Vegetation Height as the Primary Driver of Functional Changes in Orthopteran Assemblages in a Roadside Habitat
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
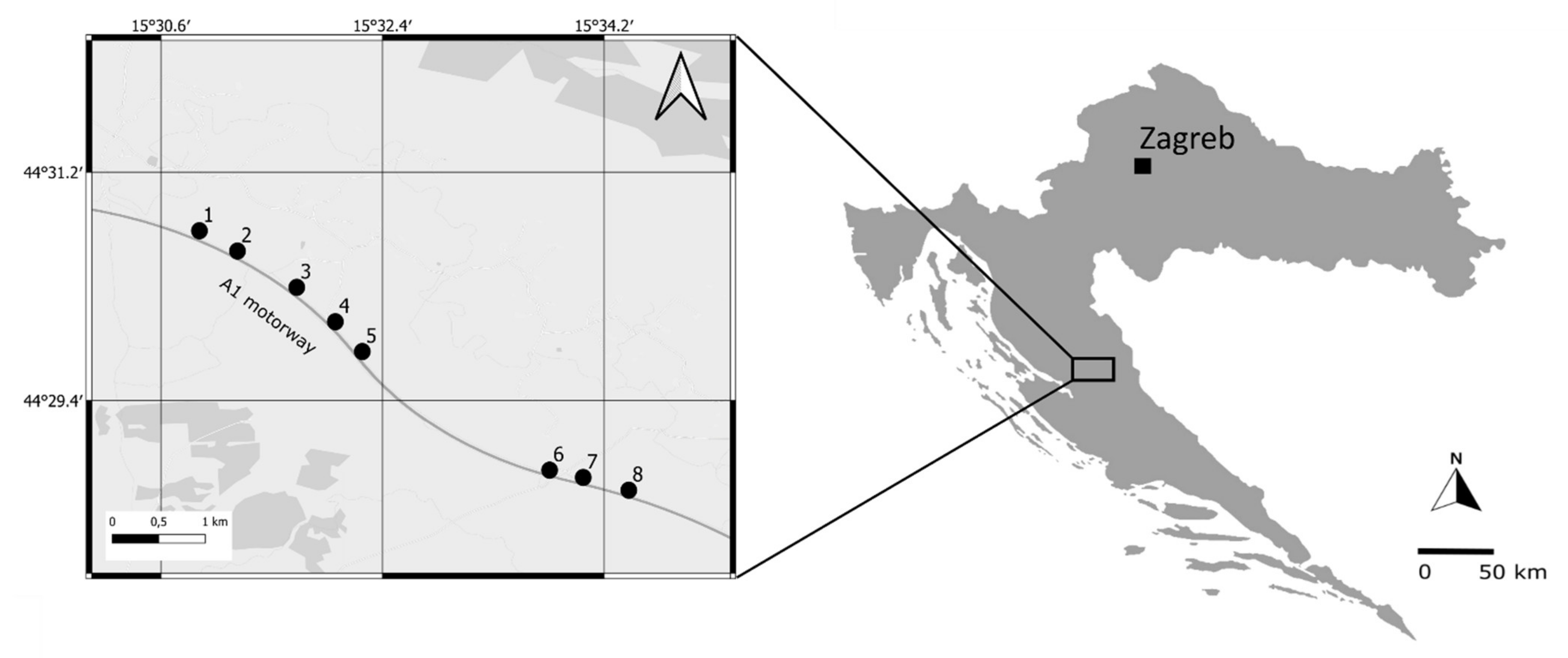
2.1. Study Area and Sampling Design
2.2. Orthoptera Sampling and Identification
2.3. Environmental Measurements
2.4. Data Analysis
3. Results
3.1. Environmental Factors
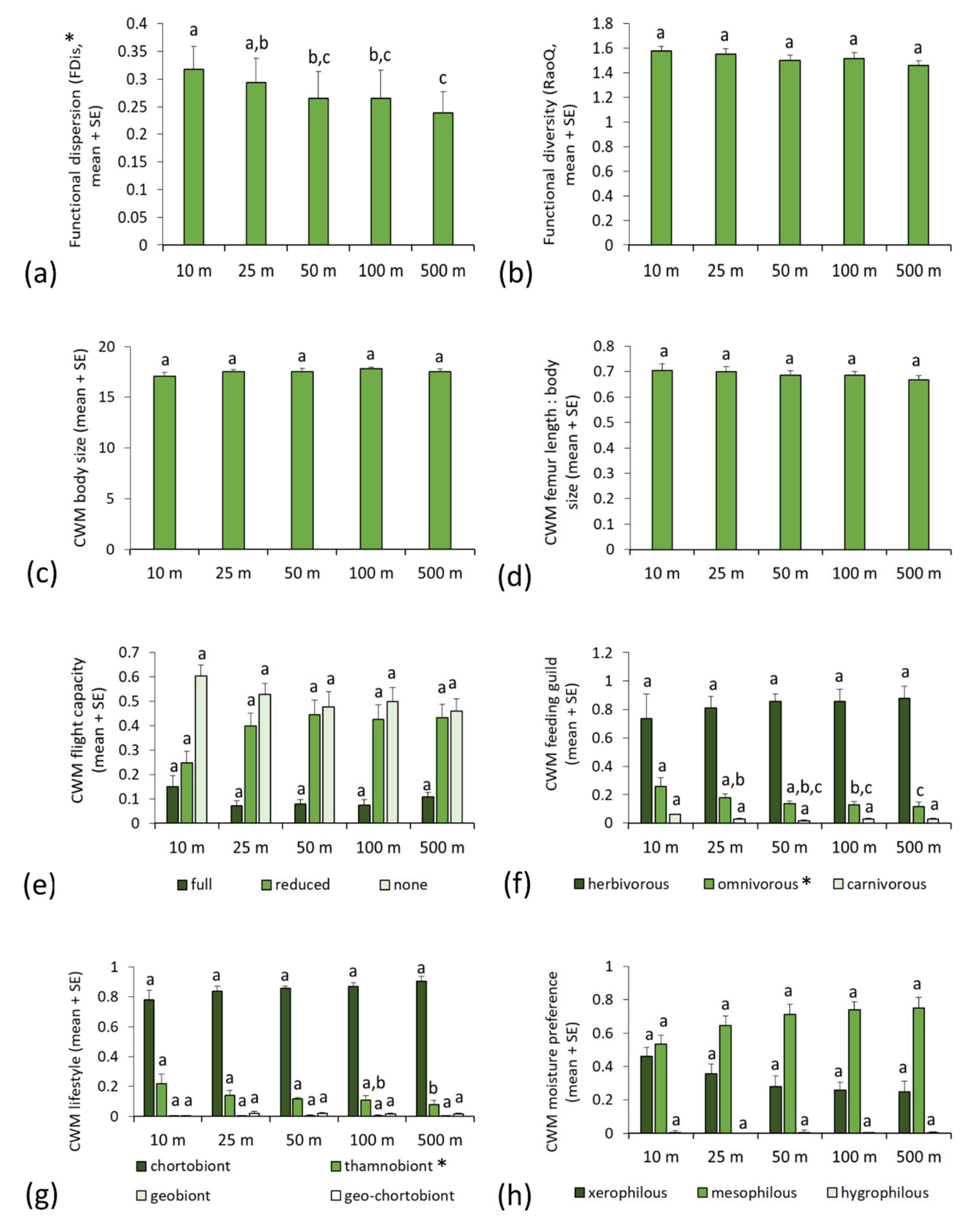
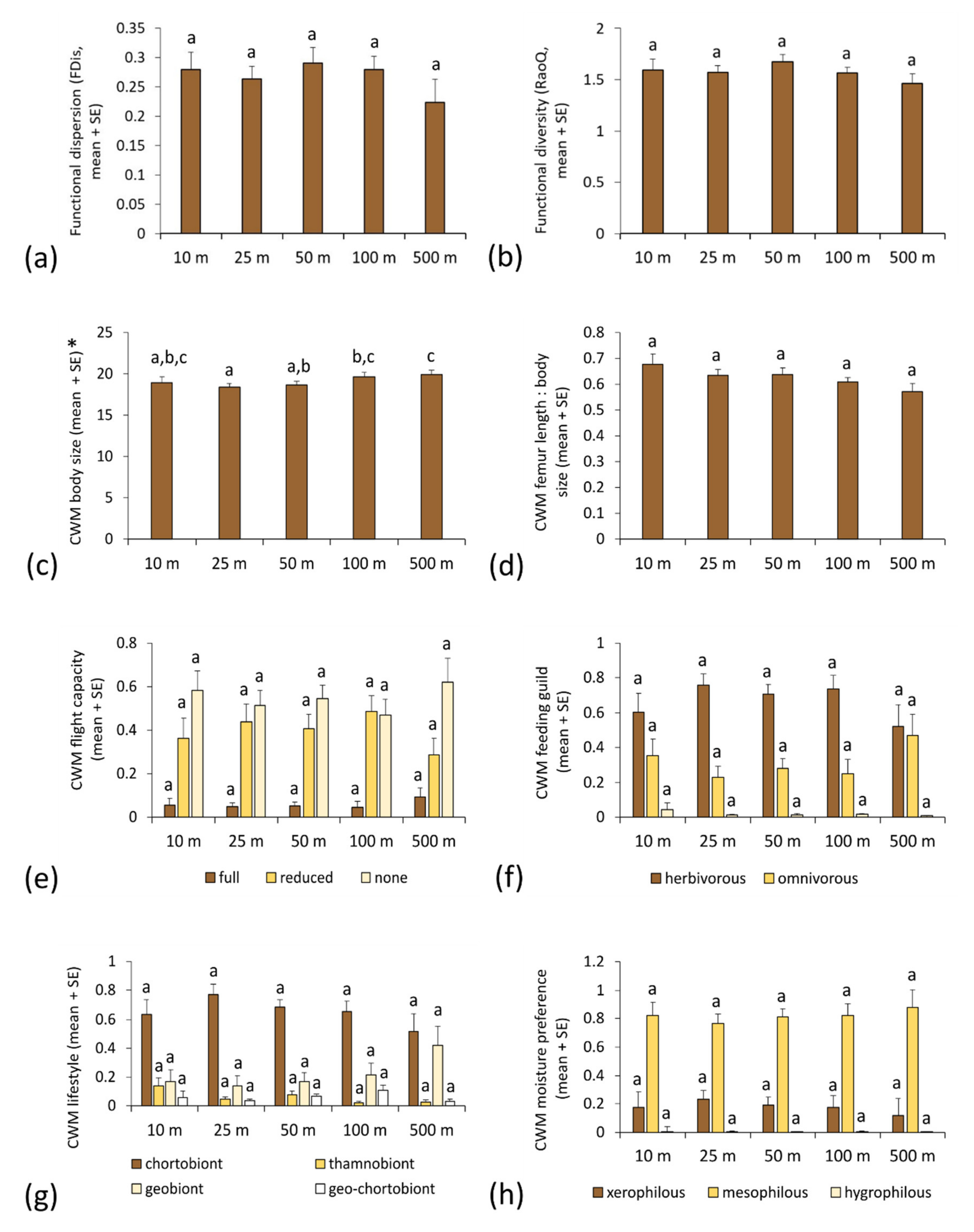
3.2. Functional Metrics of Orthopteran Assemblages and Motorway Proximity
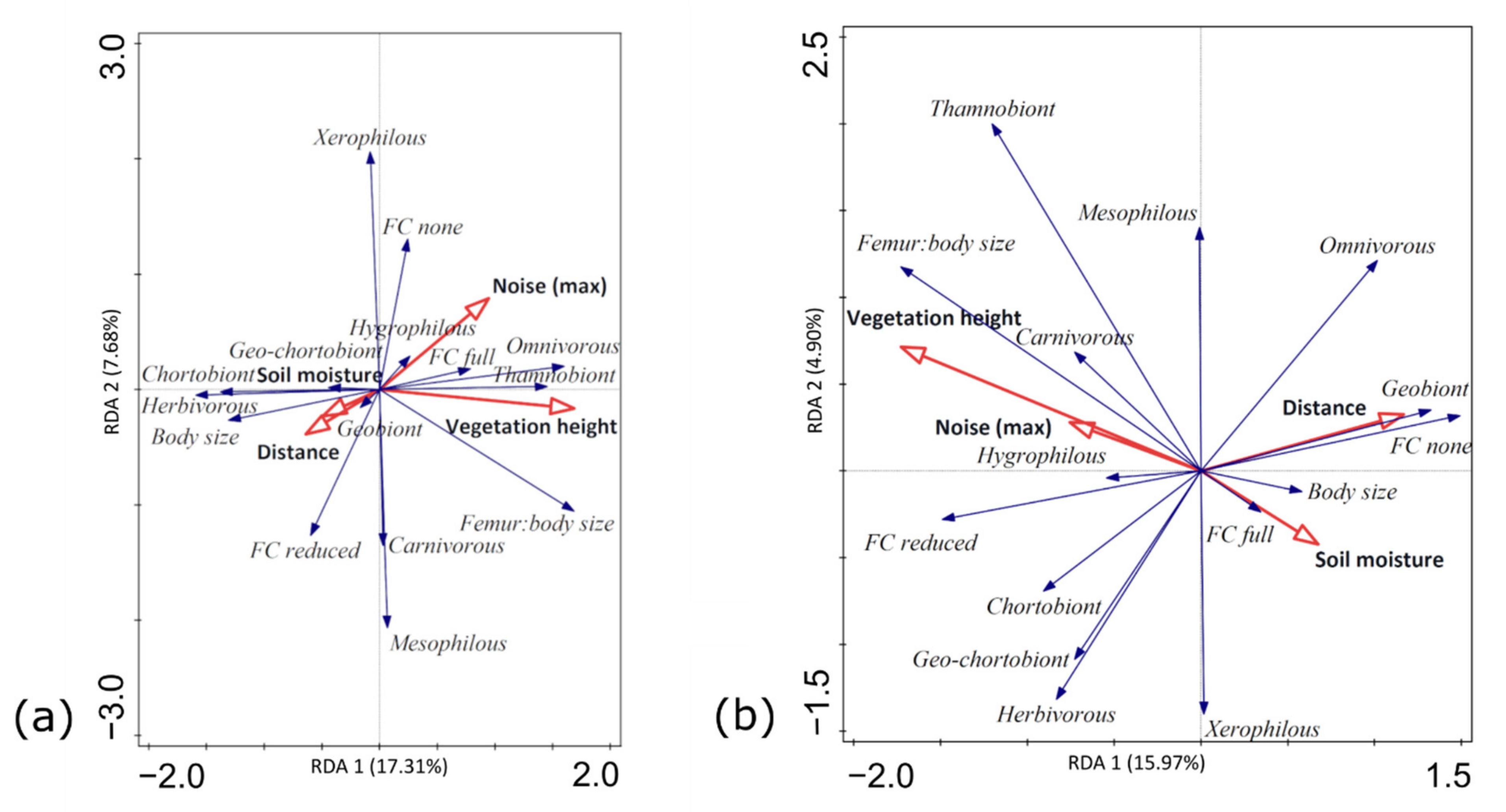
3.3. Functional Traits and Environmental Factors
4. Discussion
4.1. Functional Diversity Patterns with Respect to Road Proximity and Sampling Method
4.2. Road-Associated Changes in Functional Trait Composition
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| No. | Taxon | Body Size (mm) | Femur Length: Body Size | Flight Capacity | Feeding Guild | Lifestyle | Moisture Preference |
|---|---|---|---|---|---|---|---|
| Acrididae MacLeay, 1821 | |||||||
| 1 | Aiolopus thalassinus (Fabricius, 1781) | 20.86 | 0.59 | full | herbivorous | chortobiont | hygrophilous |
| 2 | Arcyptera brevipennis (Brunner von Wattenwyl, 1861) | 28.25 | 0.63 | none | herbivorous | geo-chortobiont | xerophilous |
| 3 | Calliptamus italicus (Linnaeus, 1758) | 21.49 | 0.60 | full | omnivorous | geo-chortobiont | xerophilous |
| 4 | Chorthippus biguttulus (Linnaeus, 1758) | 17.29 | 0.60 | full | herbivorous | chortobiont | mesophilous |
| 5 | Chorthippus biguttulus/mollis | 17.29 | 0.60 | full | herbivorous | chortobiont | mesophilous |
| 6 | Chorthippus brunneus (Thunberg, 1815) | 17.32 | 0.62 | full | herbivorous | chortobiont | mesophilous |
| 7 | Chorthippus dorsatus (Zetterstedt, 1821) | 17.15 | 0.63 | reduced | herbivorous | chortobiont | mesophilous |
| 8 | Chorthippus mollis (Charpentier, 1825) | 16.91 | 0.60 | full | herbivorous | chortobiont | mesophilous |
| 9 | Chrysochraon dispar (Germar, 1834) | 20.49 | 0.61 | reduced | herbivorous | chortobiont | hygrophilous |
| 10 | Euchorthippus declivus (Brisout de Barneville, 1848) | 19.44 | 0.59 | none | herbivorous | chortobiont | xerophilous |
| 11 | Euthystira brachyptera (Ocskay, 1826) | 17.55 | 0.65 | reduced | herbivorous | chortobiont | mesophilous |
| 12 | Odontopodisma schmidtii (Fieber, 1853) | 18.03 | 0.56 | none | herbivorous | thamnobiont | mesophilous |
| 13 | Oedipoda caerulescens (Linnaeus, 1758) | 19.42 | 0.57 | full | herbivorous | geobiont | xerophilous |
| 14 | Omocestus haemorrhoidalis (Charpentier, 1825) | 14.83 | 0.61 | reduced | herbivorous | chortobiont | mesophilous |
| 15 | Omocestus rufipes (Zetterstedt, 1821) | 14.83 | 0.71 | reduced | herbivorous | chortobiont | mesophilous |
| 16 | Pezotettix giornae (Rossi, 1794) | 13.67 | 0.55 | none | herbivorous | chortobiont | mesophilous |
| 17 | Pseudochorthippus parallelus (Zetterstedt, 1821) | 16.91 | 0.67 | reduced | herbivorous | chortobiont | mesophilous |
