Effect of Abiotic Climatic Factors on the Gonadal Maturation of the Biocontrol Agent Sphaerophoria rueppellii (Wiedemann, 1830) (Diptera: Syrphidae)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
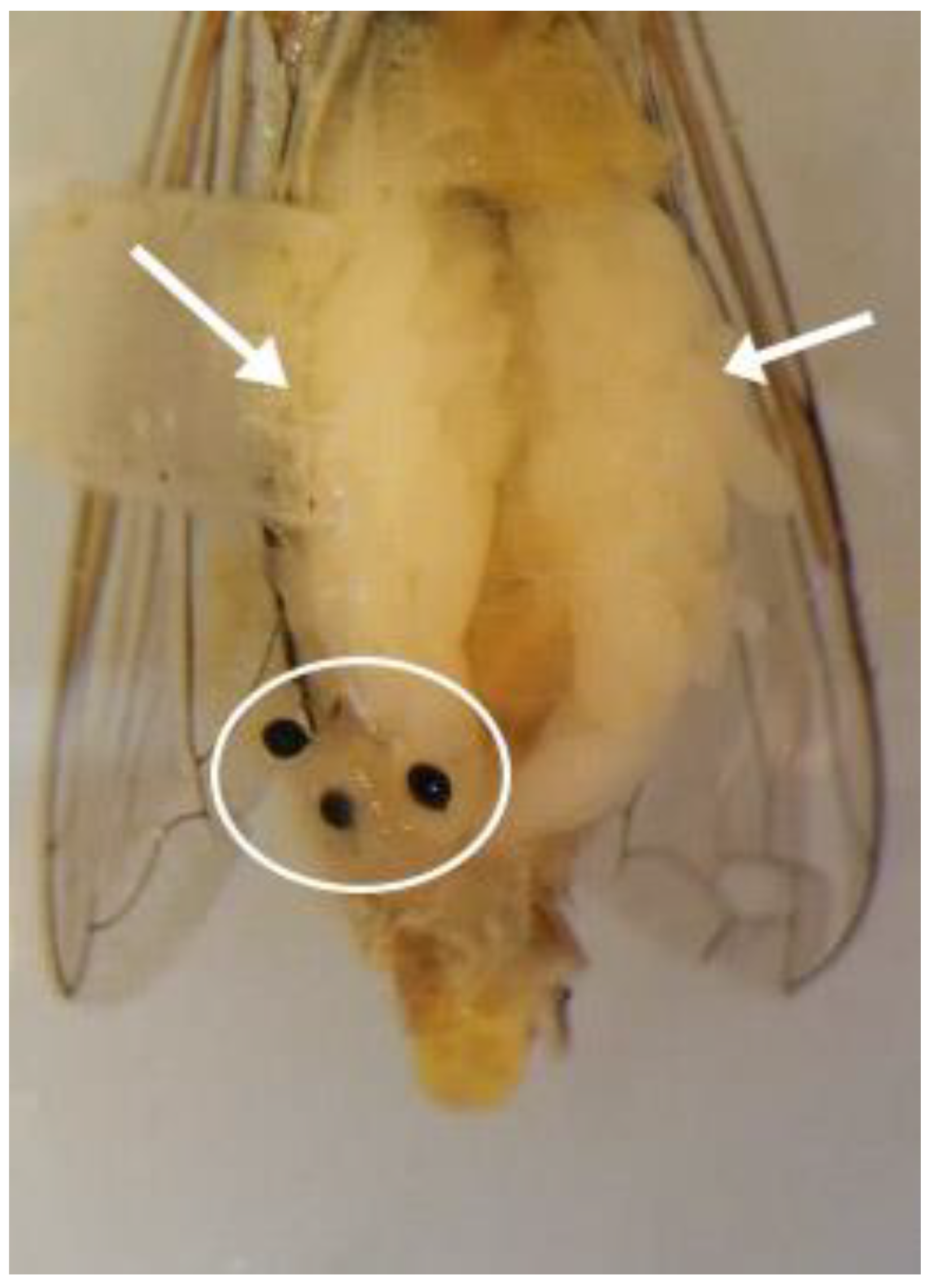
2.1. Experimental Insects and Morphological Terminology
2.2. Lifetime Oviposition
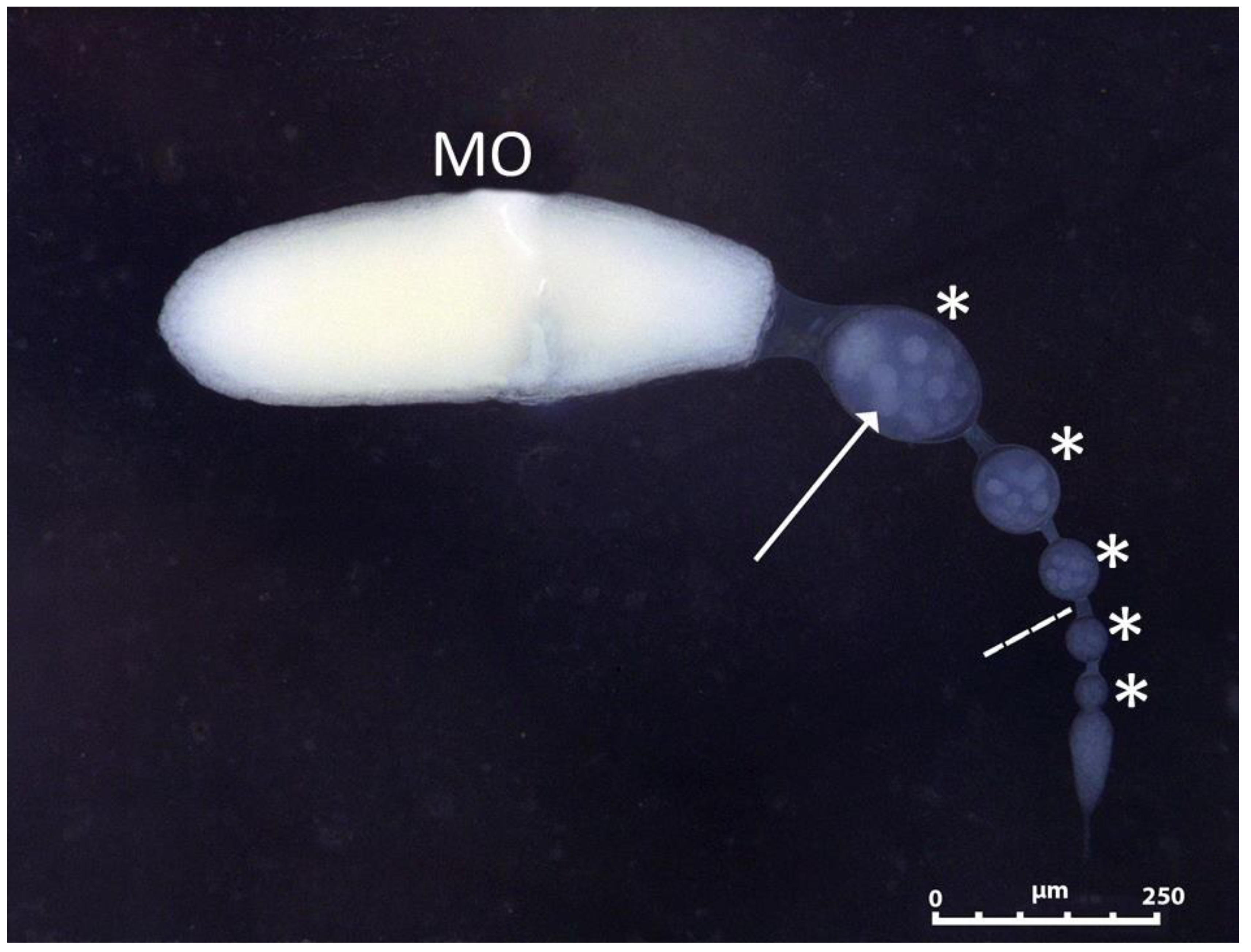
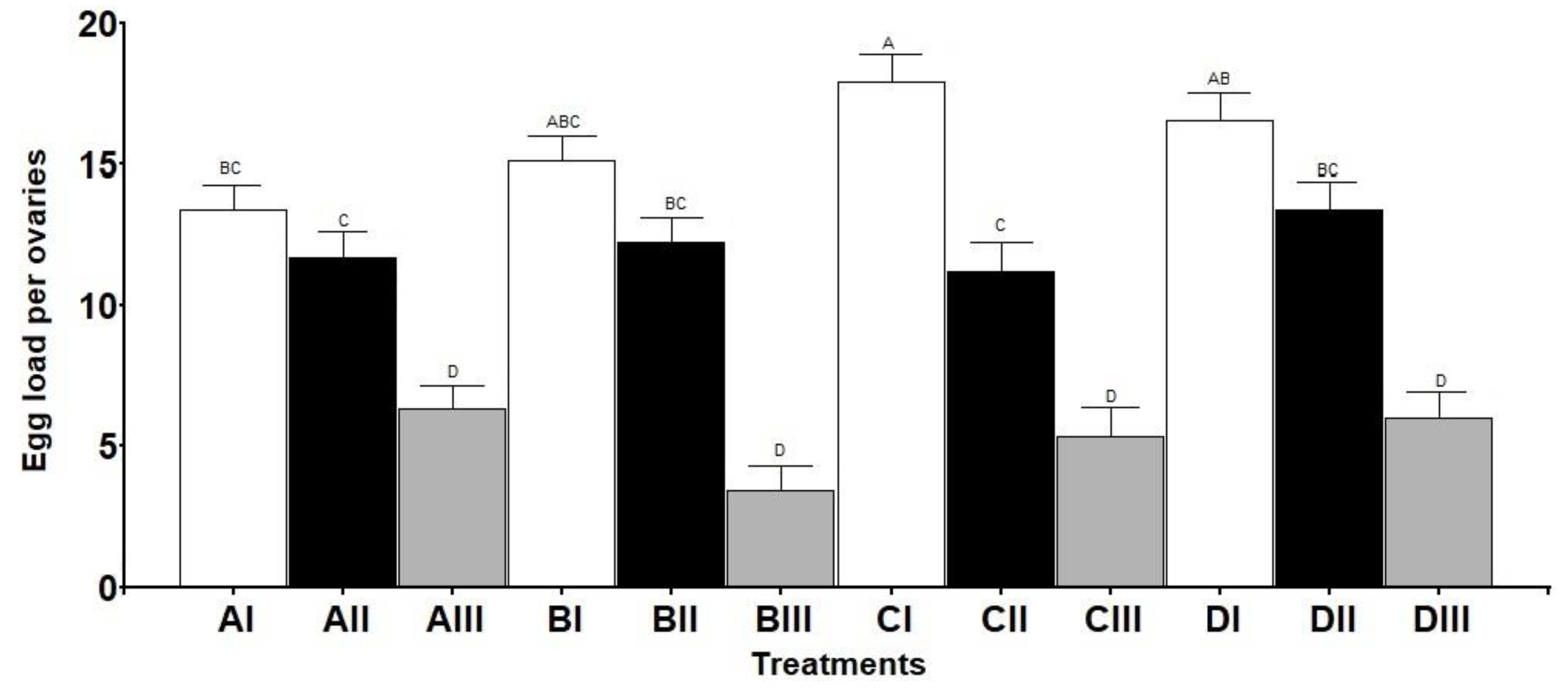
2.3. Ovaries Maturation
2.4. Maturation of the Testes
2.5. Statistical Analysis
3. Results
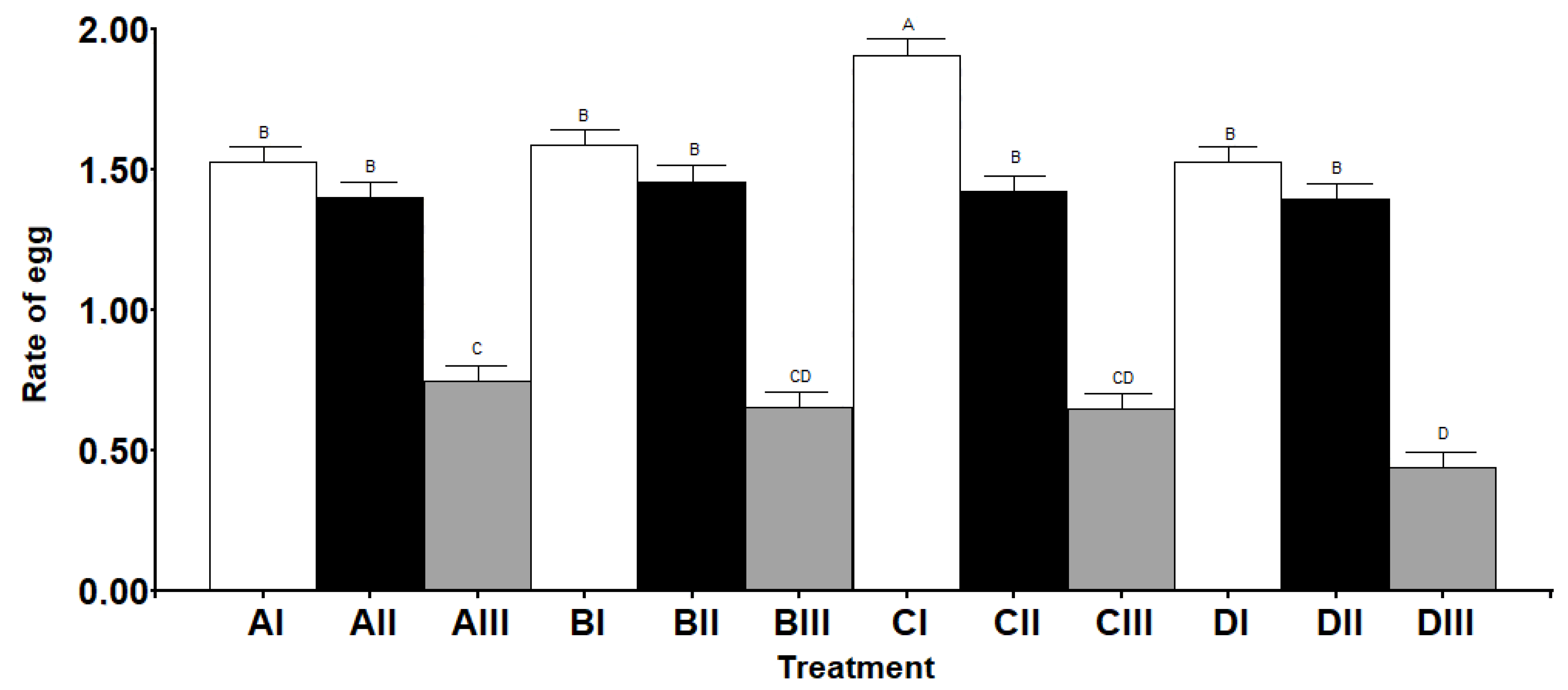
3.1. Lifetime Fecundity, Fertility, and Lifespan
3.2. Ovaries Maturation
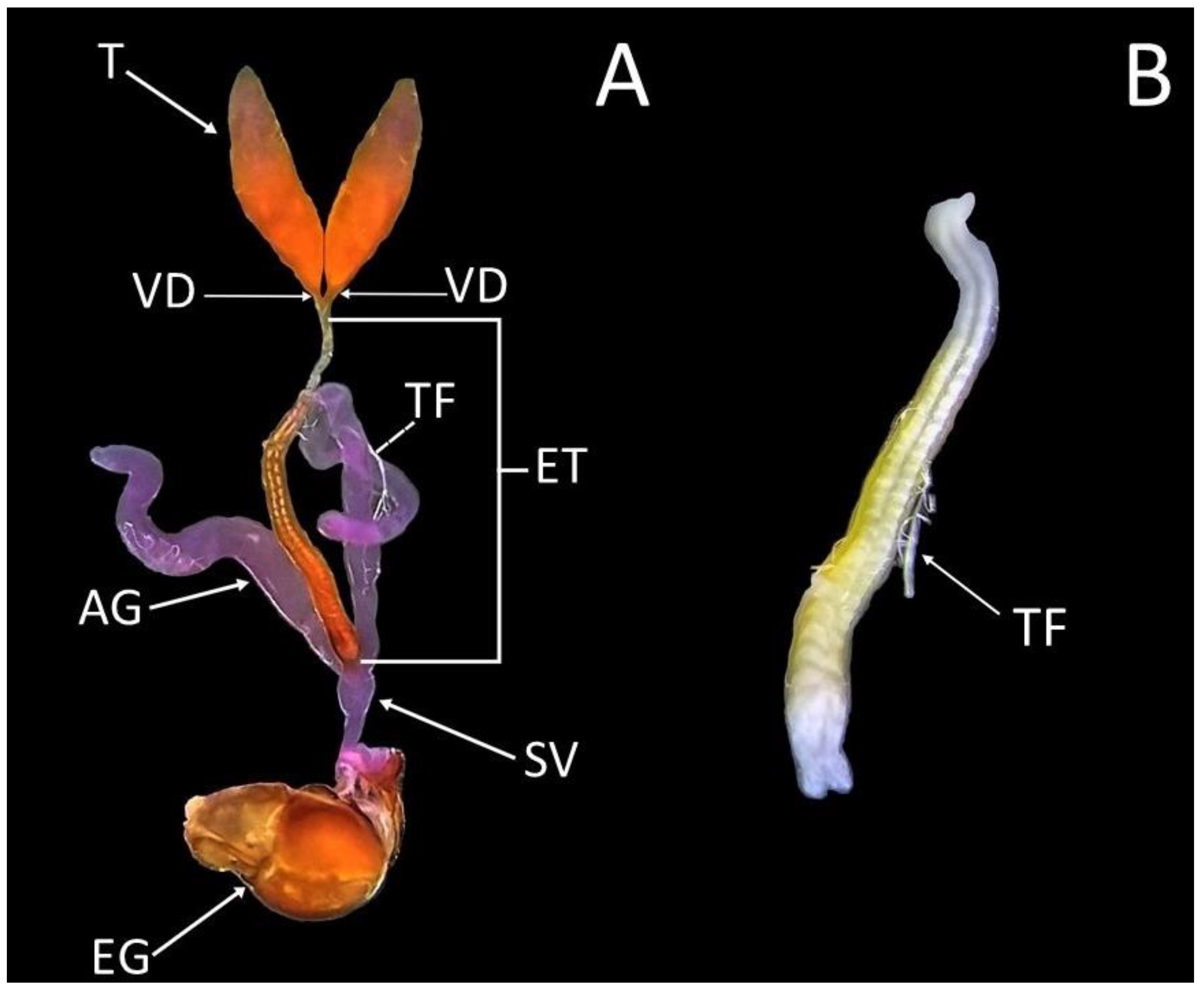
3.3. Description of the Male Reproductive System of Sphaerophoria rueppellii
3.4. Testes Maturation
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bugg, R.; Colfer, R.; Chaney, W.; Smith, H.; Cannon, J. Flower Flies (Syrphidae) and Other Biological Control Agents for Aphids in Vegetables Crops; ANR Publications: Oakland, CA, USA, 2008; pp. 1–25. [Google Scholar] [CrossRef]
- Rotheray, G.E.; Gilbert, F. The Natural History of Hoverflies; Forrest Text; Ceredigion: Wales, UK, 2011; pp. 33–623. [Google Scholar]
- Omkar, O.; Mishra, G. Syrphid flies (The hovering agents). In Ecofriendly Pest Management for Food Security; Omkar, O., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 259–279. [Google Scholar] [CrossRef]
- Klecka, J.; Hadrava, J.; Biella, P.; Akter, A. Flower visitation by hoverflies (Diptera: Syrphidae) in a temperate plant pollinator network. PeerJ 2018, 6, e6025. [Google Scholar] [CrossRef] [PubMed]
- Doyle, T.; Hawkes, W.S.L.; Massy, R.; Poweney, G.D.; Menz, M.H.M.; Wotton, K.R. Pollination by hoverflies in the Antropocene. Proc. R. Soc. B 2020, 287, 20200508. [Google Scholar] [CrossRef] [PubMed]
- Dunn, L.; Lequerica, M.; Reid, C.R.; Latty, T. Dual ecosystem service of syrphid flies (Diptera: Syrphidae): Pollinators and biological control agent. Pest. Manag. Sci. 2020, 76, 1973–1979. [Google Scholar] [CrossRef]
