Particularities of the Hermetia illucens (L.) (Diptera: Stratiomyidae) Ovipositing Behavior: Practical Applications
Abstract
:Simple Summary
Abstract
1. Introduction
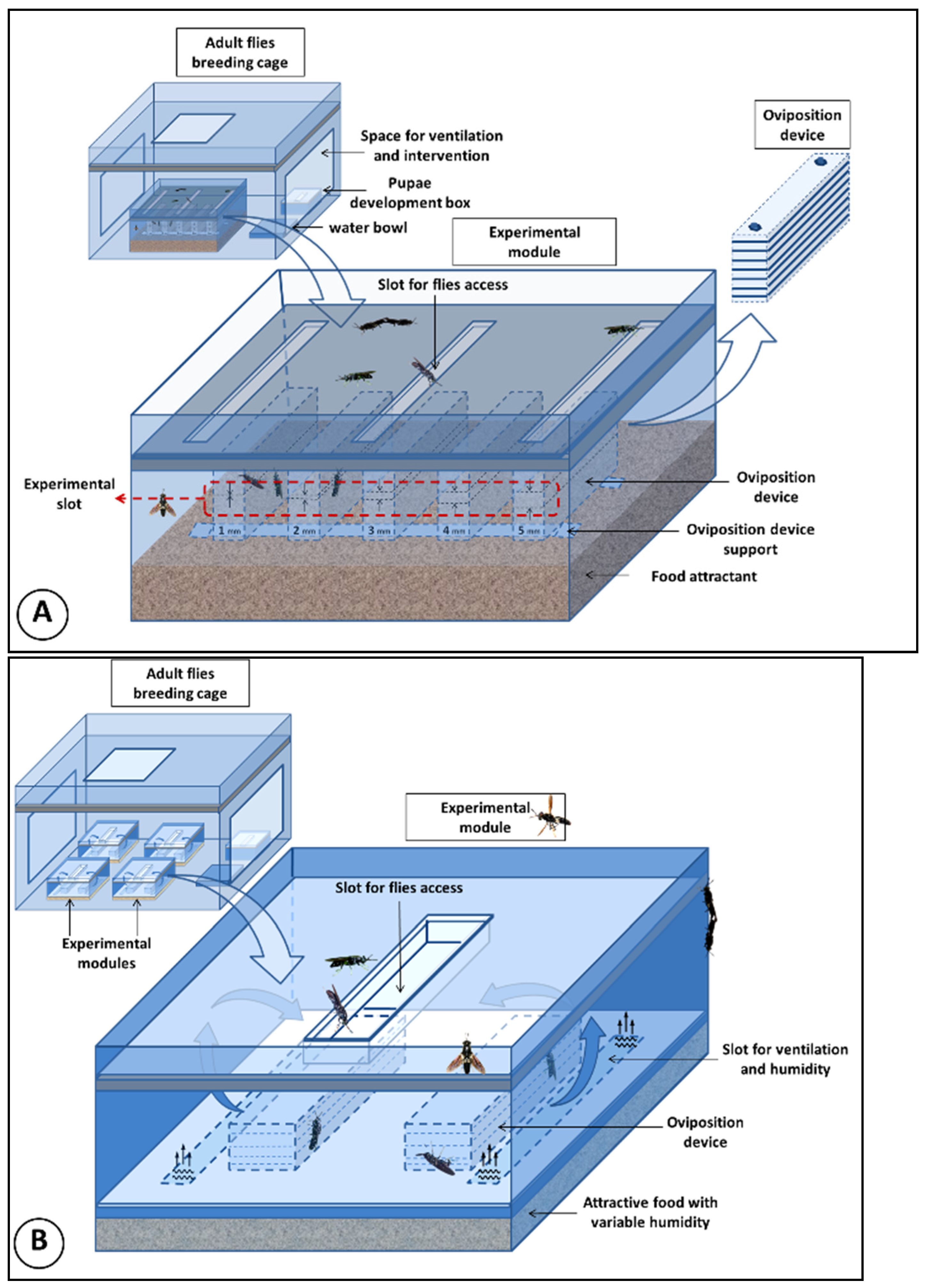
2. Materials and Methods
Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van Bavel, J. The world population explosion: Causes, backgrounds and projections for the future. Facts Views Vis. ObGyn 2013, 5, 281. [Google Scholar]
- Bongaarts, J. Development: Slow down population growth. Nature 2016, 530, 409–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lidicker, W.Z., Jr. A Scientist’s Warning to humanity on human population growth. Glob. Ecol. Conserv. 2020, 24, e01232. [Google Scholar] [CrossRef] [PubMed]
- Ganivet, E. Growth in human population and consumption both need to be addressed to reach an ecologically sustainable future. Environ. Dev. Sustain. 2020, 22, 4979–4998. [Google Scholar] [CrossRef]
- Lahane, S.; Kant, R. Investigating the sustainable development goals derived due to adoption of circular economy practices. Waste Manag. 2022, 143, 1–14. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2016. Contributing to Food Security and Nutrition for All; FAO: Rome, Italy, 2016. [Google Scholar]
- FAO. The State of World Fisheries and Aquaculture. Meeting the Sustainable Development Goals; FAO: Rome, Italy, 2018. [Google Scholar]
- FAO. The State of World Fisheries and Aquaculture 2020. Sustainability in Action; FAO: Rome, Italy, 2020. [Google Scholar]
- OECD; FAO. OECD-FAO Agricultural Outlook 2021–2030; OECD Publishing: Paris, France, 2021. [Google Scholar]
- Meneguz, M.; Schiavone, A.; Gai, F.; Dama, A.; Lussiana, C.; Renna, M.; Gasco, L. Effect of rearing substrate on growth performance, waste reduction efficiency and chemical composition of black soldier fly (Hermetia illucens) larvae. J. Sci. Food Agric. 2018, 98, 5776–5784. [Google Scholar] [CrossRef]
- Oonincx, D.; Van Huis, A.; Van Loon, J. Nutrient utilisation by black soldier flies fed with chicken, pig, or cow manure. J. Insects Food Feed 2015, 1, 131–139. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, S.; Sun, S.; Wu, M.; Bao, Y.; Tong, H.; Ren, M.; Jin, N.; Xu, J.; Zhou, H. Effects of different nitrogen sources and ratios to carbon on larval development and bioconversion efficiency in food waste treatment by black soldier fly larvae (Hermetia illucens). Insects 2021, 12, 507. [Google Scholar] [CrossRef]
- Magee, K.; Halstead, J.; Small, R.; Young, I. Valorisation of organic waste by-products using black soldier Fly (Hermetia illucens) as a bio-convertor. Sustainability 2021, 13, 8345. [Google Scholar] [CrossRef]
- Mahmood, S.; Zurbrügg, C.; Tabinda, A.B.; Ali, A.; Ashraf, A. Sustainable waste management at household level with black soldier fly larvae (Hermetia illucens). Sustainability 2021, 13, 9722. [Google Scholar] [CrossRef]
- Ceccotti, C.; Bruno, D.; Tettamanti, G.; Branduardi, P.; Bertacchi, S.; Labra, M.; Rimoldi, S.; Terova, G. New value from food and industrial wastes–Bioaccumulation of omega-3 fatty acids from an oleaginous microbial biomass paired with a brewery by-product using black soldier fly (Hermetia illucens) larvae. Waste Manag. 2022, 143, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Amrul, N.F.; Kabir Ahmad, I.; Ahmad Basri, N.E.; Suja, F.; Abdul Jalil, N.A.; Azman, N.A. A Review of organic waste treatment using black soldier fly (Hermetia illucens). Sustainability 2022, 14, 4565. [Google Scholar] [CrossRef]
