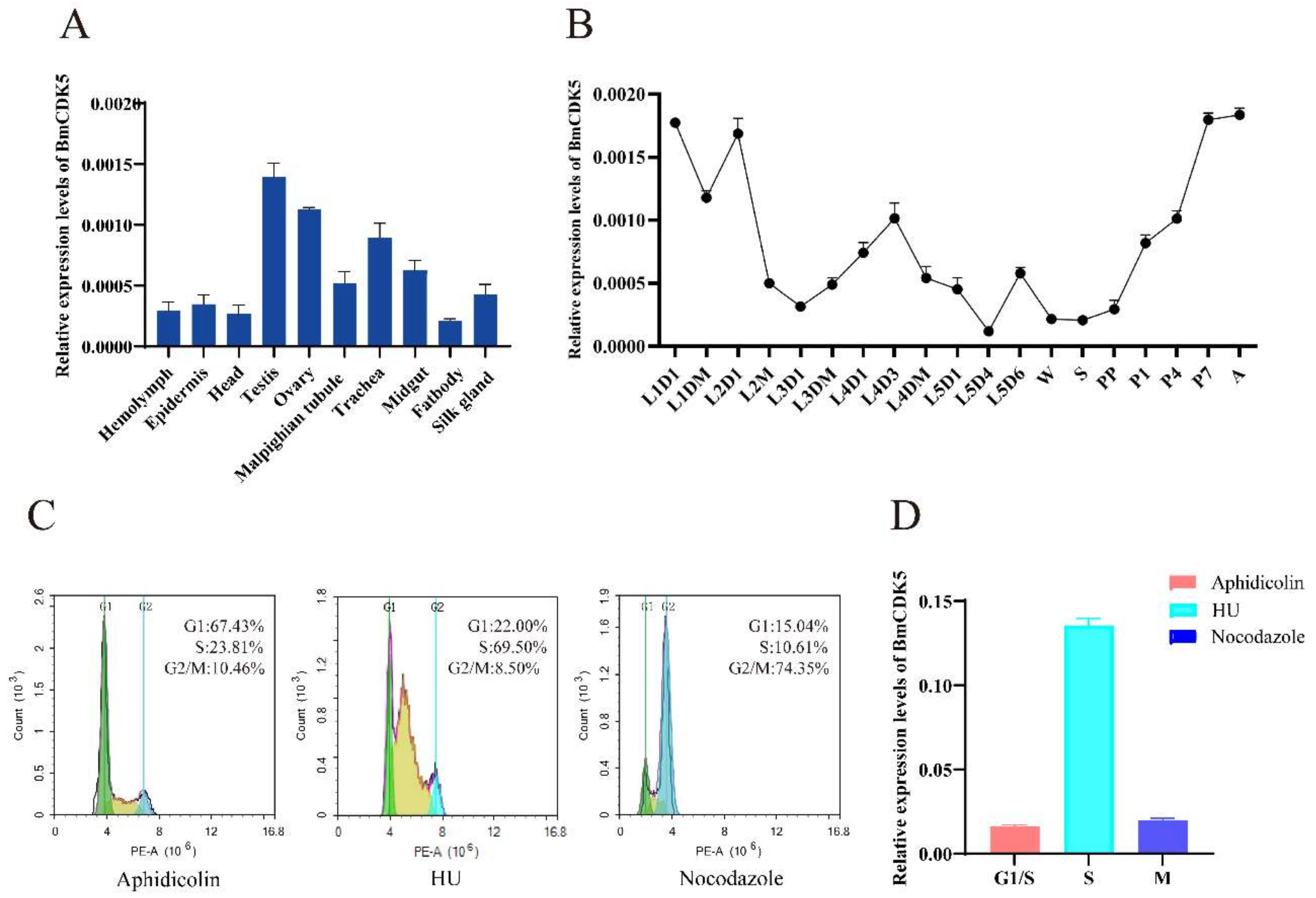
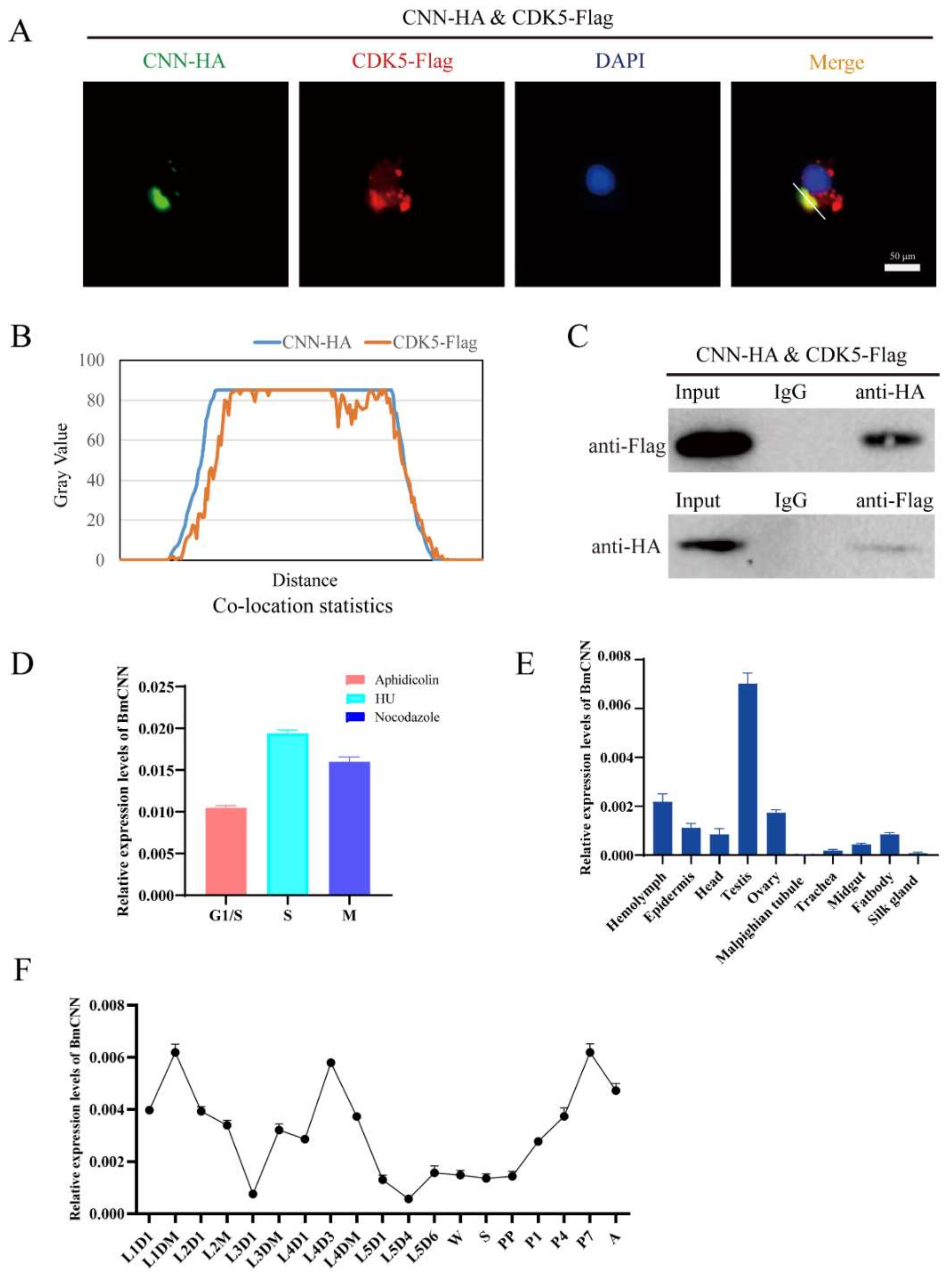
The expression levels of BmCDK5 were examined in the hemolymph, epidermis, head, testis, ovary, malpighian tubule, trachea, midgut, fat body and silk gland of the third day of fifth instar larvae (Figure 2A). We found that BmCDK5 was expressed in all larvae tissues, with the highest expression level in the gonads. It was highly expressed in the third day of fourth instar larvae, in the early and after wandering stages of the growth period (Figure 2B). Meanwhile, rigorous cell proliferation and replication occurred in the reproductive organs, which grow rapidly in the pupal stage. As a cyclin-dependent kinase, BmCDK5 was highly expressed in silkworm testes and ovaries, which indicates that BmCDK5 is particularly necessary for cell proliferation of the reproductive organs. Furthermore, we analyzed the expression of BmCDK5 in different cell cycle phases. The cell cycle was blocked at G1, S, and G2/M phases after incubating the cells with synchronous reagents Aphidicolin, Hydroxyurea (HU), and Nocodazole, respectively (Figure 2C). BmCDK5 was highly expressed in S phase (Figure 2D).