- Passaseo, A.; Pétremand, G.; Rochefort, S.; Castella, E. Pollinator emerging from the extensive green roof: Wild bees (Hymenoptera, Antophila) and hoverflies (Diptera, Syrphidae). Urban Ecosyst. 2020, 23, 1079–1086. [Google Scholar] [CrossRef]
- Freier, B.; Triltsh, H.; Möwes, M.; Moll, E. The potential of predators in natural control of aphids in wheat: Results of a ten-year field study in two German landcape. BioControl 2007, 52, 775–788. [Google Scholar] [CrossRef]
- Haenke, S.; Scheid, B.; Schaefer, M.; Tscharntke, T.; Thies, C. Increasing syrphid fly diversity and density in sown flower strips within simple vs complex landscape. J. Appl. Ecol. 2009, 46, 1106–1114. [Google Scholar] [CrossRef]
- Amorós-Jiménez, R. Biología, Interacciones y Uso del Depredador Sphaerophoria rueppellii (Diptera: Syrphidae) en el Control Integrado de Plagas de Áfidos en Cultivos de Invernadero. Ph.D. Thesis, Centro Iberoamericano de la Biodiversidad, Instituto de Investigación, Universidad de Alicante, Alicante, Spain, 2013. [Google Scholar]
- Pekas, A.; De Craecker, I.; Boonen, S.; Wäckers, F.L.; Moerkens, R. One stone; two birds: Concurrent pest control and pollination services provided by aphidophagous hoverflies. Biol. Control 2020, 149, 104328. [Google Scholar] [CrossRef]
- Rojo, S.; Gilbert, F.; Marcos-García, Mª.A.; Nieto, J.M.; Mier, M.P. A World Review of Predatory Hoverflies (Diptera, Syrphidae: Syrphinae) and Their Prey; CIBIO: Alicante, Spain, 2003. [Google Scholar]
- Singh, K.S.; Cordeiro, E.M.G.; Troczka, B.J.; Pym, A.; Mackisack, J.; Mathers, T.C.; Duarte, A.; Legeai, F.; Robin, S.; Bielza, P.; et al. Global patterns in genomic diversity underpinning the evolution of insecticide resistance in the aphid crop pest Myzus persicae. Commun. Biol. 2021, 4, 847. [Google Scholar] [CrossRef]
- Nascimento, A.V.S.; Santana, E.N.; Braz, A.S.K.; Alfenas, P.F.; Pio-Riberiro, G.; Andrade, G.P.; de Carvalho, M.G.; Murilo Zerbini, F. Cowpea aphid-borne mosaic virus (CABMV) is widespread in passionfruit in Brazil and causes passionfruit woodiness disease. Arch. Virol. 2006, 151, 1797–1809. [Google Scholar] [CrossRef] [PubMed]