| 18 | Stauroderus scalaris (Fischer von Waldheim, 1846) | 21.82 | 0.58 | full | herbivorous | chortobiont | mesophilous |
| 19 | Stenobothrus lineatus (Panzer, 1796) | 19.44 | 0.64 | reduced | herbivorous | chortobiont | mesophilous |
| Gryllidae Laicharting, 1781 | |||||||
| 20 | Gryllus campestris Linnaeus, 1758 | 22.05 | 0.46 | none | omnivorous | geobiont | mesophilous |
| 21 | Oecanthus pellucens (Scopoli, 1763) | 11.83 | 0.67 | full | omnivorous | thamnobiont | xerophilous |
| Rhaphidophoridae Walker, 1869 | |||||||
| 22 | Troglophilus sp. | 19.36 | 0.92 | none | omnivorous | geobiont | hygrophilous |
| Tetrigidae Rambur, 1838 | |||||||
| 23 | Tetrix subulata (Linnaeus, 1758) | 12.25 | 0.47 | reduced | herbivorous | geobiont | hygrophilous |
| 24 | Tetrix tenuicornis (Sahlberg, 1891) | 9.54 | 0.62 | reduced | herbivorous | geobiont | hygrophilous |
| Tettigoniidae Krauss, 1902 | |||||||
| 25 | Barbitistes yersini (Brunner von Wattenwyl, 1878) | 24.08 | 0.80 | none | herbivorous | thamnobiont | mesophilous |
| 26 | Bicolorana bicolor (Philippi, 1830) | 15.87 | 1.07 | reduced | omnivorous | chortobiont | mesophilous |
| 27 | Conocephalus fuscus (Fabricius, 1793) | 14.70 | 0.78 | full | omnivorous | chortobiont | hygrophilous |
| 28 | Decticus verrucivorus (Linnaeus, 1758) | 33.05 | 0.91 | reduced | carnivorous | geo-chortobiont | mesophilous |
| 29 | Ephippiger discoidalis (Fieber, 1853) | 26.08 | 0.73 | none | omnivorous | thamnobiont | xerophilous |
| 30 | Ephippiger ephippiger (Fiebig, 1784) | 24.49 | 0.65 | none | omnivorous | thamnobiont | mesophilous |
| 31 | Eupholidoptera schmidti (Fieber, 1861) | 25.38 | 0.98 | none | omnivorous | thamnobiont | mesophilous |
| 32 | Leptophyes albovittata (Kollar, 1833) | 12.65 | 1.00 | none | herbivorous | chortobiont | mesophilous |
| 33 | Leptophyes boscii (Fieber, 1853) | 16.12 | 1.05 | none | herbivorous | chortobiont | mesophilous |
| 34 | Modestana modesta (Fieber, 1853) | 17.89 | 1.09 | none | omnivorous | geo-chortobiont | xerophilous |
| 35 | Pachytrachis gracilis (Brunner von Wattenwyl, 1861) | 16.73 | 1.06 | none | omnivorous | thamnobiont | mesophilous |
| 36 | Pachytrachis striolatus (Fieber, 1853) | 21.02 | 1.06 | none | omnivorous | thamnobiont | mesophilous |
| 37 | Phaneroptera falcata (Poda, 1761) | 14.70 | 1.39 | full | herbivorous | thamnobiont | mesophilous |
| 38 | Pholidoptera femorata (Fieber, 1853) | 25.69 | 0.98 | none | omnivorous | thamnobiont | mesophilous |
| 39 | Pholidoptera griseoaptera (De Geer, 1773) | 17.32 | 1.05 | none | omnivorous | thamnobiont | mesophilous |
| 40 | Poecilimon sp. | 16.73 | 0.91 | none | herbivorous | chortobiont | mesophilous |
| 41 | Roeseliana roeselii (Hagenbach, 1822) | 17.32 | 0.88 | reduced | omnivorous | chortobiont | mesophilous |
| 42 | Tettigonia viridissima (Linnaeus, 1758) | 32.62 | 0.78 | full | carnivorous | thamnobiont | mesophilous |