- Kuan, Z.-J.; Chan, B.K.-N.; Gan, S.K.-E. Worming the circular economy for biowaste and plastics: Hermetia illucens, Tenebrio molitor, and Zophobas morio. Sustainability 2022, 14, 1594. [Google Scholar] [CrossRef]
- Kroeckel, S.; Harjes, A.-G.; Roth, I.; Katz, H.; Wuertz, S.; Susenbeth, A.; Schulz, C. When a turbot catches a fly: Evaluation of a pre-pupae meal of the Black Soldier Fly (Hermetia illucens) as fish meal substitute—Growth performance and chitin degradation in juvenile turbot (Psetta maxima). Aquaculture 2012, 364, 345–352. [Google Scholar] [CrossRef]
- De Marco, M.; Martínez, S.; Hernandez, F.; Madrid, J.; Gai, F.; Rotolo, L.; Belforti, M.; Bergero, D.; Katz, H.; Dabbou, S. Nutritional value of two insect larval meals (Tenebrio molitor and Hermetia illucens) for broiler chickens: Apparent nutrient digestibility, apparent ileal amino acid digestibility and apparent metabolizable energy. Anim. Feed Sci. Technol. 2015, 209, 211–218. [Google Scholar] [CrossRef]
- Renna, M.; Schiavone, A.; Gai, F.; Dabbou, S.; Lussiana, C.; Malfatto, V.; Prearo, M.; Capucchio, M.T.; Biasato, I.; Biasibetti, E. Evaluation of the suitability of a partially defatted black soldier fly (Hermetia illucens L.) larvae meal as ingredient for rainbow trout (Oncorhynchus mykiss Walbaum) diets. J. Anim. Sci. Biotechnol. 2017, 8, 57. [Google Scholar] [CrossRef]
- Ido, A.; Ali, M.-F.-Z.; Takahashi, T.; Miura, C.; Miura, T. Growth of Yellowtail (Seriola quinqueradiata) fed on a diet including partially or completely defatted black soldier fly (Hermetia illucens) Larvae Meal. Insects 2021, 12, 722. [Google Scholar] [CrossRef]
- Oteri, M.; Di Rosa, A.R.; Lo Presti, V.; Giarratana, F.; Toscano, G.; Chiofalo, B. Black soldier fly larvae meal as alternative to fish meal for aquaculture feed. Sustainability 2021, 13, 5447. [Google Scholar] [CrossRef]
- Tang, Q.; Xu, E.; Wang, Z.; Xiao, M.; Cao, S.; Hu, S.; Wu, Q.; Xiong, Y.; Jiang, Z.; Wang, F. Dietary Hermetia illucens larvae meal improves growth performance and intestinal barrier function of weaned pigs under the environment of enterotoxigenic Escherichia coli K88. Front. Nutr. 2021, 8, 812011. [Google Scholar] [CrossRef]
- Ipema, A.F.; Gerrits, W.J.; Bokkers, E.A.; Van Marwijk, M.A.; Laurenssen, B.F.; Kemp, B.; Bolhuis, J.E. Assessing the effectiveness of providing live black soldier fly larvae (Hermetia illucens) to ease the weaning transition of piglets. Front. Vet. Sci. 2022, 9, 838018. [Google Scholar] [CrossRef]
- Makkar, H.P.; Tran, G.; Heuzé, V.; Ankers, P. State-of-the-art on use of insects as animal feed. Anim. Feed Sci. Technol. 2014, 197, 1–33. [Google Scholar] [CrossRef]
- Schiavone, A.; Cullere, M.; De Marco, M.; Meneguz, M.; Biasato, I.; Bergagna, S.; Dezzutto, D.; Gai, F.; Dabbou, S.; Gasco, L. Partial or total replacement of soybean oil by black soldier fly larvae (Hermetia illucens L.) fat in broiler diets: Effect on growth performances, feed-choice, blood traits, carcass characteristics and meat quality. Ital. J. Anim. Sci. 2017, 16, 93–100. [Google Scholar] [CrossRef] [Green Version]