- Jaouannet, M.; Rodriguez, P.A.; Thorpe, P.; Lenoir, C.J.G.; MacLeod, R.; Escudero-Martinez, C.; Boss, J.I.B. Plant immunity in plant-aphid interactions. Front. Plant. Sci. 2014, 5, 663. [Google Scholar] [CrossRef]
- Ikbal, C.; Pavela, R. Essential oils as active ingredients of botanical insecticides against aphids. J. Pestic. Sci. 2019, 92, 971–986. [Google Scholar] [CrossRef]
- Nalam, V.; Louis, J.; Shah, J. Plant defense against aphids, the pest extraordinaire. Plant Sci. 2019, 279, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Almohammad, R.; Verheggen, F.J.; Haubruge, E. Searching and oviposition behavior of aphidophagous hoverflies (Diptera: Syrphidae): A review. Biotechnol. Agron. Soc. Environ. 2009, 13, 467–481. [Google Scholar]
- Brewer, M.J.; Peairs, F.B.; Elliot, N.C. Invasive Cereal Aphids of North America: Ecology and Pest Management. Annu. Rev. Entomol. 2019, 64, 73–93. [Google Scholar] [CrossRef] [PubMed]
- Amorós-Jiménez, R.; Pineda, A.; Fereres, A.; Marcos-García, Mª.A. Prey availability and abiotic requirements of immature stages of the aphid predator Sphaerophoria rueppellii. Biol. Control 2012, 63, 17–24. [Google Scholar] [CrossRef]
- Amorós-Jiménez, R.; Pineda, A.; Fereres, A.; Marcos.García, Mª.A. Feeding preference of the aphidophagous hoverfly Sphaerophoria rueppellii affect the performance of its offspring. BioControl 2014, 54, 427–435. [Google Scholar] [CrossRef]
- Branquart, E.; Hemptinne, J.L. Development of ovaries, allometry of reproductive traits and fecundity of Episyrphus balteatus (Diptera: Syrphidae). Eur. J. Entomol. 2000, 97, 165–170. [Google Scholar] [CrossRef]
- Almohammad, R.; Verheggen, F.J.; Francis, F.; Haubruge, E. Evaluation of hoverfly Episyrphus balteatus De Geer (Diptera: Syrphidae) oviposition behavior toward aphid, infested plants using a leaf disc system. Comm. Appl. Biol. Sci. 2006, 71, 403–412. [Google Scholar]