References
- van der Ree, R.; Jaeger, J.A.G.; van der Grift, E.A.; Clevenger, A.P. Effects of Roads and Traffic on Wildlife Populations and Landscape Function: Road Ecology Is Moving Toward Larger Scales. Ecol. Soc. 2011, 16, 48. [Google Scholar] [CrossRef]
- Polak, T.; Rhodes, J.R.; Jones, D.; Possingham, H.P. Optimal Planning for Mitigating the Impacts of Roads on Wildlife. J. Appl. Ecol. 2014, 51, 726–734. [Google Scholar] [CrossRef] [Green Version]
- Coffin, A.W. From Roadkill to Road Ecology: A Review of the Ecological Effects of Roads. J. Transp. Geogr. 2007, 15, 396–406. [Google Scholar] [CrossRef]
- Barbosa, P.; Schumaker, N.H.; Brandon, K.R.; Bager, A.; Grilo, C. Simulating the Consequences of Roads for Wildlife Population Dynamics. Landsc. Urban Plan. 2020, 193, 103672. [Google Scholar] [CrossRef] [PubMed]
- Rotholz, E.; Mandelik, Y. Roadside Habitats: Effects on Diversity and Composition of Plant, Arthropod, and Small Mammal Communities. Biodivers. Conserv. 2013, 22, 1017–1031. [Google Scholar] [CrossRef]
- Carr, L.W.; Fahrig, L.; Pope, S.E. Impacts of Landscape Transformation by Roads. In Applying Landscape Ecology in Biological Conservation; Gutzwiller, K.J., Ed.; Springer: New York, NY, USA, 2002; pp. 225–243. ISBN 978-0-387-98653-1. [Google Scholar]
- Bhardwaj, M.; Soanes, K.; Lahoz-Monfort, J.J.; Lumsden, L.F.; van der Ree, R. Little Evidence of a Road-Effect Zone for Nocturnal, Flying Insects. Ecol. Evol. 2019, 9, 65–72. [Google Scholar] [CrossRef] [Green Version]
- Phillips, B.B.; Bullock, J.M.; Osborne, J.L.; Gaston, K.J. Ecosystem Service Provision by Road Verges. J. Appl. Ecol. 2020, 57, 488–501. [Google Scholar] [CrossRef]
- Kaur, H.; Torma, A.; Gallé-Szpisjak, N.; Šeat, J.; Lőrinczi, G.; Módra, G.; Gallé, R. Road Verges Are Important Secondary Habitats for Grassland Arthropods. J. Insect Conserv. 2019, 23, 899–907. [Google Scholar] [CrossRef] [Green Version]
- Phillips, B.B.; Wallace, C.; Roberts, B.R.; Whitehouse, A.T.; Gaston, K.J.; Bullock, J.M.; Dicks, L.; Osborne, J.L. Enhancing Road Verges to Aid Pollinator Conservation: A Review. Biol. Conserv. 2020, 250, 108687. [Google Scholar] [CrossRef]
- Milton, S.J.; Dean, W.R.J.; Sielecki, L.E.; van der Ree, R. The Function and Management of Roadside Vegetation. In Handbook of Road Ecology; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 373–381. [Google Scholar]
- Muñoz, P.T.; Torres, F.P.; Megías, A.G. Effects of Roads on Insects: A Review. Biodivers. Conserv. 2015, 24, 659–682. [Google Scholar] [CrossRef]
- Nock, C.A.; Vogt, R.J.; Beisner, B.E. Functional Traits. In eLS; Wiley: Hoboken, NJ, USA, 2016; pp. 1–8. [Google Scholar]
- Naeem, S.; Wright, J.P. Disentangling Biodiversity Effects on Ecosystem Functioning: Deriving Solutions to a Seemingly Insurmountable Problem. Ecol. Lett. 2003, 6, 567–579. [Google Scholar] [CrossRef] [Green Version]