- Barragan-Fonseca, K.B.; Dicke, M.; van Loon, J.J. Nutritional value of the black soldier fly (Hermetia illucens L.) and its suitability as animal feed–a review. J. Insects Food Feed 2017, 3, 105–120. [Google Scholar] [CrossRef]
- Borgogno, M.; Dinnella, C.; Iaconisi, V.; Fusi, R.; Scarpaleggia, C.; Schiavone, A.; Monteleone, E.; Gasco, L.; Parisi, G. Inclusion of Hermetia illucens larvae meal on rainbow trout (Oncorhynchus mykiss) feed: Effect on sensory profile according to static and dynamic evaluations. J. Sci. Food Agric. 2017, 97, 3402–3411. [Google Scholar] [CrossRef] [PubMed]
- Murawska, D.; Daszkiewicz, T.; Sobotka, W.; Gesek, M.; Witkowska, D.; Matusevičius, P.; Bakuła, T. Partial and total replacement of soybean meal with full-fat black soldier fly (Hermetia illucens L.) Larvae meal in broiler chicken diets: Impact on growth performance, carcass quality and meat quality. Animals 2021, 11, 2715. [Google Scholar] [CrossRef] [PubMed]
- Navarro del Hierro, J.; Cantero-Bahillo, E.; Fornari, T.; Martin, D. Effect of defatting and extraction solvent on the antioxidant and pancreatic lipase inhibitory activities of extracts from Hermetia illucens and Tenebrio molitor. Insects 2021, 12, 789. [Google Scholar] [CrossRef] [PubMed]
- Cullere, M.; Tasoniero, G.; Giaccone, V.; Miotti-Scapin, R.; Claeys, E.; De Smet, S.; Dalle Zotte, A. Black soldier fly as dietary protein source for broiler quails: Apparent digestibility, excreta microbial load, feed choice, performance, carcass and meat traits. Animal 2016, 10, 1923–1930. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.-S.; Shelomi, M. Review of black soldier fly (Hermetia illucens) as animal feed and human food. Foods 2017, 6, 91. [Google Scholar] [CrossRef] [Green Version]
- Marono, S.; Loponte, R.; Lombardi, P.; Vassalotti, G.; Pero, M.; Russo, F.; Gasco, L.; Parisi, G.; Piccolo, G.; Nizza, S. Productive performance and blood profiles of laying hens fed Hermetia illucens larvae meal as total replacement of soybean meal from 24 to 45 weeks of age. Poult. Sci. 2017, 96, 1783–1790. [Google Scholar] [CrossRef]
- Yamamoto, F.Y.; Suehs, B.A.; Ellis, M.; Bowles, P.R.; Older, C.E.; Hume, M.E.; Bake, G.G.; Cammack, J.A.; Tomberlin, J.K.; Gatlin, D.M., III. Dietary fishmeal replacement by black soldier fly larvae meals affected red drum (Sciaenops ocellatus) production performance and intestinal microbiota depending on what feed substrate the insect larvae were offered. Anim. Feed Sci. Technol. 2022, 283, 115179. [Google Scholar] [CrossRef]
- Drewery, M.; Liu, X.; Wickersham, T. Black soldier fly larvae (BSFL) as a feed for beef cattle: A hedonic pricing model. J. Insects Food Feed 2022, 1–10. [Google Scholar] [CrossRef]