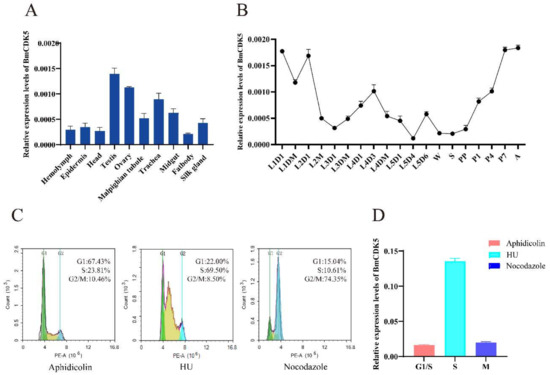
Figure 2.
Expression patterns of BmCDK5. (A) The expression patterns of BmCDK5 in different tissues of B. mori in fifth instar larvae. (B) The expression patterns of BmCDK5 in different developmental stages of B. mori. (C) BmNS cells were blocked by synchronous reagents: Aphidicolin for the first gap phase of the cell cycle (G1); Hydroxyurea (HU) for the DNA synthesis phase of the cell cycle (S); and Nocodazole for the second gap phase and the mitotic phase of the cell cycle (G2/M). (D) The expression patterns of BmCDK5 in different phases of the cell cycle in BmNS cells.
3.3. Overexpression of BmCDK5 Gene Promotes Cell Proliferation in BmNS Cells
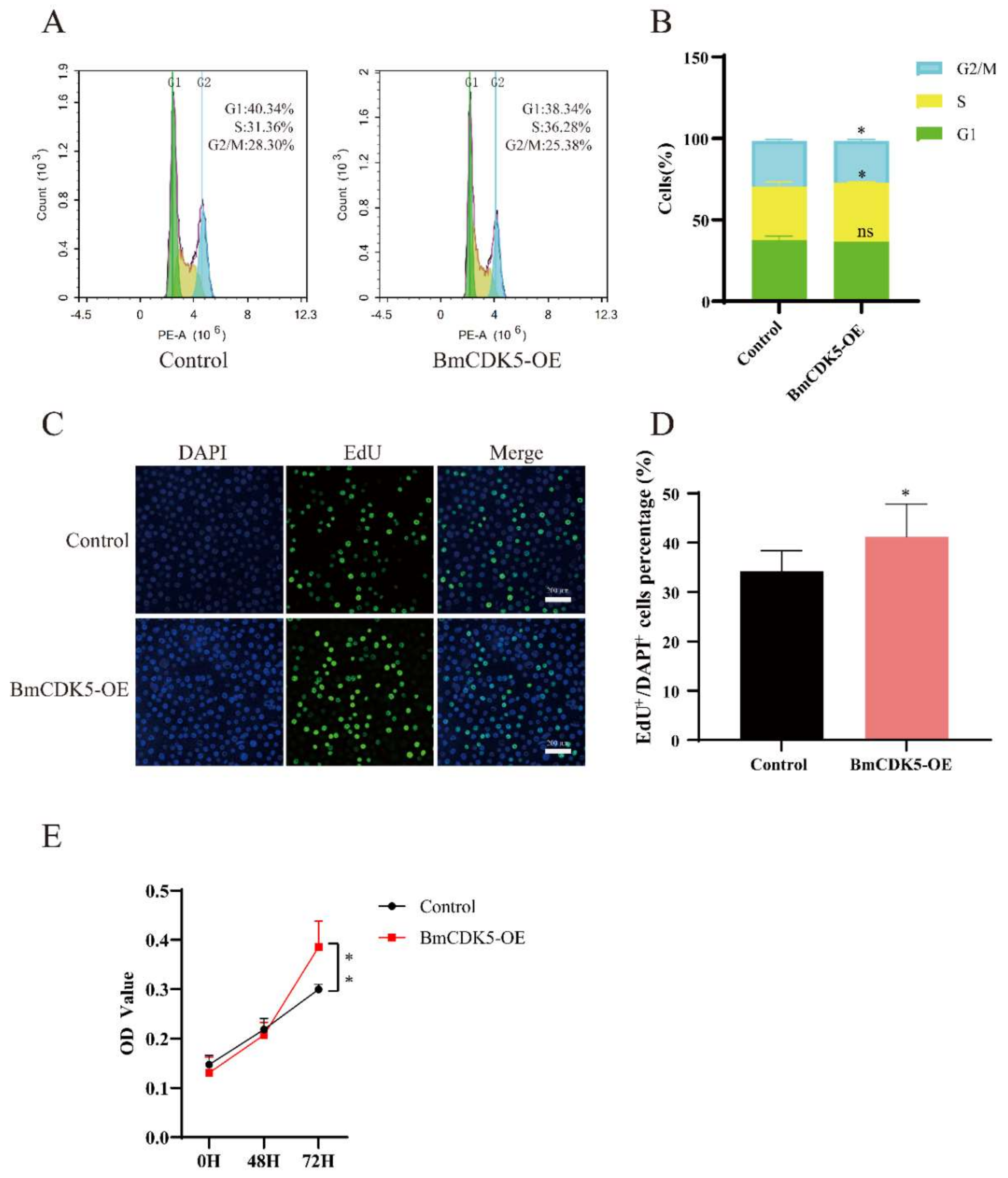
The BmCDK5 gene was overexpressed in the BmNS cells to determine its regulation in cell cycle progression. We transfected the recombinant plasmid for overexpressing BmCDK5 (BmCDK5-OE) into cells, and assessed the expression levels of BmCDK5 using qRT-PCR at 48 h after transfection (Figure S1A). Following this, flow cytometry (FCM) analysis was performed to determine the cell cycle. Overexpression of BmCDK5 did not significantly reduce the percentage of cells in the G1 stage (from 40.34% to 38.34%), significantly increased the percentage of cells in the S stage (from 31.36% to 36.28%), and significantly reduced the percentage of cells in the G2/M stage (from 28.30% to 25.38%) (Figure 3A,B).