- Zakharova, L.; Meyer, K.M.; Seifan, M. Trait-Based Modelling in Ecology: A Review of Two Decades of Research. Ecol. Model. 2019, 407, 108703. [Google Scholar] [CrossRef]
- Schowalter, T.D. Insect Ecology, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2006; ISBN 9780128030332. [Google Scholar]
- Teittinen, A.; Virta, L. Exploring Multiple Aspects of Taxonomic and Functional Diversity in Microphytobenthic Communities: Effects of Environmental Gradients and Temporal Changes. Front. Microbiol. 2021, 12, 668993. [Google Scholar] [CrossRef] [PubMed]
- Petchey, O.L.; Gaston, K.J. Functional Diversity: Back to Basics and Looking Forward. Ecol. Lett. 2006, 9, 741–758. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.; Petchey, O.L.; Askew, A.P.; Dunnett, N.P.; Beckerman, A.P.; Willis, A.J. Little Evidence for Limiting Similarity in a Long-Term Study of a Roadside Plant Community. J. Ecol. 2010, 98, 480–487. [Google Scholar] [CrossRef]
- Valladares, F.; Tena, D.; Matesanz, S.; Bochet, E.; Balaguer, L.; Costa-Tenorio, M.; Tormo, J.; García-Fayos, P. Functional Traits and Phylogeny: What Is the Main Ecological Process Determining Species Assemblage in Roadside Plant Communities? J. Veg. Sci. 2008, 19, 381–392. [Google Scholar] [CrossRef]
- Jakobsson, S.; Bernes, C.; Bullock, J.M.; Verheyen, K.; Lindborg, R. How Does Roadside Vegetation Management Affect the Diversity of Vascular Plants and Invertebrates? A Systematic Review. Environ. Evid. 2018, 7, 17. [Google Scholar] [CrossRef] [Green Version]
- Gardiner, T. Grazing and Orthoptera: A Review. J. Orthoptera Res. 2018, 27, 3–11. [Google Scholar] [CrossRef] [Green Version]
- Schirmel, J.; Gerlach, R.; Buhk, C. Disentangling the Role of Management, Vegetation Structure, and Plant Quality for Orthoptera in Lowland Meadows. Insect Sci. 2017, 26, 366–378. [Google Scholar] [CrossRef]
- Torma, A.; Császár, P.; Bozsó, M.; Deák, B.; Valkó, O.; Kiss, O.; Gallé, R. Species and Functional Diversity of Arthropod Assemblages (Araneae, Carabidae, Heteroptera and Orthoptera) in Grazed and Mown Salt Grasslands. Agric. Ecosyst. Environ. 2018, 273, 70–79. [Google Scholar] [CrossRef] [Green Version]
- Kaláb, O.; Šipoš, J.; Kočárek, P. Leaving Uncut Refuges during Meadow Harvesting Increases the Functional Diversity of Orthoptera. Entomol. Sci. 2020, 23, 95–104. [Google Scholar] [CrossRef] [Green Version]
- Dziock, F.; Gerisch, M.; Siegert, M.; Hering, I.; Scholz, M.; Ernst, R. Reproducing or Dispersing? Using Trait Based Habitat Templet Models to Analyse Orthoptera Response to Flooding and Land Use. Agric. Ecosyst. Environ. 2011, 145, 85–94. [Google Scholar] [CrossRef]
- Ferrando, C.P.R.; Podgaiski, L.R.; Costa, M.K.M.; Mendonça, M.D.S. Taxonomic and Functional Resilience of Grasshoppers (Orthoptera, Caelifera) to Fire in South Brazilian Grasslands. Neotrop. Entomol. 2016, 45, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Melliger, R.L.; Rusterholz, H.P.; Baur, B. Habitat- and Matrix-Related Differences in Species Diversity and Trait Richness of Vascular Plants, Orthoptera and Lepidoptera in an Urban Landscape. Urban Ecosyst. 2017, 20, 1095–1107. [Google Scholar] [CrossRef]