- Pérez-Pacheco, R.; Hinojosa-Garro, D.; Ruíz-Ortíz, F.; Camacho-Chab, J.C.; Ortega-Morales, B.O.; Alonso-Hernández, N.; Fonseca-Muñoz, A.; Landero-Valenzuela, N.; Loeza-Concha, H.J.; Diego-Nava, F. Growth of the black soldier fly Hermetia illucens (Diptera: Stratiomyidae) on organic-waste residues and its application as supplementary diet for Nile Tilapia Oreochromis niloticus (Perciformes: Cichlidae). Insects 2022, 13, 326. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S.; Snoeck, E.; Tello, A.; Alles, M.; Fernando, I.; Saraswati, Y.; Rahayu, T.; Grover, R.; Ullah, M.; Ristow, B. Manipulation of the black soldier fly larvae (Hermetia illucens; Diptera: Stratiomyidae) fatty acid profile through the substrate. J. Insects Food Feed 2022, 1–20. [Google Scholar] [CrossRef]
- Greenwood, M.P.; Hull, K.L.; Brink-Hull, M.; Lloyd, M.; Rhode, C. Feed and host genetics drive microbiome diversity with resultant consequences for production traits in mass-reared black soldier fly (Hermetia illucens) Larvae. Insects 2021, 12, 1082. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, M.K.; Meena, R.; Rahal, A.; Tripathi, P.; Kumar, R.; Chaudhary, U.B.; Gupta, D.L. Chemical composition, in-vitro fermentation and methane production potential of unconventional feed resources in goats. Indian J. Anim. Sci. 2016, 86, 691–695. [Google Scholar]
- Tschirner, M.; Simon, A. Influence of different growing substrates and processing on the nutrient composition of black soldier fly larvae destined for animal feed. J. Insects Food Feed 2015, 1, 249–259. [Google Scholar] [CrossRef]
- Verheyen, G.R.; Ooms, T.; Vogels, L.; Vreysen, S.; Bovy, A.; Van Miert, S.; Meersman, F. Insects as an alternative source for the production of fats for cosmetics. J. Cosmet. Sci 2018, 69, 187–202. [Google Scholar]
- Franco, A.; Salvia, R.; Scieuzo, C.; Schmitt, E.; Russo, A.; Falabella, P. Lipids from insects in cosmetics and for personal care products. Insects 2021, 13, 41. [Google Scholar] [CrossRef]
- Li, Q.; Zheng, L.; Cai, H.; Garza, E.; Yu, Z.; Zhou, S. From organic waste to biodiesel: Black soldier fly, Hermetia illucens, makes it feasible. Fuel 2011, 90, 1545–1548. [Google Scholar] [CrossRef]
- Leong, S.Y.; Kutty, S.R.M.; Malakahmad, A.; Tan, C.K. Feasibility study of biodiesel production using lipids of Hermetia illucens larva fed with organic waste. Waste Manag. 2016, 47, 84–90. [Google Scholar] [CrossRef]
- Surendra, K.; Olivier, R.; Tomberlin, J.K.; Jha, R.; Khanal, S.K. Bioconversion of organic wastes into biodiesel and animal feed via insect farming. Renew. Energy 2016, 98, 197–202. [Google Scholar] [CrossRef] [Green Version]
- Franco, A.; Scieuzo, C.; Salvia, R.; Petrone, A.M.; Tafi, E.; Moretta, A.; Schmitt, E.; Falabella, P. Lipids from Hermetia illucens, an Innovative and sustainable source. Sustainability 2021, 13, 10198. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Bouguet, V.; Spranghers, T.; Vangansbeke, D.; De Clercq, P. Beneficial effect of supplementing an artificial diet for Amblyseius swirskii with Hermetia illucens haemolymph. J. Appl. Entomol. 2015, 139, 342–351. [Google Scholar] [CrossRef]
- Gärttling, D.; Schulz, H. Compilation of black soldier fly frass analyses. J. Soil Sci. Plant Nutr. 2022, 22, 937–943. [Google Scholar] [CrossRef]
- Kawasaki, K.; Kawasaki, T.; Hirayasu, H.; Matsumoto, Y.; Fujitani, Y. Evaluation of fertilizer value of residues obtained after processing household organic waste with black soldier fly larvae (Hermetia illucens). Sustainability 2020, 12, 4920. [Google Scholar] [CrossRef]