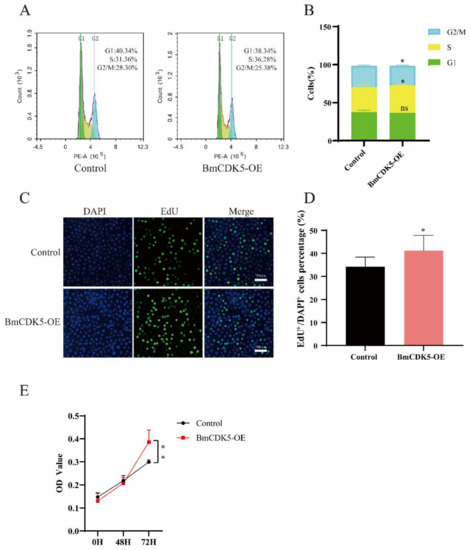
Figure 3.
Overexpression of BmCK5 gene in BmNS cells. (A) Cell cycle analysis of BmNS cells transfected with control plasmids or pIZ-BmCDK5 plasmids using flow cytometry. (B) Statistical analysis of cell percentages at G1, S, and G2/M stages. (C) EdU labeling of BmNS cells. Green fluorescence represents EdU positive or EdU+ cells. Blue fluorescence represents nuclei stained with DAPI. (D) Statistical analysis of EdU positive or EdU+ cells. (E) The CCK-8 assay was performed to detect cell proliferation ability at 0 h, 48 h, and 72 h (ns p ≥ 0.05, * 0.01 ≤ p < 0.05, ** p < 0.01).
To further determine the effect of DNA replication after BmCDK5 overexpression, the cells were labelled with 5-bromodeoxyuridine (EdU). The number of EdU-labeling cells was significantly higher in cells overexpressing BmCDK5 compared to the control (Figure 3C,D); this indicates that the relative rate of DNA synthesis was greatly increased after overexpressing BmCDK5. Meanwhile, the proliferation of cells was analyzed using a cell counting kit-8 (CCK-8) assay; the results showed that overexpression of BmCDK5 promoted the proliferation of cells at 72 h post-transfection (Figure 3E). Therefore, all results indicate that overexpression of BmCDK5 accelerates the mitotic period (M) process of the cell cycle and promotes cell proliferation.
3.4. Knock out BmCDK5 Gene Inhibiting Cell Proliferation in BmNS Cells
To further verify that BmCDK5 is involved in cell cycle regulation, CRISPR/Cas9 technology was used to knock out this gene. We transfected the recombinant plasmid to knock out BmCDK5 (BmCDK5-KO) in cells and assessed the knockout efficiency of BmCDK5 at 48 h after transfection. The result showed that BmCDK5 was successfully edited (Figure S1C).
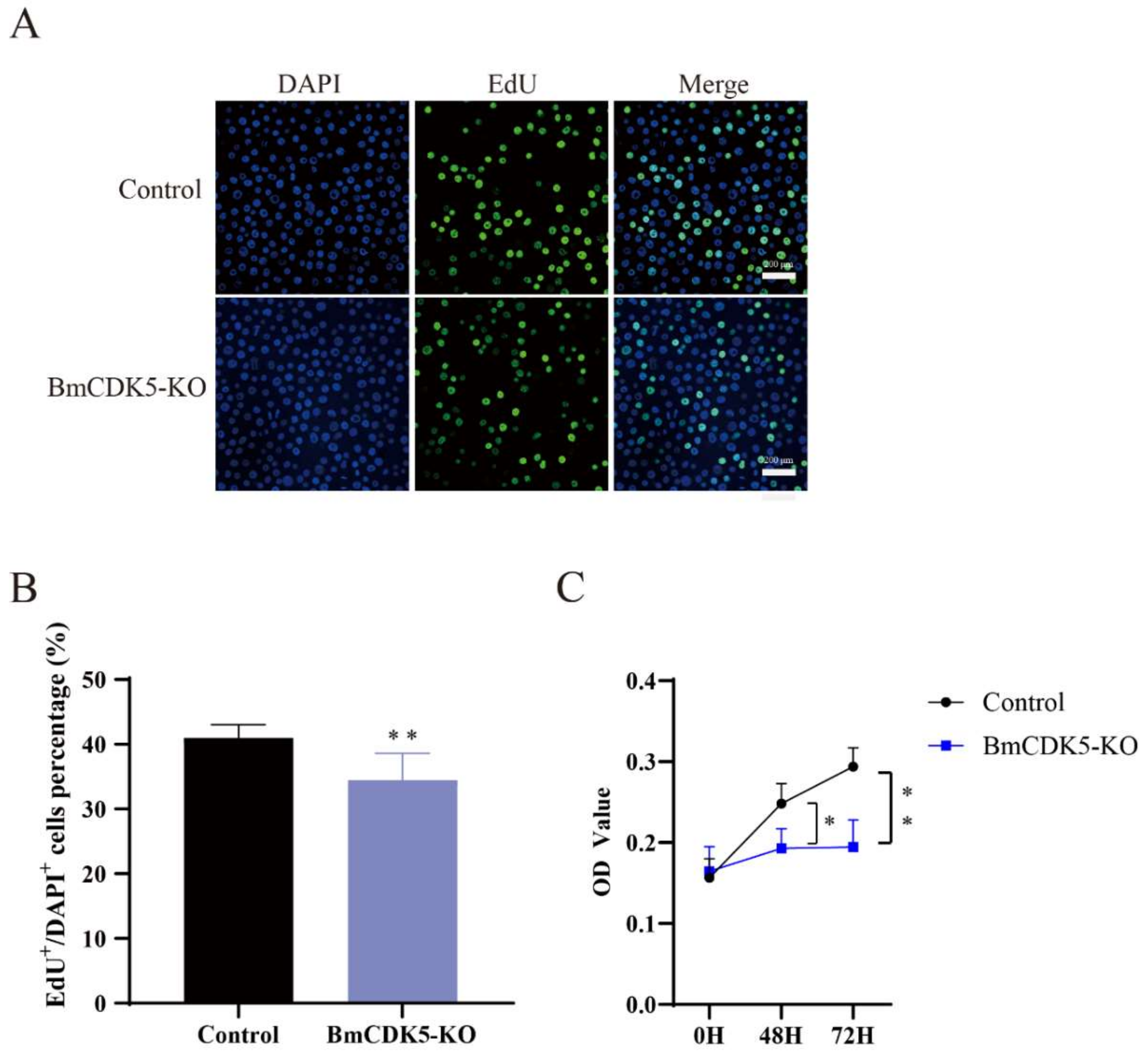
In addition, the number of EdU-labeling cells was significantly lower in cells with BmCDK5 knocked out compared to the control (Figure 4A,B), which indicates that the relative rate of DNA synthesis after BmCDK5 knockout is significantly reduced. The CCK-8 assay also showed that knockout of BmCDK5 inhibited the proliferation of cells 48 h post-transfection (Figure 4C). Therefore, all results indicate that knocking out BmCDK5 can inhibit cell proliferation.