- Schowalter, T.D. Insect Responses to Major Landscape-Level Disturbance. Annu. Rev. Entomol. 2012, 57, 1–20. [Google Scholar] [CrossRef]
- Lampe, U.; Reinhold, K.; Schmoll, T. How Grasshoppers Respond to Road Noise: Developmental Plasticity and Population Differentiation in Acoustic Signalling. Funct. Ecol. 2014, 28, 660–668. [Google Scholar] [CrossRef]
- Gurule-Small, G.A.; Tinghitella, R.M. Developmental Experience with Anthropogenic Noise Hinders Adult Mate Location in an Acoustically Signalling Invertebrate. Biol. Lett. 2018, 14, 20170714. [Google Scholar] [CrossRef] [Green Version]
- Rebrina, F.; Reinhold, K.; Tvrtković, N.; Brigić, A. Motorway Proximity Affects Spatial Dynamics of Orthopteran Assemblages in a Grassland Ecosystem. Insect Conserv. Divers. 2022, 15, 213–225. [Google Scholar] [CrossRef]
- Mody, K.; Lerch, D.; Müller, A.K.; Simons, N.K.; Blüthgen, N.; Harnisch, M. Flower Power in the City: Replacing Roadside Shrubs by Wildflower Meadows Increases Insect Numbers and Reduces Maintenance Costs. PLoS ONE 2020, 15, e0234327. [Google Scholar] [CrossRef]
- Biswas, S.R.; Mallik, A.U. Disturbance Effects on Species Diversity and Functional Diversity in Riparian and Upland Plant Communities. Ecology 2010, 91, 28–35. [Google Scholar] [CrossRef]
- Swart, R.C.; Pryke, J.S.; Roets, F. The Intermediate Disturbance Hypothesis Explains Arthropod Beta-Diversity Responses to Roads That Cut through Natural Forests. Biol. Conserv. 2019, 236, 243–251. [Google Scholar] [CrossRef]
- Simons, N.K.; Weisser, W.W.; Gossner, M.M. Multi-Taxa Approach Shows Consistent Shifts in Arthropod Functional Traits along Grassland Land-Use Intensity Gradient. Ecology 2016, 97, 754–764. [Google Scholar] [CrossRef] [PubMed]
- Whitman, D.W. The Significance of Body Size in the Orthoptera: A Review. J. Orthoptera Res. 2008, 17, 117–134. [Google Scholar] [CrossRef] [Green Version]
- Šegota, T.; Filipčić, A. Köppen’s Classification of Climates and the Problem of Corresponding Croatian Terminology. Geoadria 2003, 8, 17–37. [Google Scholar] [CrossRef] [Green Version]
- Zaninović, K.; Gajić-Čapka, M.; Tadić, M.P.; Vučetić, M.; Milković, J.; Bajić, A.; Cindrić, K.; Cvitan, L.; Katušin, Z.; Kaučić, D.; et al. Climate Atlas of Croatia: 1961–1990, 1971–2000; Croatian Meteorological and Hydrological Service: Zagreb, Croatia, 2008; ISBN 978-953-7526-01-6. [Google Scholar]
- Hochkirch, A.; Adorf, F. Effects of Prescribed Burning and Wildfires on Orthoptera in Central European Peat Bogs. Environ. Conserv. 2007, 34, 225–235. [Google Scholar] [CrossRef] [Green Version]