- Leroy, P.D.; Verhegeen, F.J.; Capella, Q.; Francias, F.; Haubruge, E. An introduction device for the aphidophagous hoverfly Episyrphus balteatus (De Geer) (Diptera: Syrphidae). Biol. Control 2010, 54, 181–188. [Google Scholar] [CrossRef]
- Singh, P.; Thakur, M.; Sharma, K.C.; Sharma, H.K.; Nayak, R.K. Larval feeding capacity and pollination efficiency of the aphidophagous syrphids, Eupeodes frequens (Matsmura) and Episyrphus balteatus (De Geer) (Diptera: Syrphidae) on the cabbage aphid (Brevicoryne brassicae L.) (Homoptera: Aphidae) on mustar crops. Egypt. J. Biol. Pest Control 2020, 30, 105. [Google Scholar] [CrossRef]
- Laubertie, E.A.; Wrateen, S.D.; Hemptinne, J.L. The contribution of potential beneficial insectary plant species to adult hoverfly (Diptera: Syrphidae) fitness. Biol. Control 2012, 61, 1–6. [Google Scholar] [CrossRef]
- Van Rijn, P.C.J.; Kooijman, J.; Wáckers, F.L. The contribution of floral resources and honeydew to the performance of predatoy hoverflies (Diptera: Syrphidae). Biol. Control 2013, 67, 32–38. [Google Scholar] [CrossRef]
- Bellefeuille, Y.; Fournier, M.; Lucas, E. Evaluation of two potential biological control agents against the foxglove aphid at low temperatures. J. Insect Sci. 2019, 19, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hurtado, P. Estudio del Ciclo de Vida de Sírfidos eristalinos (Diptera: Syrphidae) y Bases para su Cría Artificial. Ph.D. Thesis, Centro Iberoamericano de la Biodiversidad, Instituto Universitario de Investigación, Universidad de Alicante, Alicante, Spain, 2013. [Google Scholar]
- Pineda, A.; Marcos-García, Mª.A. Seasonal abundance of aphidophagous hoverflies (Diptera: Syrphidae) and their population levels in and outside Mediterranean sweet pepper greenhouse. Ann. Entomol. Soc. Am. 2008, 101, 384–391. [Google Scholar] [CrossRef]
- Lloret-Climent, M.; Amorós-Jiménez, R.; González-Franco, L.; Nescolarde-Selva, J.A. Coverage and invariance for the biological control of pest in Mediterranean greenhouses. Ecol. Modell. 2014, 292, 37–44. [Google Scholar] [CrossRef][Green Version]
- Speight, M.C.D. Species Accounts of European Syrphidae; Syrph the Net Publications: Dublin, Ireland, 2020; p. 314. [Google Scholar]