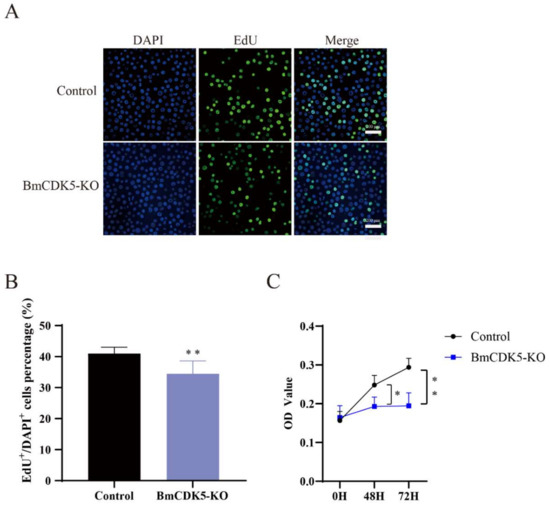
Figure 4.
Knocked out BmCK5 gene in BmNS cells. (A) EdU labeling BmNS cells. Green fluorescence represents EdU positive or EdU+ cells. Blue fluorescence represents nuclei stained with DAPI. (B) Statistical analysis of EdU positive or EdU+ cells. (C) The CCK-8 assay was performed to detect cell proliferation ability at 0 h, 48 h, and 72 h (* 0.01 ≤ p < 0.05, ** p < 0.01).
3.5. BmCDK5 Interacts with BmCNN
The phylogenetic analysis of CNN showed that BmCNN clustered together with Plutella ylostella CDK5 regulatory subunit associated protein (Figure S1A). BmCNN has two spliceosomes; functions similar to those in our previous studies were found, with differences only in length compared to our previous studies [32]. Therefore, we cloned BmCNN with a longer sequence in this study, to explore its relationship with BmCDK5. In addition, we co-transfected HA-BmCNN and Flag-BmCDK5 overexpressed plasmids into BmNS cells. Immunofluorescence (IF) was used to analyze their localization, which showed that BmCNN was expressed only in the nucleus, BmCDK5 was expressed both in cytoplasm and the nucleus, and they were co-localized in the cytoplasm (Figure 5A). Following this, we analyzed the fluorescence signal of the yellow line profile. The X-axis represents the signal position and the Y-axis represents the signal strength. It was seen that BmCDK5 and BmCNN signal positions coexist simultaneously, indicating the existence of co-location (Figure 5B). Next, Co-Immunoprecipitation (Co-IP) experiments were performed using anti-HA and anti-Flag monoclonal antibodies, which showed interaction between BmCNN and BmCDK5 (Figure 5C). The IF and Co-IP results both show that BmCDK5 interacts with BmCNN.
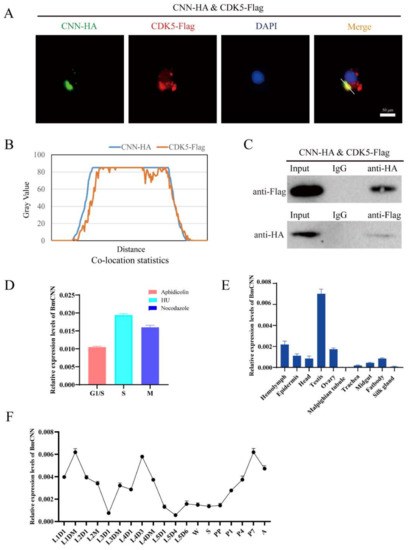
Figure 5.
Interaction of BmCDK5 and BmCNN. (A) The co-localization of BmCNN (green) with BmCDK5 (red) using IF analysis. (B) The statistics analysis of IF co-localization. (C) The interaction of BmCDK5 and BmCNN by Co-IP assays. (D) The expression pattern of BmCNN in different phase of the cell cycle in BmNS cells. (E) The expression pattern of BmCNN in different tissues of B. mori in fifth instar larvae. (F) The expression pattern of BmCNN in different development stages of B. mori.
To confirm whether there was a relationship between the expression pattern of BmCDK5 and BmCNN, we analyzed the expression profiles of BmCNN. BmCNN was also highly expressed in the S phase (Figure 5D). The expression levels of BmCNN were examined in the hemolymph, epidermis, head, testis, ovary, malpighian tubule, trachea, midgut, fat body and silk gland of the third day of fifth instar larvae using qRT-PCR. The results showed that BmCNN was also highly expressed in the gonads (Figure 5E), as was BmCDK5 (Figure 2A). Additionally, the expression pattern of BmCNN during the silkworm growth period was consistent with that of BmCDK5, which was highly expressed in the third day of fourth instar larvae, in the early and after wandering stages of the growth period (Figure 5F).