- Chia, S.Y.; Tanga, C.M.; Khamis, F.M.; Mohamed, S.A.; Salifu, D.; Sevgan, S.; Fiaboe, K.K.; Niassy, S.; van Loon, J.J.; Dicke, M. Threshold temperatures and thermal requirements of black soldier fly Hermetia illucens: Implications for mass production. PLoS ONE 2018, 13, e0206097. [Google Scholar] [CrossRef] [Green Version]
- Gligorescu, A.; Fischer, C.H.; Larsen, P.F.; Nørgaard, J.V.; Heckman, L.-H.L. Production and optimization of Hermetia illucens (L.) larvae reared on food waste and utilized as feed ingredient. Sustainability 2020, 12, 9864. [Google Scholar] [CrossRef]
- Van, J.C.F.; Tham, P.E.; Lim, H.R.; Khoo, K.S.; Chang, J.-S.; Show, P.L. Integration of Internet-of-Things as sustainable smart farming technology for the rearing of black soldier fly to mitigate food waste. J. Taiwan Inst. Chem. Eng. 2022, 7, 104235. [Google Scholar] [CrossRef]
- Pastor, B.; Velasquez, Y.; Gobbi, P.; Rojo, S. Conversion of organic wastes into fly larval biomass: Bottlenecks and challenges. J. Insects Food Feed 2015, 1, 179–193. [Google Scholar] [CrossRef]
- Bertinetti, C.; Samayoa, A.C.; Hwang, S.-Y. Effects of feeding adults of Hermetia illucens (Diptera: Stratiomyidae) on longevity, oviposition, and egg hatchability: Insights into optimizing egg production. J. Insect Sci. 2019, 19, 19. [Google Scholar] [CrossRef] [Green Version]
- Addeo, N.; Li, C.; Rusch, T.; Dickerson, A.; Tarone, A.; Bovera, F.; Tomberlin, J. Impact of age, size, and sex on adult black soldier fly Hermetia illucens L.(Diptera: Stratiomyidae) thermal preference. J. Insects Food Feed 2022, 8, 129–139. [Google Scholar] [CrossRef]
- Li, C.; Addeo, N.; Rusch, T.; Dickerson, A.; Tarone, A.; Hu, W.; Tomberlin, J. Impact of age, sex, and size on the thermal tolerance of the adult black soldier fly (Diptera: Stratiomyidae). J. Insects Food Feed 2021, 8, 681–691. [Google Scholar] [CrossRef]
- Oonincx, D.; Volk, N.; Diehl, J.; Van Loon, J.; Belušič, G. Photoreceptor spectral sensitivity of the compound eyes of black soldier fly (Hermetia illucens) informing the design of LED-based illumination to enhance indoor reproduction. J. Insect Physiol. 2016, 95, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Park, K.-H.; Han, M.-H.; Lee, S.; Kim, E.-S.; Song, M.-H.; Kim, W.-T.; Choi, J.-Y.; Kim, H.G. Oviposition activity of black soldier fly (Hermetia illucens) under artificial illumination. Int. J. Ind. Entomol. 2017, 35, 100–105. [Google Scholar]
- Heussler, C.D.; Walter, A.; Oberkofler, H.; Insam, H.; Arthofer, W.; Schlick-Steiner, B.C.; Steiner, F.M. Influence of three artificial light sources on oviposition and half-life of the Black Soldier Fly, Hermetia illucens (Diptera: Stratiomyidae): Improving small-scale indoor rearing. PLoS ONE 2018, 13, e0197896. [Google Scholar] [CrossRef] [Green Version]
- Bekker, N.S.; Heidelbach, S.; Vestergaard, S.Z.; Nielsen, M.E.; Riisgaard-Jensen, M.; Zeuner, E.J.; Bahrndorff, S.; Eriksen, N.T. Impact of substrate moisture content on growth and metabolic performance of black soldier fly larvae. Waste Manag. 2021, 127, 73–79. [Google Scholar] [CrossRef]