- Van Rijn, P.C.J.; Wäckers, F.L. Nectar accessibility determines fitness, flower choice and abundance of hoverflies that provide natural pest control. J. Appl. Ecol. 2016, 53, 925–933. [Google Scholar] [CrossRef]
- Hart, A.J.; Bale, J.S.; Fenlon, J.S. Development threshold, day-degree requirements and voltinism of the aphid predator Episyrphus balteatus (Diptera: Syrphidae). Ann. Appl. Biol. 1997, 130, 427–437. [Google Scholar] [CrossRef]
- Hondelmann, P.; Poehling, H.-M. Diapause and overwintering of the hoverfly Episyrphus balteatus. Entomol. Exp. Appl. 2007, 124, 189–200. [Google Scholar] [CrossRef]
- Stenberg, J.A. A conceptual framework for Integrated Pest Management. Trends Plants Sci. 2017, 22, P759–P769. [Google Scholar] [CrossRef]
- Sinclair, B.J.; Borkent, A.; Monty Wood, D. The male genital tract and aedeagal component of the Diptera with a discussion of their phylogenetic significance. J. Linn. Soc. Lond. Zool. 2007, 150, 711–742. [Google Scholar] [CrossRef]
- Minkenberg, O.P.J.M.; Tatar, M.; Rosenheim, J.A. Egg load as a major source of variability in insect foraging and oviposition behavior. Oikos 1992, 65, 134–142. [Google Scholar] [CrossRef]
- Duffield, R.M. Biology of Microdon fuscipennis (Diptera: Syrphidae) with interpretations of the reproductive strategies of Microdon species found North of Mexico. Proc. Entomol. Soc. Wash. 1981, 83, 716–724. [Google Scholar]
- Gilbert, F.F. Hoverflies Naturalists’ Handbooks; Pelagic Publishing: Exeter, UK, 2015; Volume 5, pp. 1–67. [Google Scholar]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; González, L.; Tablada, M.; Robledo, C.M. InfoStat Versión 2018e. Grupo InfoStat, FCA, Universidad Nacional de Córdoba, Argentina. Available online: http://infostat.com.ar (accessed on 17 May 2021).