3.6. BmCDK5 and BmCNN Both Affect the Cytoskeleton Morphology of BmNS Cells
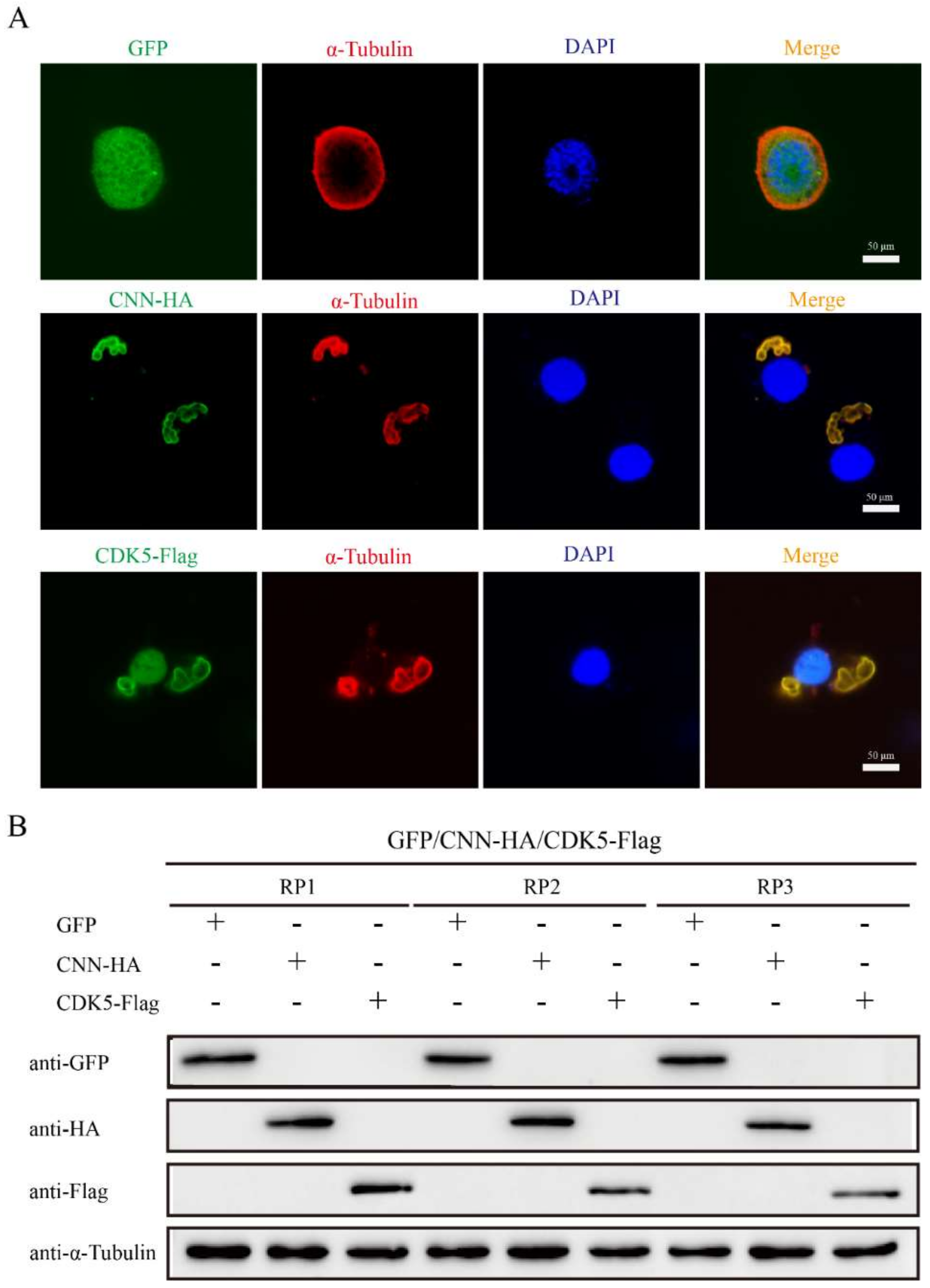
The effects of BmCDK5 and BmCNN overexpression on cytoskeleton morphology were verified by transfecting HA-BmCNN and Flag-BmCDK5 recombinant plasmids into the cells. IF was conducted after 48 h of cell transfection; the results show an abnormal cytoskeleton morphology which was caused by the overexpression of BmCDK5 and BmCNN (Figure 6A). Meanwhile, the expression levels of α-Tubulin did not change compared to the control groups (Figure 6B). The results suggest that overexpression of BmCDK5 and BmCNN did not induce changes in microtubule protein expression, but significantly changed microtubule morphology.
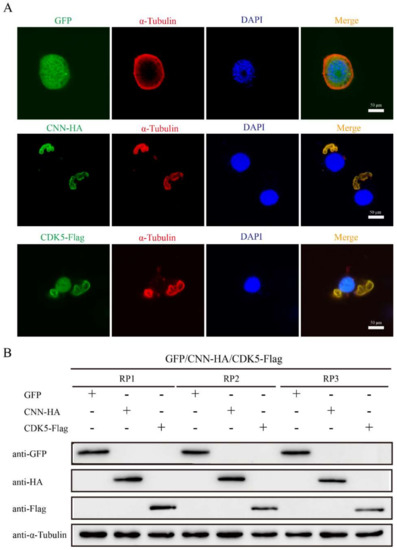
Figure 6.
Effect of BmCDK5-BmCNN complex on cytoskeletal morphology. (A) IF analysis of cytoskeletal changes after overexpressing BmCNN and BmCDK5. Cytoskeleton was labeled using α-Tubulin (red). (B). Western blotting analysis of cytoskeletal changes after overexpressing BmCNN and BmCDK5. Three biological repeats, including RP1, RP2, and RP3.
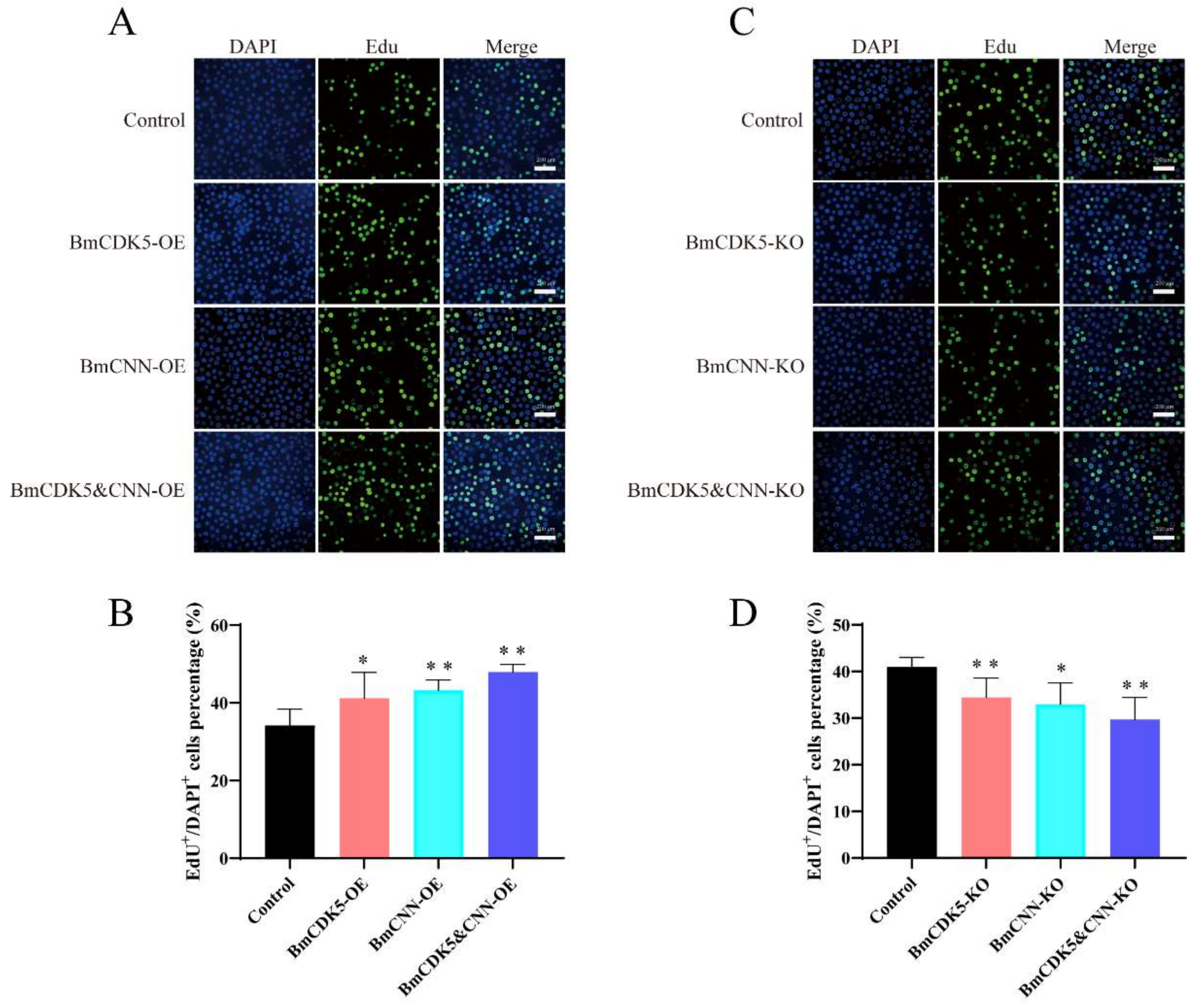
In addition, to determine the effect of DNA replication after BmCDK5-BmCNN complex overexpression, the complex transfected cells were labelled with EdU (Figure 7A,B). The number of EdU-labelling cells was drastically higher in cells overexpressing BmCDK5-BmCNN complex compared to the control. Interestingly, the knockout groups had the opposite effect (Figure 7C,D), which indicates that expression of the BmCDK5-BmCNN complex greatly increased the relative rate of DNA synthesis.