- Bardi, A.; Papini, P.; Quaglino, E.; Biondi, E.; Topić, J.; Milović, M.; Pandža, M.; Kaligarič, M.; Oriolo, G.; Roland, V.; et al. Karta Prirodnih i Poluprirodnih Ne-Šumskih Kopnenih i Slatkovodnih Staništa Republike Hrvatske; AGRISTUDIO s.r.l., TEMI S.r.l., TIMESIS S.r.l., HAOP; Republic of Croatia, Ministry of Environmental Protection and Energy: Zagreb, Croatia, 2016. [Google Scholar]
- Knapp, M.; Saska, P.; Knappová, J.; Vonička, P.; Moravec, P.; Kůrka, A.; Anděl, P. The Habitat-Specific Effects of Highway Proximity on Ground-Dwelling Arthropods: Implications for Biodiversity Conservation. Biol. Conserv. 2013, 164, 22–29. [Google Scholar] [CrossRef]
- Rada, S. Comparison of Two Methods for Sampling Orthopterans in Grassland: Differences in Species Representation and Sex Ratios. Turk. J. Zool. 2017, 41, 1105–1110. [Google Scholar] [CrossRef]
- Schirmel, J.; Buchholz, S.; Fartmann, T. Is Pitfall Trapping a Valuable Sampling Method for Grassland Orthoptera? J. Insect Conserv. 2010, 14, 289–296. [Google Scholar] [CrossRef]
- Harz, K. The Orthoptera of Europe II. Junk, W.N.V., Ed.; Pemberley Publishers: Austin, TX, USA, 1975; ISBN 9789401019491. [Google Scholar]
- Harz, K. The Orthoptera of Europe I. Junk, W.N.V., Ed.; Pemberley Publishers: Austin, TX, USA, 1969; ISBN 9789048185122. [Google Scholar]
- Sardet, É.; Roesti, C.; Braud, Y. Cahier d’identification Des Orthoptères de France, Belgique, Luxembourg et Suisse; Biotope Éditions; La Boutique des Naturalistes: Quebec, QC, Canada, 2015. [Google Scholar]
- Auestad, I.; Rydgren, K.; Austad, I. Road Verges: Potential Refuges for Declining Grassland Species despite Remnant Vegetation Dynamics. Ann. Bot. Fenn. 2011, 48, 289–303. [Google Scholar] [CrossRef]
- Deljouei, A.; Sadeghi, S.M.M.; Abdi, E.; Bernhardt-Römermann, M.; Pascoe, E.L.; Marcantonio, M. The Impact of Road Disturbance on Vegetation and Soil Properties in a Beech Stand, Hyrcanian Forest. Eur. J. For. Res. 2018, 137, 759–770. [Google Scholar] [CrossRef]
- Samara, T.; Tsitsoni, T. The Effects of Vegetation on Reducing Traffic Noise from a City Ring Road. Noise Control Eng. J. 2011, 59, 68–74. [Google Scholar] [CrossRef]
- Bazelet, C.S.; Samways, M.J. Habitat Quality of Grassland Fragments Affects Dispersal Ability of a Mobile Grasshopper, Ornithacris cyanea (Orthoptera: Acrididae). Afr. Entomol. 2014, 22, 714–725. [Google Scholar] [CrossRef]
- Gossner, M.M.; Simons, N.K.; Achtziger, R.; Blick, T.; Dorow, W.H.O.; Dziock, F.; Köhler, F.; Rabitsch, W.; Weisser, W.W. A Summary of Eight Traits of Coleoptera, Hemiptera, Orthoptera and Araneae, Occurring in Grasslands in Germany. Sci. Data 2015, 2, 150013. [Google Scholar] [CrossRef]
- Iorgu, I.S.; Iorgu, E.I. Bush-Crickets, Crickets and Grasshoppers from Moldavia (Romania); Pim Iași: Iași, Romania, 2008; ISBN 978-606-520-112-5. [Google Scholar]
- Bellmann, H.; Rutschmann, F.; Roesti, C.; Hochkirch, A. Der Kosmos Heuschreckenführer; Franckh-Kosmos Verlags-GmbH & Co. KG: Stuttgart, Germany, 2019. [Google Scholar]
- Wagner, W. Orthoptera and Their Ecology. Available online: http://www.pyrgus.de (accessed on 26 April 2021).