- Holmes, L.; Vanlaerhoven, S.; Tomberlin, J. Substrate effects on pupation and adult emergence of Hermetia illucens (Diptera: Stratiomyidae). Environ. Entomol. 2013, 42, 370–374. [Google Scholar] [CrossRef] [Green Version]
- Park, K.; Kim, W.; Kim, E.; Kwak, K.-W.; Choi, J.-Y.; Lee, S.; Song, M.; Kim, S.-H. Oviposition site preference in black soldier fly, Hermetia illucens (Diptera: Stratiomyidae), in artificial rearing system. Int. J. Ind. Entomol. 2016, 33, 54–58. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.-C.; Lee, S.-B.; Park, K.-H.; Nam, S.-H.; Kim, J.-G.; Kim, W.-T.; Choi, J.-Y. Artificial Multiplication of the Black Soldier Fly (BSF), Hermetia illucens (Diptera: Stratmyidae) Using the Livestock Feces. J. Sericult. Entomol. Sci. 2012, 50, 63–70. [Google Scholar]
- Boaru, A.; Vig, A.; Ladoşi, D.; Păpuc, T.; Struţi, D.; Georgescu, B. The use of various oviposition structures for the black soldier fly, Hermetia illucens L.(Diptera: Stratiomydae) in improving the reproductive process in captivity. Anim. Biol. Anim. Husb. 2019, 11, 12–20. [Google Scholar]
- Tomberlin, J.K.; Adler, P.H.; Myers, H.M. Development of the black soldier fly (Diptera: Stratiomyidae) in relation to temperature. Environ. Entomol. 2009, 38, 930–934. [Google Scholar] [CrossRef]
- Holmes, L.; VanLaerhoven, S.; Tomberlin, J. Lower temperature threshold of black soldier fly (Diptera: Stratiomyidae) development. J. Insects Food Feed 2016, 2, 255–262. [Google Scholar] [CrossRef]
- Georgescu, B.; Struţi, D.; Păpuc, T.; Ladoşi, D.; Boaru, A. Body weight loss of black soldier fly Hermetia illucens (Diptera: Stratiomyidae) during development in non-feeding stages: Implications for egg clutch parameters. Eur. J. Entomol. 2020, 117, 216–225. [Google Scholar] [CrossRef]
- Lupi, D.; Savoldelli, S.; Leonardi, M.G.; Jucker, C. Feeding in the adult of Hermetia illucens (Diptera Stratiomyidae): Reality or fiction? J. Entomol. Acarol. Res. 2019, 51, 27–32. [Google Scholar] [CrossRef]
- Nakamura, S.; Ichiki, R.T.; Shimoda, M.; Morioka, S. Small-scale rearing of the black soldier fly, Hermetia illucens (Diptera: Stratiomyidae), in the laboratory: Low-cost and year-round rearing. Appl. Entomol. Zool. 2016, 51, 161–166. [Google Scholar] [CrossRef]
- Tomberlin, J.K.; Sheppard, D.C. Factors influencing mating and oviposition of black soldier flies (Diptera: Stratiomyidae) in a colony. J. Entomol. Sci. 2002, 37, 345–352. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, L.; He, J.; Tomberlin, J.K.; Li, J.; Lei, C.; Sun, M.; Liu, Z.; Yu, Z. An artificial light source influences mating and oviposition of black soldier flies, Hermetia illucens. J. Insect Sci. 2010, 10, 202. [Google Scholar] [CrossRef] [Green Version]
- Scieuzo, C.; Nardiello, M.; Farina, D.; Scala, A.; Cammack, J.A.; Tomberlin, J.K.; Vogel, H.; Salvia, R.; Persaud, K.; Falabella, P. Hermetia illucens (L.)(Diptera: Stratiomyidae) odorant binding proteins and their interactions with selected volatile organic compounds: An in silico approach. Insects 2021, 12, 814. [Google Scholar] [CrossRef]
- Julita, U.; Fitri, L.L.; Putra, R.E.; Permana, A.D. Research article ovitrap preference in the black soldier fly, Hermetia illucens (L.)(Diptera: Stratiomyidae). J. Biol. Sci 2021, 24, 562–570. [Google Scholar]