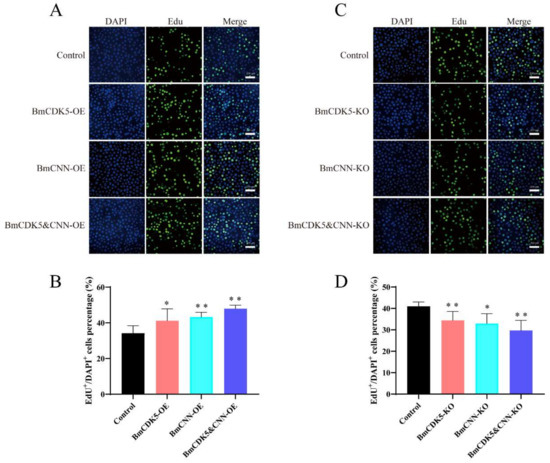
Figure 7.
Cell proliferation regulated by BmCDK5-BmCNN complex in BmNS cells. (A,C) The EdU labeling experiment in BmNS cells. (B,D) Statistical analysis of EdU positive or EdU+ cells (* 0.01 ≤ p < 0.05, ** p < 0.01).
4. Discussion
As a CDKs, CDK5 plays an important role in regulating the progress of cell cycles and cell division [11,33]. However, the regulation mode of CDK5 differs from that of other CDKs, which mainly control cell growth by affecting changes in the cytoskeleton [34]. There are few studies of CDK5 in insects compared to mammals and humans. Studies of CDK5 in B. mori are crucial due to this insect’s economic importance and because it is a model of the order Lepidoptera [35]. Several cyclins and CDK proteins are identified in B. mori, such as cyclin B, cyclin E, CDK1, CDK2, and CDK4 [36,37]; however, little is known about the function of CDK5 in B. mori. In this article, we systematically analyzed the function of the BmCDK5 gene, and demonstrated that BmCDK5 is involved in the cell cycle of the silkworm through interaction with BmCNN to regulate cytoskeleton structure.
CDK5 gradually became a popular research topic amongst scholars due to its role in the regulation of the cell cycle and its regulatory mechanism, which are different from those of other CDKs [7,8]. In the past, it was reported that CDK5 had no function in regulating the cell cycle [9,10]. Further studies of CDK5 and its regulatory proteins were carried out and the results revealed protein interactions between CDK5 and CyclinD1, D3, and Cyclin l. These combinations activated the protein kinase and were indirectly involved in cell cycle regulation [38,39]. In our study, overexpression of the BmCDK5 gene accelerates the process of the mitotic period (M) of the cell cycle and promotes cell proliferation. Knocking out the BmCDK5 gene inhibited cell proliferation. Our results proved that BmCDK5 plays an important role in regulating the cell cycle and promotes cell proliferation in BmNS cells.
Further to this, we analyzed the expression of BmCDK5 in different cell cycle phases, and found that BmCDK5 was highly expressed in S phase. CDK5 expression and its influence on the cell cycle were analyzed comprehensively. It is inferred that CDK5 is highly expressed and accumulated in the S phase, which accelerates the process of the M phase and promotes the cell cycle. Analysis of CDK5 expression in various tissues showed that BmCDK5 was expressed in all larval tissues, with the highest expression level in the gonads. CDK5 was highly expressed in the third day of fourth instar larvae, in the early and after wandering stages of the growth period. As a cyclin-dependent kinase, BmCDK5 was highly expressed in silkworm testes and ovaries, which indicates that BmCDK5 is particularly necessary for cell proliferation of the reproductive organs.
Previous studies proved that CDK5 plays an important role in neurite outgrowth, neuronal migration, and neuronal cell survival [40,41]. CDK5 contributes to the migration of the developing central nervous system, the cytoskeleton structure, and organization of the nerve cell [13]. CDK5 plays an important role in cytoskeletal regulation and is closely associated with tubulin [13,19,20]. Moreover, microtubules are highly dynamic polymers that form a major filament system of the cytoskeleton in eukaryotic cells [22]. Centrosomin (CNN) is a core component in mitotic centrosomes during syncytial development, which is abundantly distributed in the PCM [25,26]. We identified a protein called BmCNN that can interact with BmCDK5, and follows the same trends as BmCDK5 both in the cell cycle phase and in the space-time expression pattern of B. mori. BmCDK5 and BmCNN can regulate cell proliferation, and affect cytoskeleton morphology, but they do not induce changes in the quantity of cytoskeleton protein expression.