- R Core Team, R. A Language and Environment for Statistical Computing; R Core Team R: Vienna, Austria, 2020. [Google Scholar]
- Laliberte, E.; Legendre, P. A Distance-Based Framework for Measuring Functional Diversity from Multiple Traits. Ecology 2010, 91, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Ricotta, C.; Moretti, M. CWM and Rao’s Quadratic Diversity: A Unified Framework for Functional Ecology. Oecologia 2011, 167, 181–188. [Google Scholar] [CrossRef]
- ter Braak, C.J.F.; Šmilauer, P. Canoco Reference Manual and User’s Guide: Software for Ordination; Microcomputer Power: Ithaca, NY, USA, 2012. [Google Scholar]
- TIBCO Software Inc. Data Science Textbook; TIBCO Software Inc.: Palo Alto, CA, USA, 2020. [Google Scholar]
- IBM Corp. IBM SPSS Statistics for Windows, Version 27.0 2020; IBM Corp.: Armonk, NY, USA, 2020. [Google Scholar]
- Field, A. Discovering Statistics Using SPSS, 3rd ed.; SAGE Publications Ltd.: New York, NY, USA, 2009; ISBN 978-1847879073. [Google Scholar]
- Lepš, J.; Šmilauer, P. Multivariate Analysis of Ecological Data Using CANOCO 5, 2nd ed.; Cambridge University Press: Cambridge, UK, 2014; ISBN 9781139627061. [Google Scholar]
- Zhou, T.; Luo, X.; Hou, Y.; Xiang, Y.; Peng, S. Quantifying the Effects of Road Width on Roadside Vegetation and Soil Conditions in Forests. Landsc. Ecol. 2020, 35, 69–81. [Google Scholar] [CrossRef] [Green Version]
- Schirmel, J.; Mantilla-Contreras, J.; Blindow, I.; Fartmann, T. Impacts of Succession and Grass Encroachment on Heathland Orthoptera. J. Insect Conserv. 2011, 15, 633–642. [Google Scholar] [CrossRef]
- Rebrina, F.; Petek, M.; Gulin, V.; Brigić, A. Ground Beetle Assemblages Respond to Motorway Proximity through Changes in Functional Rather than Taxonomic Diversity in a Grassland Ecosystem. Glob. Ecol. Conserv. 2022. under review. [Google Scholar]
- Karadimou, E.K.; Kallimanis, A.S.; Tsiripidis, I.; Dimopoulos, P. Functional Diversity Exhibits a Diverse Relationship with Area, Even a Decreasing One. Sci. Rep. 2016, 6, 35420. [Google Scholar] [CrossRef] [Green Version]
- Pakeman, R.J. Functional Trait Metrics Are Sensitive to the Completeness of the Species’ Trait Data? Methods Ecol. Evol. 2014, 5, 9–15. [Google Scholar] [CrossRef]
- Marini, L.; Bommarco, R.; Fontana, P.; Battisti, A. Disentangling Effects of Habitat Diversity and Area on Orthopteran Species with Contrasting Mobility. Biol. Conserv. 2010, 143, 2164–2171. [Google Scholar] [CrossRef]
- Hidasi-Neto, J.; Barlow, J.; Cianciaruso, M.V. Bird Functional Diversity and Wildfires in the Amazon: The Role of Forest Structure. Anim. Conserv. 2012, 15, 407–415. [Google Scholar] [CrossRef]
- Gerisch, M.; Agostinelli, V.; Henle, K.; Dziock, F. More Species, but All Do the Same: Contrasting Effects of Flood Disturbance on Ground Beetle Functional and Species Diversity. Oikos 2012, 121, 508–515. [Google Scholar] [CrossRef]
- Neff, F.; Blüthgen, N.; Chisté, M.N.; Simons, N.K.; Steckel, J.; Weisser, W.W.; Westphal, C.; Pellissier, L.; Gossner, M.M. Cross-Scale Effects of Land Use on the Functional Composition of Herbivorous Insect Communities. Landsc. Ecol. 2019, 34, 2001–2015. [Google Scholar] [CrossRef]
- McCabe, L.M.; Cobb, N.S.; Butterfield, B.J. Environmental Filtering of Body Size and Darker Coloration in Pollinator Communities Indicate Thermal Restrictions on Bees, but Not Flies, at High Elevations. PeerJ 2019, 7, e7867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribera, I.; Dolédec, S.; Downie, I.S.; Foster, G.N. Effect of Land Disturbance and Stress on Species Traits of Ground Beetle Assemblages. Ecology 2001, 82, 1112–1129. [Google Scholar] [CrossRef]
- Schmidt, A.K.D.; Balakrishnan, R. Ecology of Acoustic Signalling and the Problem of Masking Interference in Insects. J. Comp. Physiol.-A Sens. Neural Behav. Physiol. 2015, 201, 133–142. [Google Scholar] [CrossRef]
- Montealegre-Z, F.; Ogden, J.; Jonsson, T.; Soulsbury, C.D. Morphological Determinants of Signal Carrier Frequency in Katydids (Orthoptera): A Comparative Analysis Using Biophysical Evidence of Wing Vibration. J. Evol. Biol. 2017, 30, 2068–2078. [Google Scholar] [CrossRef] [Green Version]
- Rentch, J.S.; Fortney, R.H.; Stephenson, S.L.; Adams, H.S.; Grafton, W.N.; Anderson, J.T. Vegetation-Site Relationships of Roadside Plant Communities in West Virginia, USA. J. Appl. Ecol. 2005, 42, 129–138. [Google Scholar] [CrossRef]
- Vandevelde, J.-C.; Penone, C. Ecological Roles of Railway Verges in Anthropogenic Landscapes: A Synthesis of Five Case Studies in Northern France. In Railway Ecology; Borda-de-Água, L., Barrientos, R., Beja, P., Pereira, H.M., Eds.; Springer: Cham, Switzerland, 2017; pp. 261–276. [Google Scholar]
- Morris, E.K.; Caruso, T.; Buscot, F.; Fischer, M.; Hancock, C.; Maier, T.S.; Meiners, T.; Müller, C.; Obermaier, E.; Prati, D.; et al. Choosing and Using Diversity Indices: Insights for Ecological Applications from the German Biodiversity Exploratories. Ecol. Evol. 2014, 4, 3514–3524. [Google Scholar] [CrossRef] [Green Version]
| Functional Parameter | F | p | d.f. | p | |||||
|---|---|---|---|---|---|---|---|---|---|
| 10–25 | 10–50 | 10–100 | 10–500 | 25–50 | |||||
| Functional dispersion (FDis) | 3.189 | 0.025 | 4 | 0.310 | 0.034 | 0.035 | 0.002 | 0.249 | |
| Functional diversity (RaoQ) | 1.109 | 0.368 | 4 | / | / | / | / | / | |
| CWM body size | 0.805 | 0.530 | 4 | / | / | / | / | / | |
| CWM femur length: body size | 0.594 | 0.669 | 4 | / | / | / | / | / | |
| CWM flight capacity | full | 1.339 | 0.275 | 4 | / | / | / | / | / |