- Holmes, L.; Vanlaerhoven, S.; Tomberlin, J. Relative humidity effects on the life history of Hermetia illucens (Diptera: Stratiomyidae). Environ. Entomol. 2012, 41, 971–978. [Google Scholar] [CrossRef] [Green Version]
- Gougbedji, A.; Agbohessou, P.; Lalèyè, P.A.; Francis, F.; Megido, R.C. Technical basis for the small-scale production of black soldier fly, Hermetia illucens (L. 1758), meal as fish feed in Benin. J. Agric. Food Res. 2021, 4, 100153. [Google Scholar] [CrossRef]
- Hoc, B.; Noël, G.; Carpentier, J.; Francis, F.; Caparros Megido, R. Optimization of black soldier fly (Hermetia illucens) artificial reproduction. PLoS ONE 2019, 14, e0216160. [Google Scholar] [CrossRef] [Green Version]
- Klüber, P.; Bakonyi, D.; Zorn, H.; Rühl, M. Does light color temperature influence aspects of oviposition by the black soldier fly (Diptera: Stratiomyidae)? J. Econ. Entomol. 2020, 113, 2549–2552. [Google Scholar] [CrossRef] [PubMed]
- Booth, D.C.; Sheppard, C. Oviposition of the black soldier fly, Hermetia illucens (Diptera: Stratiomyidae): Eggs, masses, timing, and site characteristics. Environ. Entomol. 1984, 13, 421–423. [Google Scholar] [CrossRef]
- Hogsette, J.A. New diets for production of house flies and stable flies (Diptera: Muscidae) in the laboratory. J. Econ. Entomol. 1992, 85, 2291–2294. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Najar-Rodriguez, A.J.; Minor, M.A.; Hedderley, D.I.; Morel, P.C. Mating success of the black soldier fly, Hermetia illucens (Diptera: Stratiomyidae), under four artificial light sources. J. Photochem. Photobiol. B Biol. 2020, 205, 111815. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.; Tomberlin, J. Effects of adult body size on mating success of the black soldier fly, Hermetia illucens (L.)(Diptera: Stratiomyidae). J. Insects Food Feed 2021, 7, 5–20. [Google Scholar] [CrossRef]
- Jucker, C.; Erba, D.; Leonardi, M.G.; Lupi, D.; Savoldelli, S. Assessment of vegetable and fruit substrates as potential rearing media for Hermetia illucens (Diptera: Stratiomyidae) larvae. Environ. Entomol. 2017, 46, 1415–1423. [Google Scholar] [CrossRef]
| Specification | Distance from the Substrate | TOTAL | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 4 mm | 8 mm | 12 mm | 16 mm | 20 mm | 24 mm | 28 mm | Total Clutches (N = 140) | Clutch Weight (mg) | ||
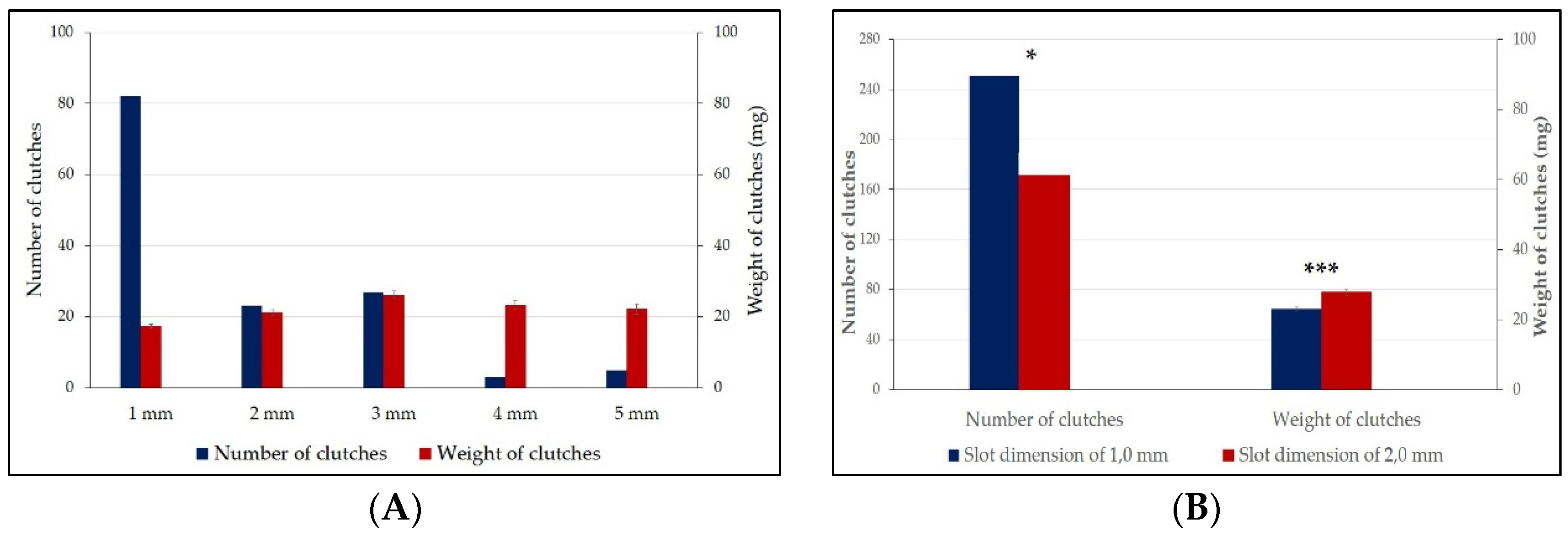
| Slot dimensions | 1 mm | 18 | 23 | 2 | 0 | 17 | 5 | 13 | 82 a | 17.47 ± 0.55 a |
| 2 mm | 10 | 0 | 0 | 4 | 1 | 0 | 6 | 23 b | 21.16 ± 0.83 b | |
| 3 mm | 0 | 0 | 7 | 17 | 0 | 3 | 0 | 27 b | 26.16 ± 1.04 c | |
| 4 mm | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 3 b | 23.27 ± 1.13 b | |
| 5 mm | 1 | 0 | 2 | 1 | 0 | 1 | 0 | 5 b | 22.17 ± 1.42 b | |
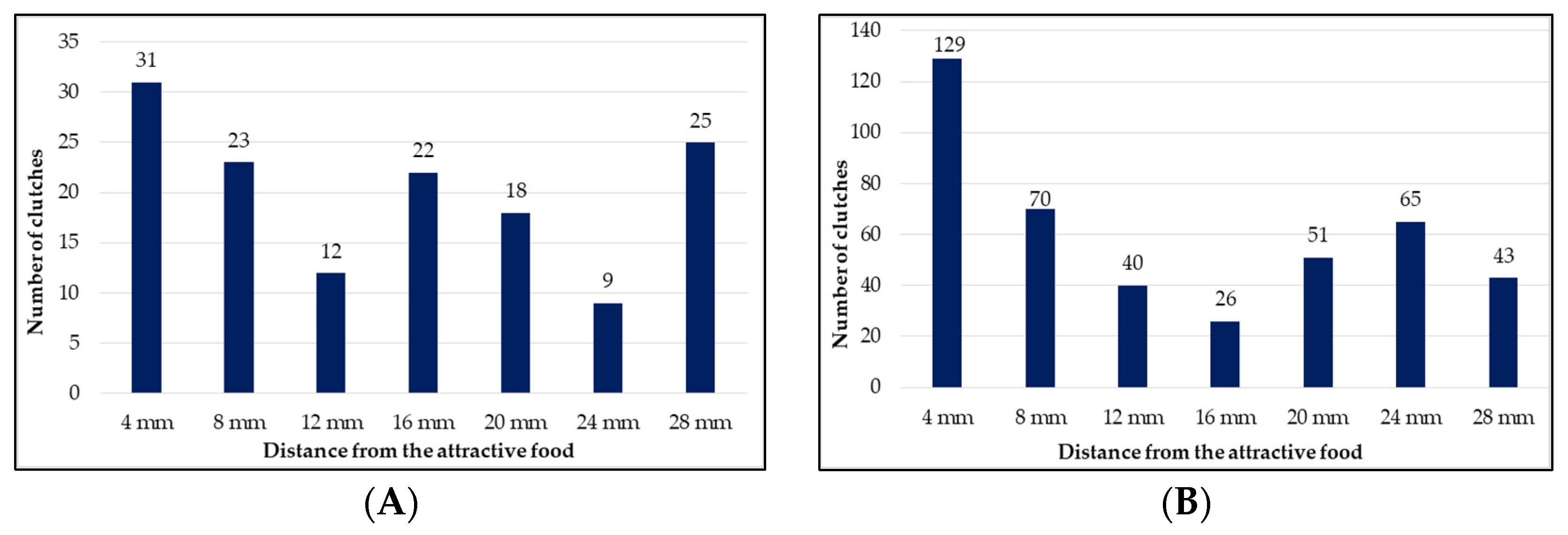
| Total clutches (N = 140) | 31 a | 23 a | 12 b | 22 a | 18 a | 9 b | 25 a | Mann–Whitney | F = 18.03 p = 0.001 | |
| Clutch weight (mg) | 18.74 ± 0.88 a | 16.73 ± 0.99 a | 25.73 ± 2.03 b | 24.18 ± 1.26 b | 17.83 ± 1.22 a | 22.83 ± 1.55 b | 19.78 ± 0.97 a | F = 7.145 p = 0.001 | one-way ANOVA | |