- R’Kha, S.; Moreteau, B.; Coyne, J.A.; David, J.R. Evolution of a lesser fitness trait: Egg production in the specialist Drosophila sechellia. Genet. Res. Camb. 1997, 69, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Sarikaya, D.P.; Belay, A.A.; Ahuja, A.; Dorta, A.; Green, D.A., II; Extavour, C.G. The roles of cell size and cell number in determining ovariole number in Drosophila. Dev. Biol. 2012, 363, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Jervis, M.A.; Copland, M.J.W.; Harvey, J.A. The life-cycle. In Insects as Natural Enemies. A Practical Perspective; Jervis, M.A., Ed.; Springer: Dordrecht, The Netherlands, 2007; pp. 72–87. [Google Scholar]
- Dixon, T.J. Studies on oviposition behaviour of Syrphidae (Diptera). Trans. R. Ent. Soc. Lond. 1959, 111, 57–80. [Google Scholar] [CrossRef]
- Chandler, A.E.F. The relationship between aphid infestations and oviposition by aphidophagous Syrphidae (Diptera). Ann. Appl. Biol. 1968, 61, 425–434. [Google Scholar] [CrossRef]
- Zheng, Z.; Liu, H.; Wang, X.; Wu, X.; Chen, Y.; Deng, J.; Chen, X.; Li, Y.; Pu, D. Development and reproduction of the hoverfly Eupeodes corollae (Diptera: Syrphidae). J. Earth Sci. Environ. Stud. 2019, 4, 654–660. [Google Scholar]
- Belliure, B.; Michaud, J.P. Biology and behavior of Pseudodorus clavatus (Diptera: Syrphidae), and important predator of citrus aphids. Ann. Entomol. Soc. Am. 2001, 94, 91–96. [Google Scholar] [CrossRef]
- Sánchez-Galván, I.R.; Quinto, J.; Micó, E.; Galante, E.; Marcos-García, Mª.A. Facilitation among saproxylic insects inhabiting tree hollows in a Mediterranean forrest: The case of cetonids (Coleoptera: Cetoniidae) and syrphids (Diptera: Syrphidae). Environ. Entomol. 2014, 43, 336–343. [Google Scholar] [CrossRef]
- McCalla, K.A.; Keçeci, M.; Milosavljević, I.; Ratkowsky, D.A.; Hoodle, M.S. The influence of temperature variation on life history parameters and therman performance curves of Tamarixia radiata (Hymenoptera: Eulophidae), a parasitoid of the Asian Citrus Psyllid (Hemiptera: Liviidae). J. Econ. Entomol. 2019, 112, 1560–1574. [Google Scholar] [CrossRef]
- Scheneider, F. Bionomics and physiology of aphidophagous syrphidae. Annu. Rev. Entomol. 1969, 14, 103–124. [Google Scholar] [CrossRef]