| reduced | 2.357 | 0.073 | 4 | / | / | / | / | / | |
| none | 1.575 | 0.203 | 4 | / | / | / | / | / | |
| CWM feeding guild | herbivorous | 1.750 | 0.161 | 4 | / | / | / | / | / |
| omnivorous | 2.790 | 0.041 | 4 | 0.451 | 0.126 | 0.041 | 0.004 | 0.426 | |
| carnivorous | 0.965 | 0.439 | 4 | / | / | / | / | / | |
| CWM lifestyle | chortobiont | 0.412 | 0.799 | 4 | / | / | / | / | / |
| thamnobiont | 2.710 | 0.046 | 4 | 0.606 | 0.686 | 0.238 | 0.006 | 0.911 | |
| geobiont | 0.681 | 0.610 | 4 | / | / | / | / | / | |
| geo-chortobiont | 1.592 | 0.198 | 4 | / | / | / | / | / | |
| CWM moisture preference | xerophilous | 2.409 | 0.068 | 4 | / | / | / | / | / |
| mesophilous | 2.428 | 0.066 | 4 | / | / | / | / | / | |
| hygrophilous | 1.632 | 0.188 | 4 | / | / | / | / | / | |
| Functional Parameter | F | p | d.f. | p | |||||
| 25–100 | 25–500 | 50–100 | 50–500 | 100–500 | |||||
| Functional dispersion (FDis) | 3.189 | 0.025 | 4 | 0.250 | 0.030 | 0.998 | 0.280 | 0.279 | |
| Functional diversity (RaoQ) | 1.109 | 0.368 | 4 | / | / | / | / | / | |
| CWM body size | 0.805 | 0.530 | 4 | / | / | / | / | / | |
| CWM femur length: body size | 0.594 | 0.669 | 4 | / | / | / | / | / | |
| CWM flight capacity | full | 1.339 | 0.275 | 4 | / | / | / | / | / |
| reduced | 2.357 | 0.073 | 4 | / | / | / | / | / | |
| none | 1.575 | 0.203 | 4 | / | / | / | / | / | |
| CWM feeding guild | herbivorous | 1.750 | 0.161 | 4 | / | / | / | / | / |
| omnivorous | 2.790 | 0.041 | 4 | 0.182 | 0.028 | 0.583 | 0.148 | 0.361 | |
| carnivorous | 0.965 | 0.439 | 4 | / | / | / | / | / | |
| CWM lifestyle | chortobiont | 0.412 | 0.799 | 4 | / | / | / | / | / |
| thamnobiont | 2.710 | 0.046 | 4 | 0.501 | 0.020 | 0.433 | 0.016 | 0.089 | |
| geobiont | 0.681 | 0.610 | 4 | / | / | / | / | / | |
| geo-chortobiont | 1.592 | 0.198 | 4 | / | / | / | / | / | |
| CWM moisture preference | xerophilous | 2.409 | 0.068 | 4 | / | / | / | / | / |
| mesophilous | 2.428 | 0.066 | 4 | / | / | / | / | / | |
| hygrophilous | 1.632 | 0.188 | 4 | / | / | / | / | / | |
| Functional Parameter | F | p | d.f. | p | |||||
|---|---|---|---|---|---|---|---|---|---|
| 10–25 | 10–50 | 10–100 | 10–500 | 25–50 | |||||
| Functional dispersion (FDis) | 0.871 | 0.491 | 4 | / | / | / | / | / | |
| Functional diversity (RaoQ) | 0.866 | 0.494 | 4 | / | / | / | / | / | |
| CWM body size | 2.908 | 0.035 | 4 | 0.208 | 0.537 | 0.404 | 0.084 | 0.514 | |
| CWM femur length: body size | 2.048 | 0.109 | 4 | / | / | / | / | / | |
| CWM flight capacity | full | 0.495 | 0.740 | 4 | / | / | / | / | / |
| reduced | 0.913 | 0.467 | 4 | / | / | / | / | / | |
| none | 0.486 | 0.746 | 4 | / | / | / | / | / | |
| CWM feeding guild | herbivorous | 1.584 | 0.200 | 4 | / | / | / | / | / |
| omnivorous | 0.725 | 0.581 | 4 | / | / | / | / | / | |
| carnivorous | 1.131 | 0.358 | 4 | / | / | / | / | / | |
| CWM lifestyle | chortobiont | 2.323 | 0.076 | 4 | / | / | / | / | / |
| thamnobiont | 0.974 | 0.434 | 4 | / | / | / | / | / | |
| geobiont | 0.954 | 0.445 | 4 | / | / | / | / | / | |
| geo-chortobiont | 2.511 | 0.059 | 4 | / | / | / | / | / | |
| CWM moisture preference | xerophilous | 1.147 | 0.351 | 4 | / | / | / | / | / |
| mesophilous | 1.177 | 0.338 | 4 | / | / | / | / | / | |
| hygrophilous | 1.126 | 0.360 | 4 | / | / | / | / | / | |
| Functional Parameter | F | p | d.f. | p | |||||
| 25–100 | 25–500 | 50–100 | 50–500 | 100–500 | |||||
| Functional dispersion (FDis) | 0.871 | 0.491 | 4 | / | / | / | / | / | |
| Functional diversity (RaoQ) | 0.866 | 0.494 | 4 | / | / | / | / | / | |
| CWM body size | 2.908 | 0.035 | 4 | 0.041 | 0.004 | 0.151 | 0.022 | 0.355 | |
| CWM femur length: body size | 2.048 | 0.109 | 4 | / | / | / | / | / | |
| CWM flight capacity | full | 0.495 | 0.740 | 4 | / | / | / | / | / |
| reduced | 0.913 | 0.467 | 4 | / | / | / | / | / | |
| none | 0.486 | 0.746 | 4 | / | / | / | / | / | |
| CWM feeding guild | herbivorous | 1.584 | 0.200 | 4 | / | / | / | / | / |
| omnivorous | 0.725 | 0.581 | 4 | / | / | / | / | / | |
| carnivorous | 1.131 | 0.358 | 4 | / | / | / | / | / | |
| CWM lifestyle | chortobiont | 2.323 | 0.076 | 4 | / | / | / | / | / |
| thamnobiont | 0.974 | 0.434 | 4 | / | / | / | / | / | |
| geobiont | 0.954 | 0.445 | 4 | / | / | / | / | / | |
| geo-chortobiont | 2.511 | 0.059 | 4 | / | / | / | / | / | |
| CWM moisture preference | xerophilous | 1.147 | 0.351 | 4 | / | / | / | / | / |
| mesophilous | 1.177 | 0.338 | 4 | / | / | / | / | / | |
| hygrophilous | 1.126 | 0.360 | 4 | / | / | / | / | / | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rebrina, F.; Reinhold, K.; Tvrtković, N.; Gulin, V.; Brigić, A. Vegetation Height as the Primary Driver of Functional Changes in Orthopteran Assemblages in a Roadside Habitat. Insects 2022, 13, 572. https://doi.org/10.3390/insects13070572
Rebrina F, Reinhold K, Tvrtković N, Gulin V, Brigić A. Vegetation Height as the Primary Driver of Functional Changes in Orthopteran Assemblages in a Roadside Habitat. Insects. 2022; 13(7):572. https://doi.org/10.3390/insects13070572
Chicago/Turabian StyleRebrina, Fran, Klaus Reinhold, Nikola Tvrtković, Vesna Gulin, and Andreja Brigić. 2022. "Vegetation Height as the Primary Driver of Functional Changes in Orthopteran Assemblages in a Roadside Habitat" Insects 13, no. 7: 572. https://doi.org/10.3390/insects13070572
APA StyleRebrina, F., Reinhold, K., Tvrtković, N., Gulin, V., & Brigić, A. (2022). Vegetation Height as the Primary Driver of Functional Changes in Orthopteran Assemblages in a Roadside Habitat. Insects, 13(7), 572. https://doi.org/10.3390/insects13070572