| Specification | Distance from the Substrate | Total | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 mm | 8 mm | 12 mm | 16 mm | 20 mm | 24 mm | 28 mm | Ʃ Clutches | Ʃ 1 + 2 mm Mann–Whitney | ||||
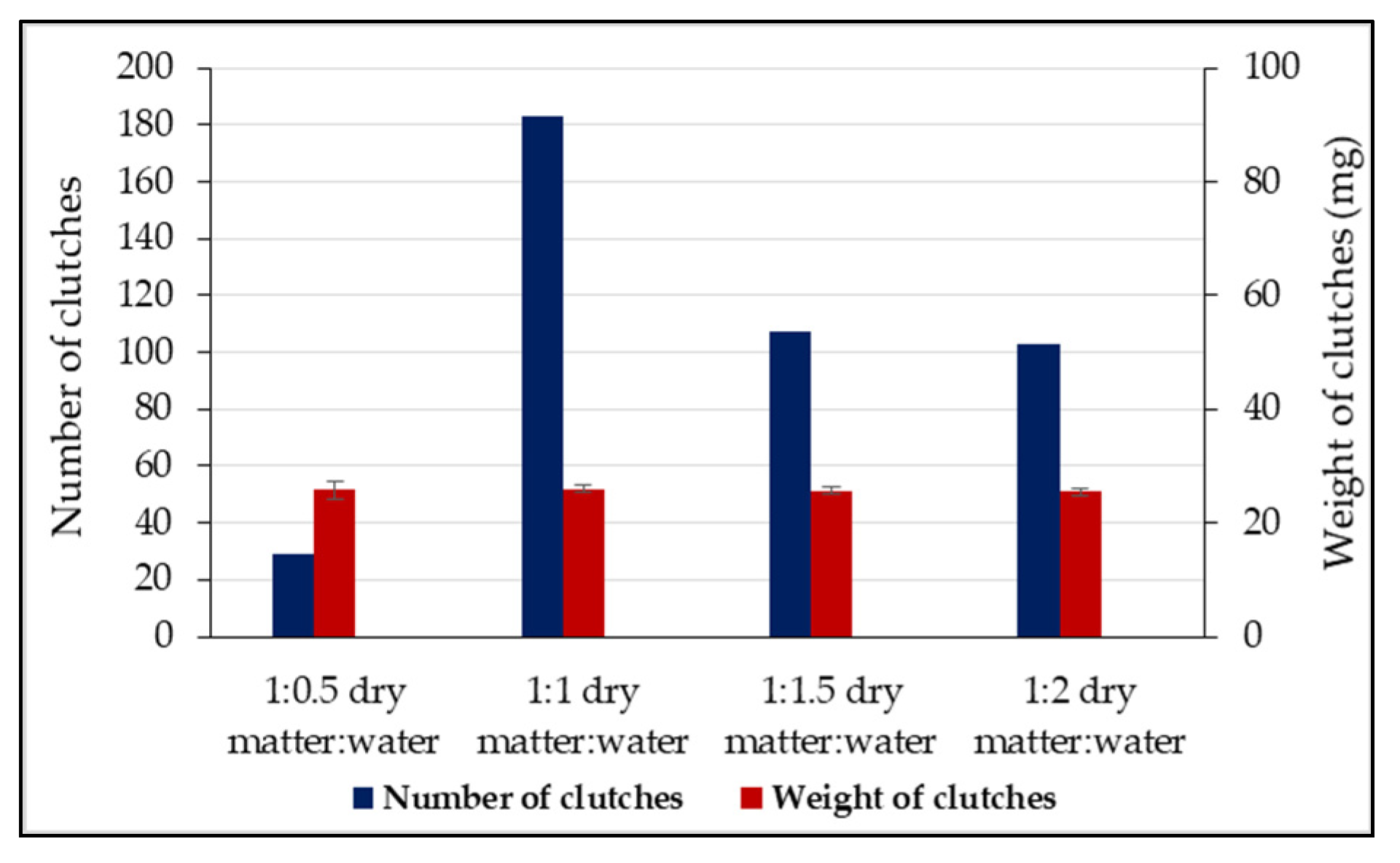
| Ratio dry matter:water | 1:0.5 | Slot | 1 mm | 9 | 1 | 1 | - | 3 | 3 | 0 | 17 | 31 a |
| 2 mm | 6 | 2 | 0 | 3 | 2 | 0 | 1 | 14 | ||||
| 1:1 | Slot | 1 mm | 35 | 30 | 22 | 0 | 12 | 11 | 13 | 123 | 183 b | |
| 2 mm | 14 | 7 | 0 | 16 | 0 | 16 | 7 | 60 | ||||
| 1:1.5 | Slot | 1 mm | 16 | 12 | 9 | 0 | 12 | 15 | 0 | 64 | 107 c | |
| 2 mm | 17 | 3 | 1 | 2 | 0 | 7 | 13 | 43 | ||||
| 1:2 | Slot | 1 mm | 15 | 3 | 7 | 2 | 7 | 13 | 0 | 47 | 103 c | |
| 2 mm | 17 | 12 | 0 | 3 | 15 | 0 | 9 | 56 | ||||
| Total clutches Mann–Whitney | 1 mm | 75 a | 46 ab | 39 ab | 2 c | 34 ab | 42 ab | 13 b | 251 | 424 | ||
| 2 mm | 54 a | 24 ab | 1 c | 24 ab | 17 ab | 23 ab | 30 ab | 173 | ||||
| Ʃ1 + 2 mm | 129 a | 70 ab | 40 b | 26 b | 51 b | 65 ab | 43 b | - | ||||
| Specification | Clutch Number | Clutch Weight (mg) | The Average Clutch Weight According to the Distance from the Substrate | One-Way ANOVA F = 0.090 p-Value 0.966 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 mm | 8 mm | 12 mm | 16 mm | 20 mm | 24 mm | 28 mm | ||||||
| Substrate (DM)/water | 1:0.5 | 1 mm | 17 | 21.06 ± 1.88 | 22.96 | 26.47 | 23.10 | 28.27 | 24.65 | 24.57 | 30.30 | 25.76 ± 1.69 |
| 2 mm | 14 | 30.79 ± 2.22 | ||||||||||
| 1:1 | 1 mm | 123 | 24.72 ± 0.84 | 25.92 | 24.95 | 25.53 | 30.09 | 22.17 | 23.03 | 29.76 | 25.92 ± 0.66 | |
| 2 mm | 60 | 28.05 ± 1.01 | ||||||||||
| 1:1.5 | 1 mm | 64 | 24.36 ± 0.70 | 23.64 | 24.33 | 23.83 | 26.59 | 25.10 | 26.45 | 29.12 | 25.58 ± 0.62 | |
| 2 mm | 43 | 27.40 ± 1.0 | ||||||||||
| 1:2 | 1 mm | 47 | 22.08 ± 0.72 | 25.64 | 25.25 | 22.09 | 24.36 | 25.33 | 23.15 | 32.48 | 25.47 ± 0.65 | |
| 2 mm | 56 | 28.31 ± 0.87 | ||||||||||
| Clutch weight (mg) one-way ANOVA: F = 5.88 p = 0.001 | 24.54 ± 6.25 a | 25.25 ± 6.35 ab | 23.64 ± 6.27 a | 27.33 ± 7.52 b | 24.31 ± 6.96 a | 24.30 ± 6.57 a | 30.41 ± 8.37 c | 25.68 mg | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bogdan, G.; Ioan, S.D.; Mihai, Ș.; Elena, M.L.; Vasile, M.D.; Mihaela, B.A. Particularities of the Hermetia illucens (L.) (Diptera: Stratiomyidae) Ovipositing Behavior: Practical Applications. Insects 2022, 13, 611. https://doi.org/10.3390/insects13070611
Bogdan G, Ioan SD, Mihai Ș, Elena ML, Vasile MD, Mihaela BA. Particularities of the Hermetia illucens (L.) (Diptera: Stratiomyidae) Ovipositing Behavior: Practical Applications. Insects. 2022; 13(7):611. https://doi.org/10.3390/insects13070611
Chicago/Turabian StyleBogdan, Georgescu, Struți Dănuț Ioan, Șuteu Mihai, Moldovan Lavinia Elena, Moldovan Dorin Vasile, and Boaru Anca Mihaela. 2022. "Particularities of the Hermetia illucens (L.) (Diptera: Stratiomyidae) Ovipositing Behavior: Practical Applications" Insects 13, no. 7: 611. https://doi.org/10.3390/insects13070611
APA StyleBogdan, G., Ioan, S. D., Mihai, Ș., Elena, M. L., Vasile, M. D., & Mihaela, B. A. (2022). Particularities of the Hermetia illucens (L.) (Diptera: Stratiomyidae) Ovipositing Behavior: Practical Applications. Insects, 13(7), 611. https://doi.org/10.3390/insects13070611