5. Conclusions
In conclusion, we identified a homologous gene of the Cyclin-dependent kinase family, BmCDK5, in the silkworm, B. mori. We verified BmCDK5 expression patterns at different developmental stages and in several tissues. Additionally, we determined the expression patterns of BmCDK5 in different phases of the BmNS cell cycle. Moreover, we obtained evidence for the interaction between BmCDK5 and BmCNN. Based on our results, it is speculated that BmCDK5 interacts with BmCNN to regulate cell proliferation by affecting microtubule morphology in BmNS cells. Our findings provide insight into the relationship between the cell cycle functions of the Cyclin-dependent kinase family and cytoskeleton morphology.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/insects13070609/s1, Figure S1: (A) The level of transcription of BmCDK5 and BmCNN in BmCDK5 overexpressing BmNS cells and empty vector control cells was determined using qRT-PCR. The level of transcription of BmCNN in BmCNN overexpressing BmNS cells and empty vector control cells was determined using qRT-PCR (* p < 0.05, ** p < 0.01). (B,C) DNA sequencing analysis of the CRISPR/Cas9 editing target genes (BmCDK5 and BmCNN). The red sequence is the target sequence. WT is the unknocked sample, as a reference. (D) Phylogenetic tree of CNN.; Table S1: The RT-qPCR primer sequences.
Author Contributions
Writing-original draft, Y.W. and X.Z.; methodology, Y.W., X.Z., Z.J., P.C., X.J. and Z.D.; data curation, Y.W., M.P. and C.L.; supervision, P.C., X.J. and Z.D.; funding acquisition, M.P. and C.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Nos. 31872428, 31872427) and the Natural Science Foundation of Chongqing (cstc2021ycjh-bgzxm0191 and cstc2021ycjh-bgzxm0190).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data is contained within the article and supplementary material.
Acknowledgments
We thank Nur Fazleen Binti Idris for polishing this paper’s English.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Malumbres, M. Cyclin-dependent kinases. Genome Biol. 2014, 15, 122. [Google Scholar] [CrossRef] [PubMed]
- Malumbres, M.; Harlow, E.; Hunt, T.; Hunter, T.; Lahti, J.M.; Manning, G.; Morgan, D.O.; Tsai, L.H.; Wolgemuth, D.J. Cyclin-dependent kinases: A family portrait. Nat. Cell Biol. 2009, 11, 1275–1276. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Kaldis, P. Cdks, cyclins and CKIs: Roles beyond cell cycle regulation. Development 2013, 140, 3079–3093. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.; Rossie, S. Tale of the Good and the Bad Cdk5: Remodeling of the Actin Cytoskeleton in the Brain. Mol. Neurobiol. 2017, 55, 3426–3438. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.-L.; Li, B.-S.; Amin, N.D.; Albers, W.; Pant, H.C. A peptide derived from cyclin-dependent kinase activator (p35) specifically inhibits Cdk5 activity and phosphorylation of tau protein in transfected cells. JBIC J. Biol. Inorg. Chem. 2002, 269, 4427–4434. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.-L.; Kesavapany, S.; Gravell, M.; Hamilton, R.S.; Schubert, M.; Amin, N.; Albers, W.; Grant, P.; Pant, H.C. A Cdk5 inhibitory peptide reduces tau hyperphosphorylation and apoptosis in neurons. EMBO J. 2004, 24, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, X.; Lv, P.; Yu, H.; Jiang, X. CDKKnockdown inhibits proliferation and induces apoptosis and Cell Cycle Arrest in Human Glioblastoma. J. Cancer 2021, 12, 3958–3966. [Google Scholar] [CrossRef]
- Zhang, S.; Lu, Z.; Mao, W.; Ahmed, A.A.; Yang, H.; Zhou, J.; Jennings, N.; Rodriguez-Aguayo, C.; Lopez-Berestein, G.; Miranda, R.; et al. CDKRegulates Paclitaxel Sensitivity in Ovarian Cancer Cells by Modulating AKT Activation, p21Cip1- and p27Kip1-Mediated GCell Cycle Arrest and Apoptosis. PLoS ONE 2015, 10, e0131833. [Google Scholar]
- Liu, W.; Li, J.; Song, Y.-S.; Li, Y. The regulation mechanism of CDK5 in cells. J. Shanxi Agric. Sci. 2014, 42, 5. [Google Scholar]
- Li, X.; Mao, Y.; Wang, Z.; Li, Q.; Hu, G. Cdk5: Advances in new tumor targets and inhibitors. Acta Pharm. Sin. 2016, 51, 8. [Google Scholar]
- Dhavan, R.; Tsai, L.H. A decade of CDK5. Nat. Rev. Mol. Cell Biol. 2001, 2, 749–759. [Google Scholar] [CrossRef]
- Feldmann, G.; Mishra, A.; Hong, S.-M.; Bisht, S.; Strock, C.J.; Ball, D.W.; Goggins, M.; Maitra, A.; Nelkin, B.D. Inhibiting the Cyclin-Dependent Kinase CDK5 Blocks Pancreatic Cancer Formation and Progression through the Suppression of Ras-Ral Signaling. Cancer Res. 2010, 70, 4460–4469. [Google Scholar] [CrossRef]
- Ohshima, T.; Ward, J.M.; Huh, C.G.; Longenecker, G.; Veeranna; Pant, H.C.; Brady, R.O.; Martin, L.J.; Kulkarni, A.B. Targeted disruption of the cyclin-dependent kinase 5 gene results in abnormal corticogenesis, neuronal pathology and perinatal death. Proc. Natl. Acad. Sci. USA 1996, 93, 11173–11178. [Google Scholar] [CrossRef]
- Cortés, N.; Guzmán-Martínez, L.; Andrade, V.; González, A.; Maccioni, R.B. CDK5: A Unique CDK and Its Multiple Roles in the Nervous System. J. Alzheimer’s Dis. 2019, 68, 843–855. [Google Scholar] [CrossRef]
- Cheung, Z.H.; Ip, N.Y. Cdk5: A multifaceted kinase in neurodegenerative diseases. Trends Cell Biol. 2012, 22, 169–175. [Google Scholar] [CrossRef]
- Shukla, V.; Skuntz, S.; Pant, H.C. Deregulated Cdk5 Activity Is Involved in Inducing Alzheimer’s Disease. Arch. Med Res. 2012, 43, 655–662. [Google Scholar] [CrossRef]
- Meyer, D.A.; Torres-Altoro, M.I.; Tan, Z.; Tozzi, A.; Di Filippo, M.; DiNapoli, V.; Plattner, F.; Kansy, J.W.; Benkovic, S.A.; Huber, J.D.; et al. Ischemic Stroke Injury Is Mediated by Aberrant Cdk5. J. Neurosci. 2014, 34, 8259–8267. [Google Scholar] [CrossRef]