- Svensson, B.G.; Janzon, L.A. Why does the hoverfly Metasyrphus corollae migrate? Ecol. Entomol. 1984, 9, 329–335. [Google Scholar] [CrossRef]
- Jervis, M.A.; Kidd, N.A.C. Host-feeding strategies in hymenopteran parasitoids. Biol. Rev. 1986, 61, 395–434. [Google Scholar] [CrossRef]
- Rosenheim, J.A.; Heimpel, G.E.; Mangel, M. Egg maturation, egg resorption and the costliness of transient egg limitation in insects. Proc. R. Soc. Lond. B 2000, 267, 1565–1573. [Google Scholar] [CrossRef] [PubMed]
- Kunkel, J.G. Models of pattern formation in Insect Oocytes. In Vivo 1991, 5, 443–456. [Google Scholar] [PubMed]
- Klowden, M.J. Reproduction Systems. In Physiological Systems in Insects, 3rd ed.; Klowden, M.J., Ed.; Academic Press: Cambridge, MA, USA, 2013; Chapter 4; pp. 197–254. [Google Scholar] [CrossRef]
- Hong, B.M.; Hung, H.Q. Effect of temperature and diet on the life cycle and predatory capacity of Episyrphus balteatus (De Geer) (Syrphidae: Diptera) cultured on Aphis gossypii (Glover). J. ISSAAS 2010, 16, 98–103. [Google Scholar]
- Milosavljević, I.; McCalla, K.A.; Ratkowsky, D.A.; Hoddle, M.S. Effect of constant and fluctuating temperatures on development rates and logenvity of Diaphorencyrtus aligarhensis (Hymenoptera: Encyrtidae). J. Econ. Entomol. 2019, 112, 1062–1072. [Google Scholar] [CrossRef]
- Spiegel, C.N.; Bretas, J.A.C.; Peixoto, A.A.; Vigoder, F.M.; Bruno, R.V.; Soared, M.J. Fine structure of the male reproductive system and reproductive behavior of Lutzomyia longipalpis Sandflies (Diptera: Psychodidae: Phlebotominae). PLoS ONE 2013, 8, e74898. [Google Scholar] [CrossRef]
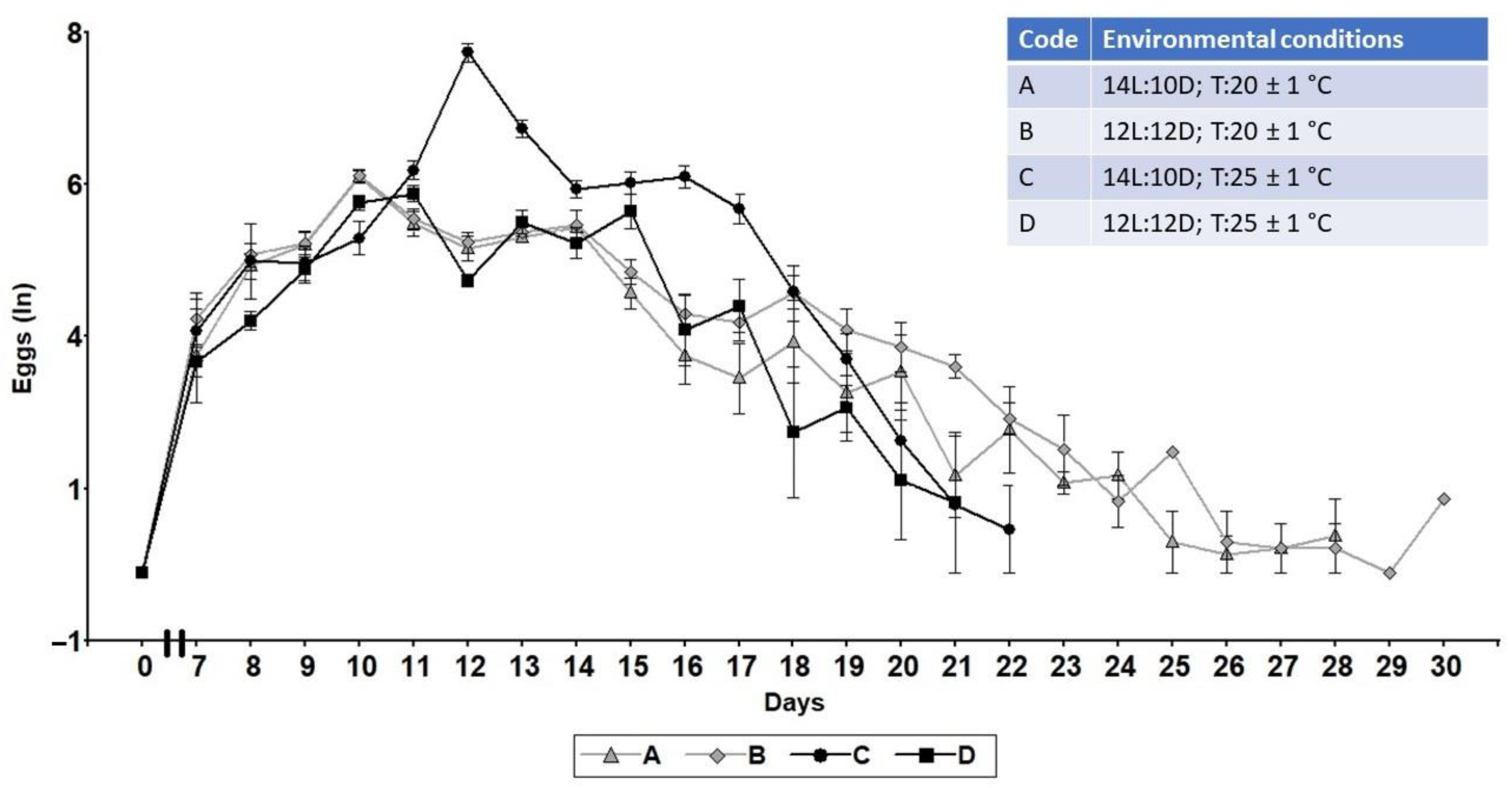
| Code | Environmental Conditions |
|---|---|
| A | 14L:10D; T:20 ± 1 °C |
| B | 12L:12D; T:20 ± 1 °C |
| C | 14L:10D; T:25 ± 1 °C |
| D | 12L:12D; T:25 ± 1 °C |
| Code | Environmental Conditions | Mean Total Eggs | Hatching (%) |
|---|---|---|---|
| A | 14L:10D, 20 ± 1 °C | 62.72 ± 24.06 b | 88.6 ± 0.87 a |
| B | 12L:10D, 20 ± 1 °C | 70.91 ± 24.06 b | 91 ± 0.55 a |
| C | 14L:10D, 25 ± 1 °C | 294.67 ± 28.35 a | 96 ± 1.38 b |
| D | 12L:12D, 25 ± 1 °C | 94.05 ± 30.07 b | 81 ± 0.73 a |
| Code | Environmental Conditions | Lifespan (Days) | |
|---|---|---|---|
| Males (n = 50) | Female (n = 150) | ||
| A | 14L:10D, 20 ± 1 °C | 28 ± 2.3 a,b | 27.1 ± 0.72 a,b |
| B | 12L:10D, 20 ± 1 °C | 23 ± 0.89 b,c | 29.6 ± 0.92 a |
| C | 14L:10D, 25 ± 1 °C | 20.8 ± 0.86 c | 22.4 ± 1.25 c |
| D | 12L:12D, 25 ± 1 °C | 21.8 ± 0.80 c | 20.7 ± 1.24 c |
| Code | Environmental Conditions | Treatment * | Maturation (Day) | Peak of Ovarioles (Day) |
|---|---|---|---|---|
| A | 14L:10D, 20 ± 1 °C | I | 8th | 11th |
| II | 11th | 15th | ||
| III | 11th | 15th | ||
| B | 12L:10D,20 ± 1 °C | I | 8th | 11th |
| II | 8th | 11th | ||
| III | 8th | 11th | ||
| C | 14L:10D, 25 ± 1 °C | I | 8th | 11th |
| II | 8th | 11th | ||
| III | 8th | 15th | ||
| D | 12L:12D, 25 ± 1 °C | I | 8th | 11th |
| II | 8th | 11th | ||
| III | 11th | 15th |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orengo-Green, J.J.; Casas, J.L.; Marcos-García, M.Á. Effect of Abiotic Climatic Factors on the Gonadal Maturation of the Biocontrol Agent Sphaerophoria rueppellii (Wiedemann, 1830) (Diptera: Syrphidae). Insects 2022, 13, 573. https://doi.org/10.3390/insects13070573
Orengo-Green JJ, Casas JL, Marcos-García MÁ. Effect of Abiotic Climatic Factors on the Gonadal Maturation of the Biocontrol Agent Sphaerophoria rueppellii (Wiedemann, 1830) (Diptera: Syrphidae). Insects. 2022; 13(7):573. https://doi.org/10.3390/insects13070573
Chicago/Turabian StyleOrengo-Green, José J., José L. Casas, and Mª. Ángeles Marcos-García. 2022. "Effect of Abiotic Climatic Factors on the Gonadal Maturation of the Biocontrol Agent Sphaerophoria rueppellii (Wiedemann, 1830) (Diptera: Syrphidae)" Insects 13, no. 7: 573. https://doi.org/10.3390/insects13070573
APA StyleOrengo-Green, J. J., Casas, J. L., & Marcos-García, M. Á. (2022). Effect of Abiotic Climatic Factors on the Gonadal Maturation of the Biocontrol Agent Sphaerophoria rueppellii (Wiedemann, 1830) (Diptera: Syrphidae). Insects, 13(7), 573. https://doi.org/10.3390/insects13070573