- Reinhardt, L.; Kordes, S.; Reinhardt, P.; Glatza, M.; Baumann, M.; Drexler, H.C.A.; Menninger, S.; Zischinsky, G.; Eickhoff, J.; Frob, C.; et al. Dual Inhibition of GSK3beta and CDKProtects the Cytoskeleton of Neurons from Neuroinflammatory-Mediated Degeneration In Vitro and In Vivo. Stem Cell Rep. 2019, 12, 502–517. [Google Scholar] [CrossRef]
- Nandi, N.; Tyra, L.K.; Stenesen, D.; Krämer, H. Stress-induced Cdk5 activity enhances cytoprotective basal autophagy in Drosophila melanogaster by phosphorylating acinus at serine437. eLife 2017, 6, e30760. [Google Scholar] [CrossRef]
- Smith-Trunova, S.; Prithviraj, R.; Spurrier, J.; Kuzina, I.; Gu, Q.; Giniger, E. Cdk5 regulates developmental remodeling of mushroom body neurons in Drosophila. Dev. Dyn. 2015, 244, 1550–1563. [Google Scholar] [CrossRef]
- Kapitein, L.C.; Hoogenraad, C.C. Building the neuronal microtubule cytoskeleton. Neuron 2015, 87, 492–506. [Google Scholar] [CrossRef]
- Kellogg, D.R.; Moritz, M.; Alberts, B.M. The centrosome and cellular organization. Annu. Rev. Biochem. 1994, 63, 639–674. [Google Scholar] [CrossRef]
- Eisman, R.C.; Phelps, M.A.S.; Kaufman, T.C. Centrosomin: A Complex Mix of Long and Short Isoforms Is Required for Centrosome Function During Early Development in Drosophila melanogaster. Genetics 2009, 182, 979–997. [Google Scholar] [CrossRef]
- Mack, G.J.; Ou, Y.; Rattner, J.B. Integrating centrosome structure with protein composition and function in animal cells. Microsc. Res. Tech. 2000, 49, 409–419. [Google Scholar] [CrossRef]
- Lange, B.M.H.; Kirfel, G.; Gestmann, I.; Herzog, V.; González, C. Structure and microtubule-nucleation activity of isolated Drosophila embryo centrosomes characterized by whole mount scanning and transmission electron microscopy. Histochem. Cell Biol. 2005, 124, 325–334. [Google Scholar] [CrossRef][Green Version]
- Lange, B.M.; Bachi, A.; Wilm, M.; Gonzalez, C. Hsp90 is a core centrosomal component and is required at different stages of the centrosome cycle in Drosophila and vertebrates. EMBO J. 2000, 19, 1252–1262. [Google Scholar] [CrossRef]
- Eisman, R.C.; Phelps, M.A.S.; Kaufman, T.C. An Amino-Terminal Polo Kinase Interaction Motif Acts in the Regulation of Centrosome Formation and Reveals a Novel Function for centrosomin (cnn) in Drosophila. Genetics 2015, 201, 685–706. [Google Scholar] [CrossRef][Green Version]
- Chen, J.V.; Buchwalter, R.A.; Kao, L.-R.; Megraw, T.L. A Splice Variant of Centrosomin Converts Mitochondria to Microtubule-Organizing Centers. Curr. Biol. 2017, 27, 1928–1940.e6. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Tong, X.; Zeng, J.; Meng, G.; Sun, F.; Hu, H.; Song, J.; Lu, C.; Dai, F. Hippo pathway regulates somatic development and cell proliferation of silkworm. Genomics 2019, 111, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.-H.; Wu, Y.-F.; Dong, X.-L.; Pan, C.-X.; Du, G.-Y.; Yang, J.-G.; Wang, W.; Bao, X.-Y.; Chen, P.; Pan, M.-H.; et al. Identification and characterization of the BmCyclin L1-BmCDK11A/B complex in relation to cell cycle regulation. Cell Cycle 2017, 16, 861–868. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wei, Y.; Zhou, X.-L.; Liu, T.-H.; Chen, P.; Jiang, X.; Dong, Z.-Q.; Pan, M.-H.; Lu, C. A Matrix Metalloproteinase Mediates Tracheal Development in Bombyx mori. Int. J. Mol. Sci. 2021, 22, 5618. [Google Scholar] [CrossRef]
- Jiang, X.; Zhou, X.L.; Cheng, P.; Wei, Y.; Pan, M.H.; Lu, C. Identification and expression analysis of centrosome protein BmCNN gene in Bombyx mori. Sericult. Sci. 2022, 48, 0103–0110. [Google Scholar]
- Pozo, K.; Bibb, J.A. The Emerging Role of Cdk5 in Cancer. Trends Cancer 2016, 2, 606–618. [Google Scholar] [CrossRef]
- Dhariwala, F.A.; Rajadhyaksha, M.S. An Unusual Member of the Cdk Family: Cdk5. Cell. Mol. Neurobiol. 2008, 28, 351–369. [Google Scholar] [CrossRef]
- Biology Analysis Group; Xia, Q.Y.; Zhou, Z.Y.; Lu, C.; Cheng, D.; Dai, F.-Y.; Liu, B.; Zhao, P.; Zha, X.; Cheng, T.; et al. A Draft Sequence for the Genome of the Domesticated Silkworm (Bombyx mori). Science 2004, 306, 1937–1940. [Google Scholar]
- Zhou, X.-L.; Wei, Y.; Chen, X.-Y.; Chen, P.; Tang, X.-F.; Zhang, Q.; Dong, Z.-Q.; Pan, M.-H.; Lu, C. BmGeminin2 interacts with BmRRS1 and regulates Bombyx mori cell proliferation. Cell Cycle 2019, 18, 1498–1512. [Google Scholar] [CrossRef]
- Zhou, X.-L. Study on the Mechanism of BmZFP67 in Regulating Mitosis—Mitosis Transition of Silk Gland. Master’s Thesis, Southwest University, Chongqing, China, 2019. [Google Scholar]
- Contreras-Vallejos, E.; Utreras, E.; Gonzalez-Billault, C. Going out of the brain: Non-nervous system physiological and pathological functions of Cdk5. Cell Signal 2012, 24, 44–52. [Google Scholar] [CrossRef]
- Brinkkoetter, P.T.; Olivier, P.; Wu, J.S.; Henderson, S.; Krofft, R.D.; Pippin, J.W.; Hockenbery, D.; Roberts, J.M.; Shankland, S.J. Cyclin I activates Cdk5 and regulates expression of Bcl-2 and Bcl-XL in postmitotic mouse cells. J. Clin. Investig. 2009, 119, 3089–3101. [Google Scholar] [CrossRef]
- Goodwin, P.R.; Sasaki, J.M.; Juo, P. Cyclin-Dependent Kinase 5 Regulates the Polarized Trafficking of Neuropeptide-Containing Dense-Core Vesicles in Caenorhabditis elegans Motor Neurons. J. Neurosci. 2012, 32, 8158–8172. [Google Scholar] [CrossRef]
- Zhang, P.; Yu, P.-C.; Tsang, A.H.K.; Chen, Y.; Fu, A.K.Y.; Fu, W.-Y.; Chung, K.K.; Ip, N.Y. S-Nitrosylation of Cyclin-Dependent Kinase 5 (Cdk5) Regulates Its Kinase Activity and Dendrite Growth During Neuronal Development. J. Neurosci. 2010, 30, 14366–14370. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).